ITK329 Kinetika Katalisis Chapter 7 HETEROGENEOUS CATALYST CATALYSIS

- Slides: 64

ITK-329 Kinetika & Katalisis Chapter 7 HETEROGENEOUS CATALYST & CATALYSIS Dicky Dermawan www. dickydermawan. net 78. net dickydermawan@gmail. com 135

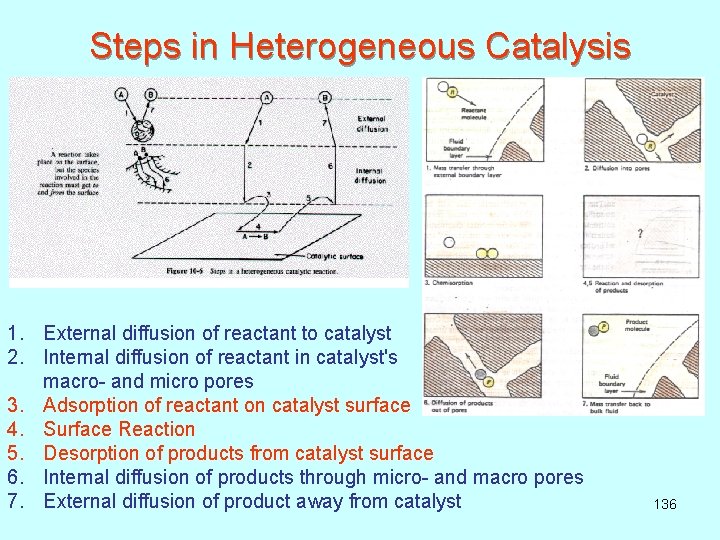
Steps in Heterogeneous Catalysis 1. External diffusion of reactant to catalyst 2. Internal diffusion of reactant in catalyst's macro- and micro pores 3. Adsorption of reactant on catalyst surface 4. Surface Reaction 5. Desorption of products from catalyst surface 6. Internal diffusion of products through micro- and macro pores 7. External diffusion of product away from catalyst 136

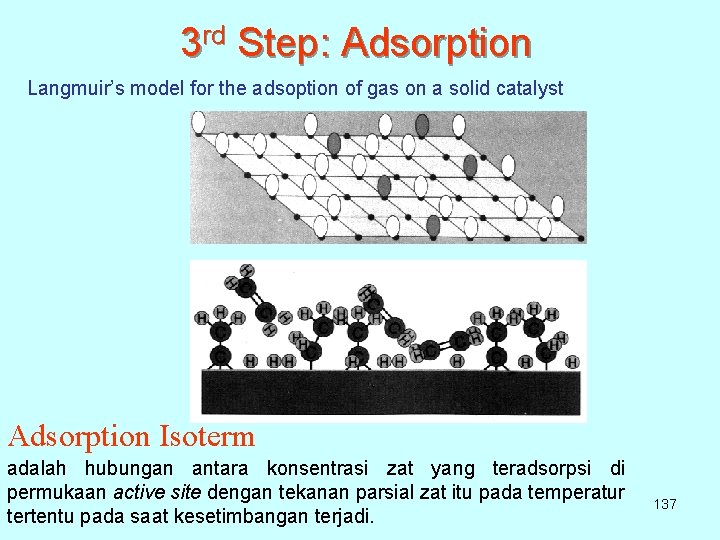
3 rd Step: Adsorption Langmuir’s model for the adsoption of gas on a solid catalyst Adsorption Isoterm adalah hubungan antara konsentrasi zat yang teradsorpsi di permukaan active site dengan tekanan parsial zat itu pada temperatur tertentu pada saat kesetimbangan terjadi. 137

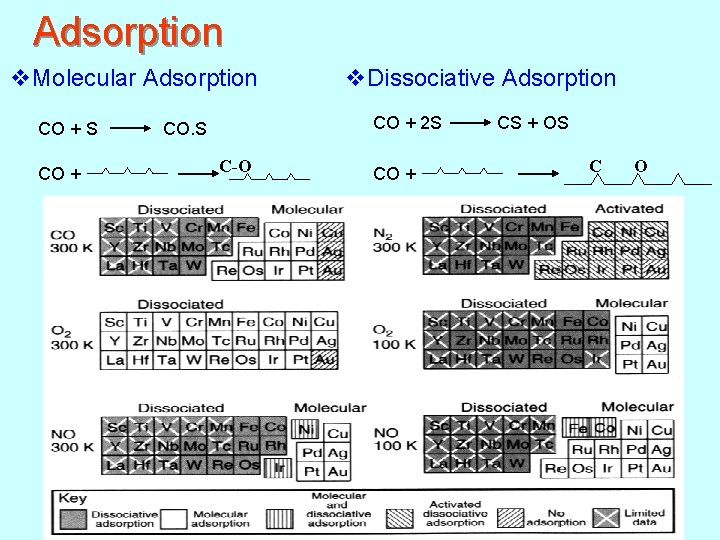
Adsorption v. Molecular Adsorption v. Dissociative Adsorption CO + S CO. S CO + 2 S CS + OS C-O CO + C O 138

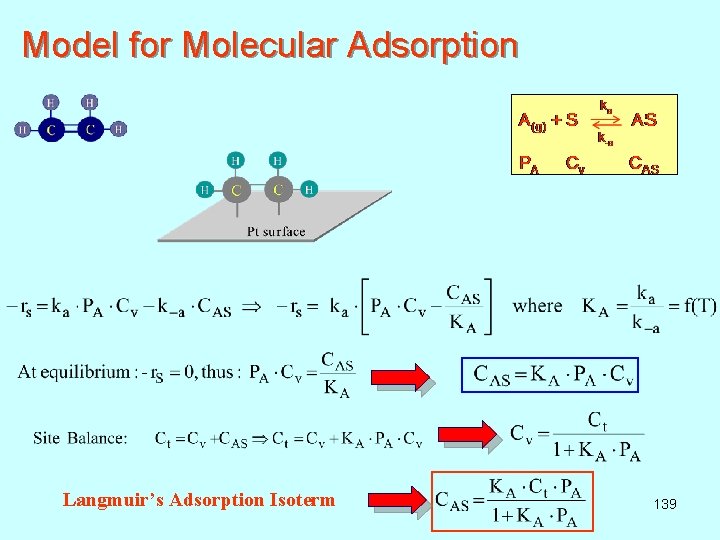
Model for Molecular Adsorption Langmuir’s Adsorption Isoterm 139

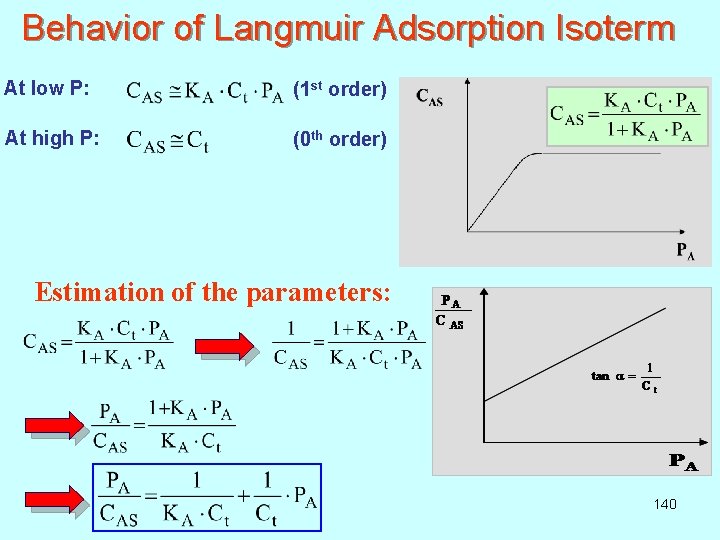
Behavior of Langmuir Adsorption Isoterm At low P: (1 st order) At high P: (0 th order) Estimation of the parameters: 140

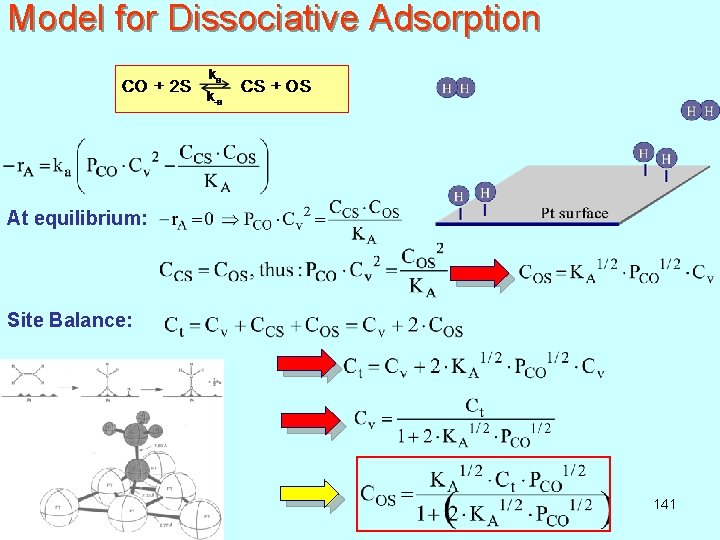
Model for Dissociative Adsorption At equilibrium: Site Balance: 141

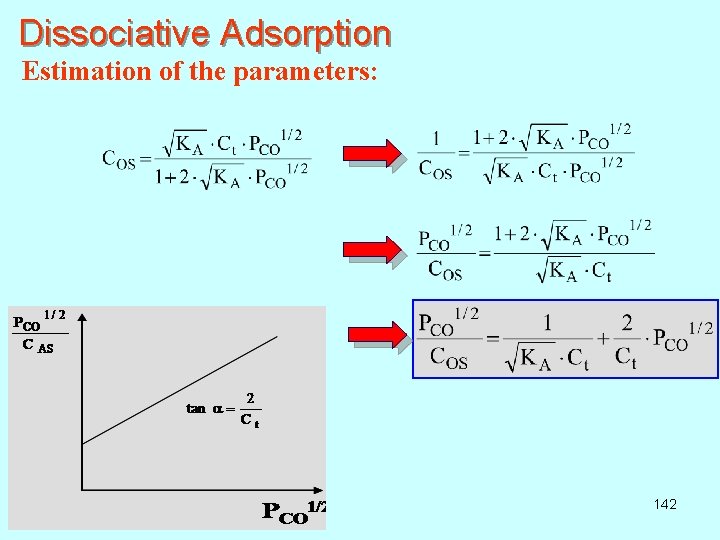
Dissociative Adsorption Estimation of the parameters: 142

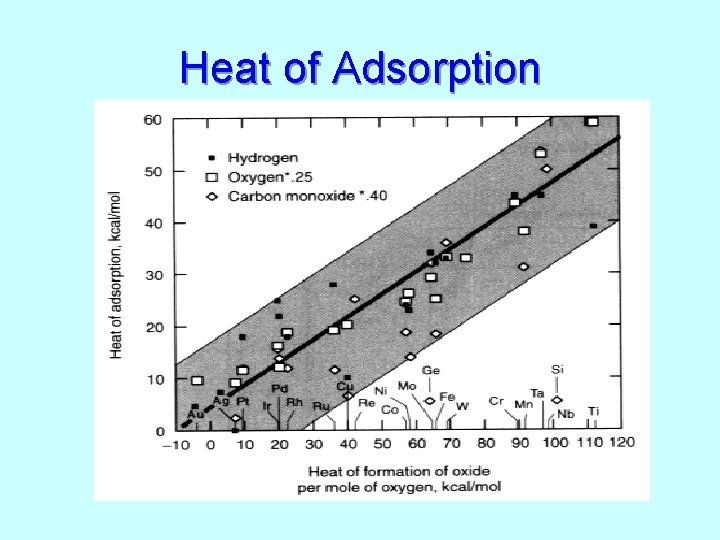
Heat of Adsorption

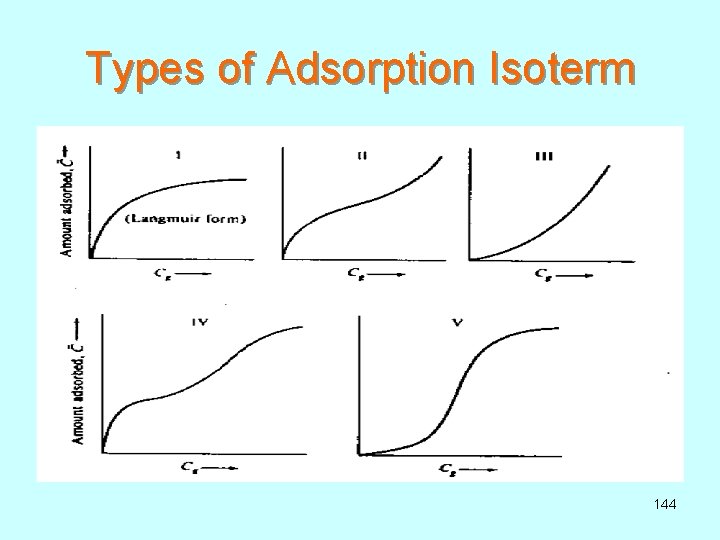
Types of Adsorption Isoterm 144

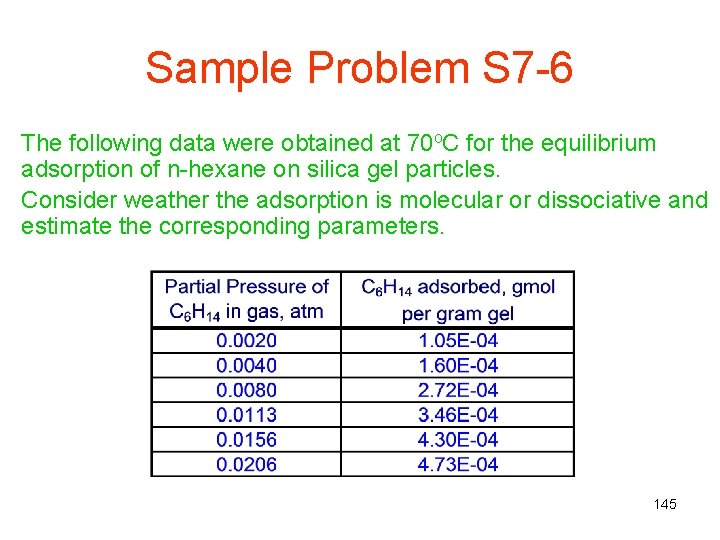
Sample Problem S 7 -6 The following data were obtained at 70 o. C for the equilibrium adsorption of n-hexane on silica gel particles. Consider weather the adsorption is molecular or dissociative and estimate the corresponding parameters. 145

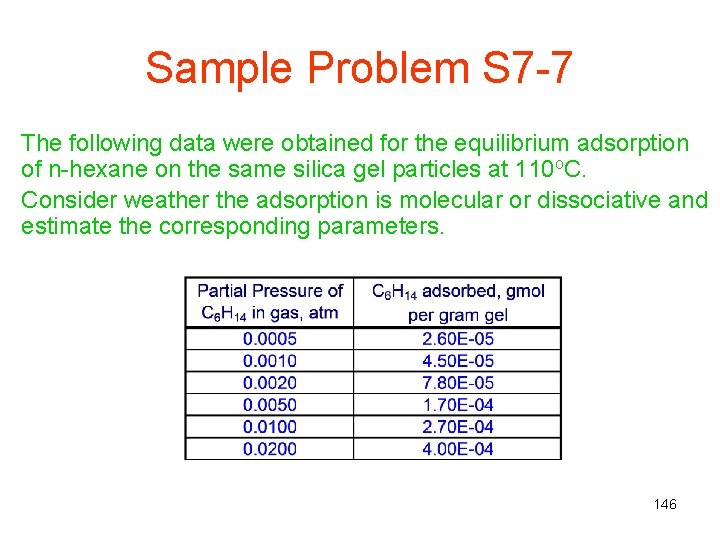
Sample Problem S 7 -7 The following data were obtained for the equilibrium adsorption of n-hexane on the same silica gel particles at 110 o. C. Consider weather the adsorption is molecular or dissociative and estimate the corresponding parameters. 146

Sample Problem S 7 -10 147

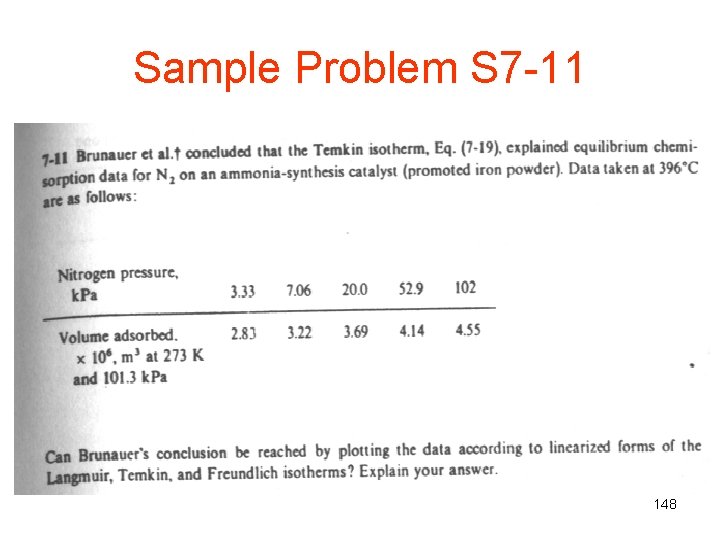
Sample Problem S 7 -11 148

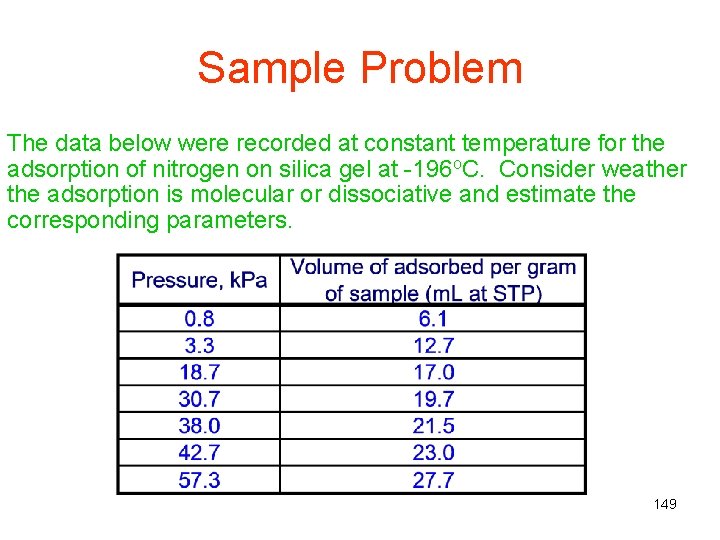
Sample Problem The data below were recorded at constant temperature for the adsorption of nitrogen on silica gel at -196 o. C. Consider weather the adsorption is molecular or dissociative and estimate the corresponding parameters. 149

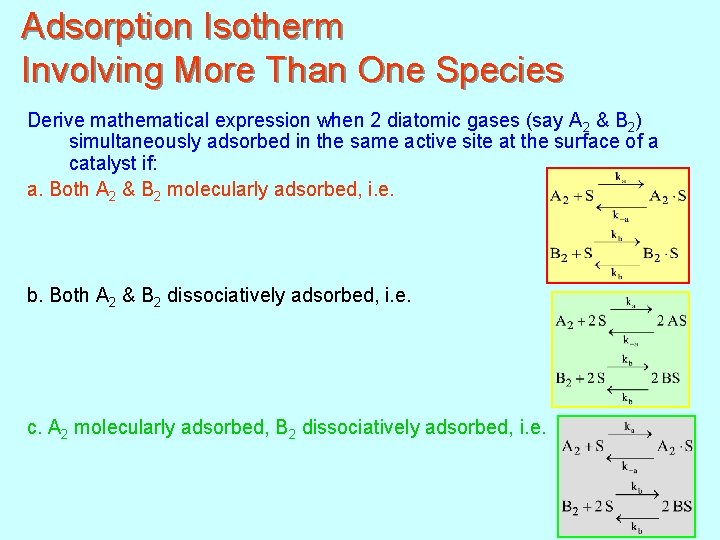
Adsorption Isotherm Involving More Than One Species Derive mathematical expression when 2 diatomic gases (say A 2 & B 2) simultaneously adsorbed in the same active site at the surface of a catalyst if: a. Both A 2 & B 2 molecularly adsorbed, i. e. b. Both A 2 & B 2 dissociatively adsorbed, i. e. c. A 2 molecularly adsorbed, B 2 dissociatively adsorbed, i. e. 150

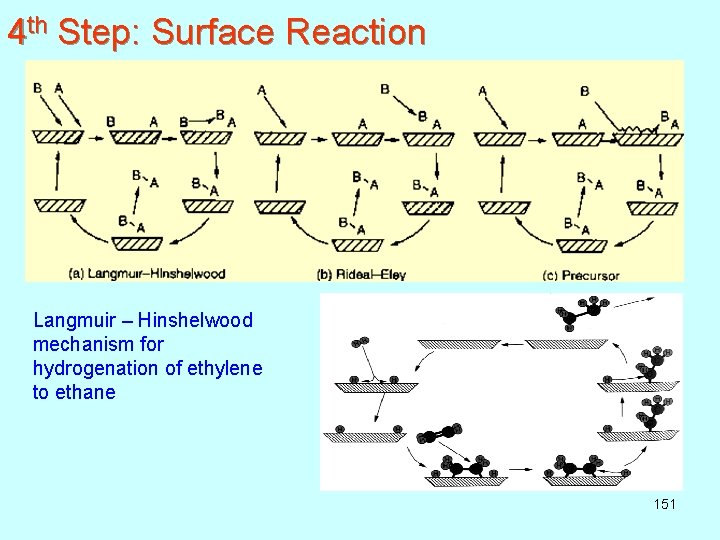
4 th Step: Surface Reaction Langmuir – Hinshelwood mechanism for hydrogenation of ethylene to ethane 151

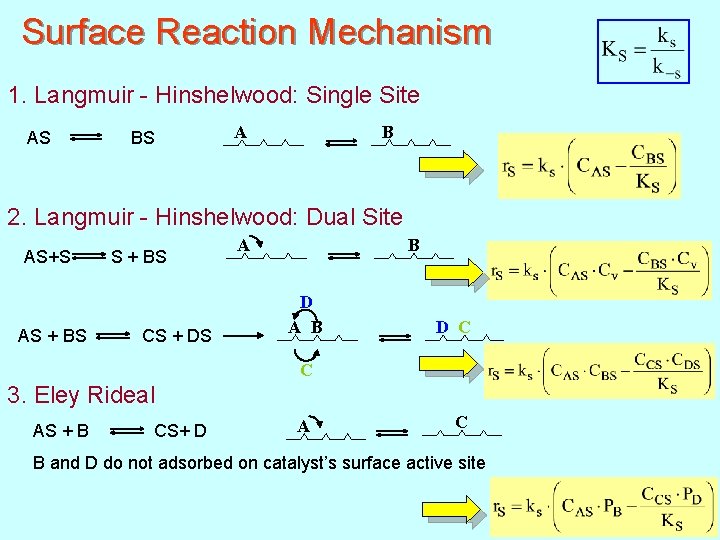
Surface Reaction Mechanism 1. Langmuir - Hinshelwood: Single Site AS BS A B 2. Langmuir - Hinshelwood: Dual Site AS+S S + BS AS + BS CS + DS B A D A B D C C 3. Eley Rideal AS + B CS+ D A C B and D do not adsorbed on catalyst’s surface active site 152

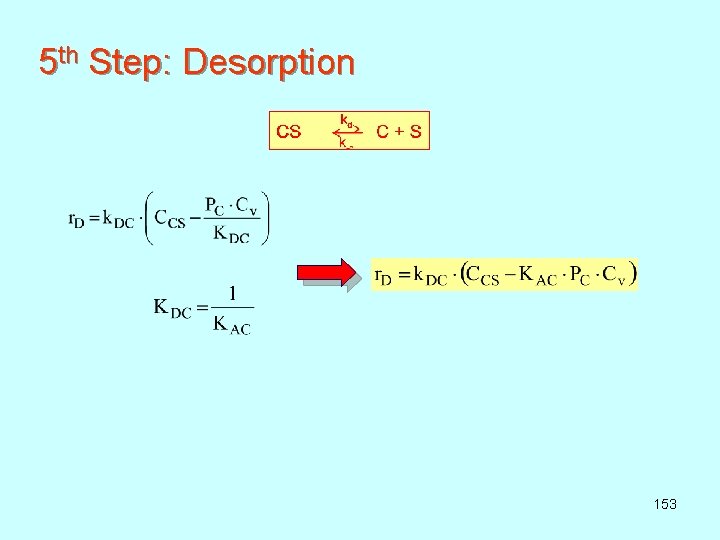
5 th Step: Desorption 153

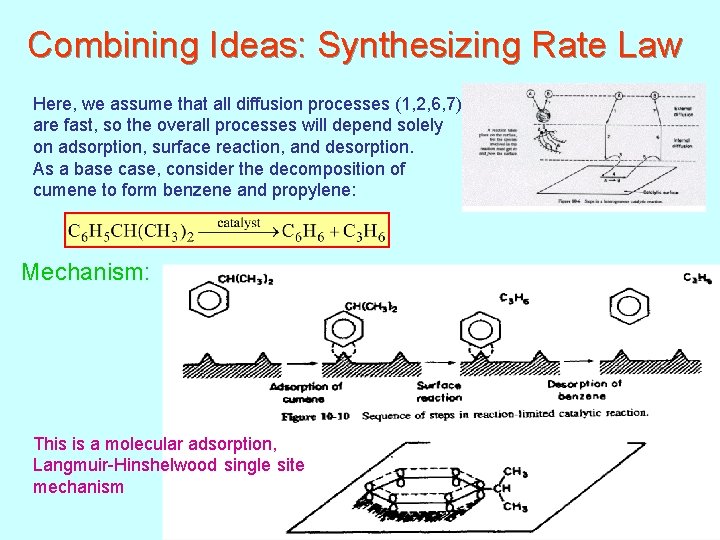
Combining Ideas: Synthesizing Rate Law Here, we assume that all diffusion processes (1, 2, 6, 7) are fast, so the overall processes will depend solely on adsorption, surface reaction, and desorption. As a base case, consider the decomposition of cumene to form benzene and propylene: Mechanism: This is a molecular adsorption, Langmuir-Hinshelwood single site mechanism 154

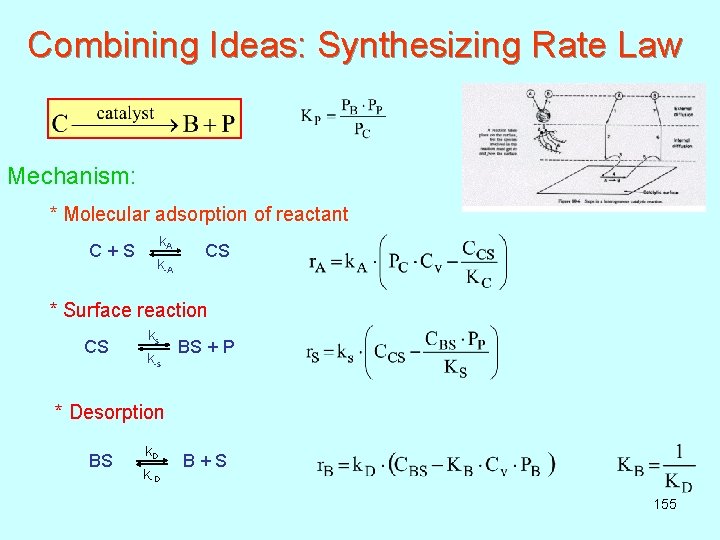
Combining Ideas: Synthesizing Rate Law Mechanism: * Molecular adsorption of reactant k A C + S CS k-A * Surface reaction ks CS BS + P k-s * Desorption k D BS B + S k-D 155

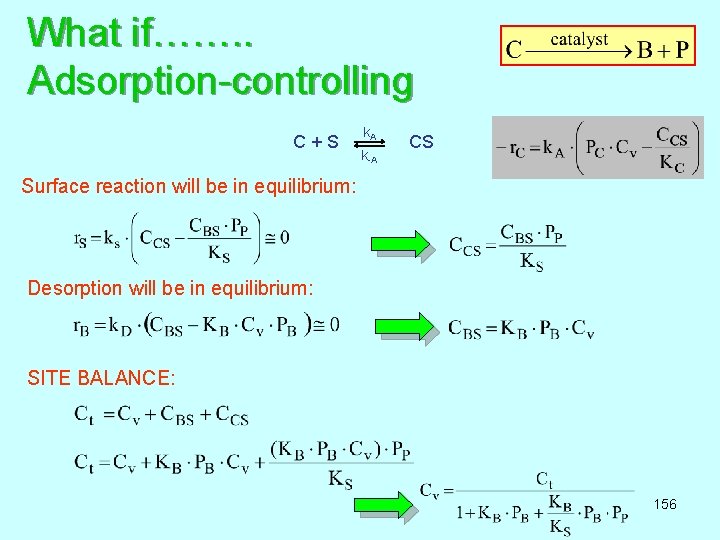
What if……. . Adsorption-controlling k A C + S CS k-A Surface reaction will be in equilibrium: Desorption will be in equilibrium: SITE BALANCE: 156

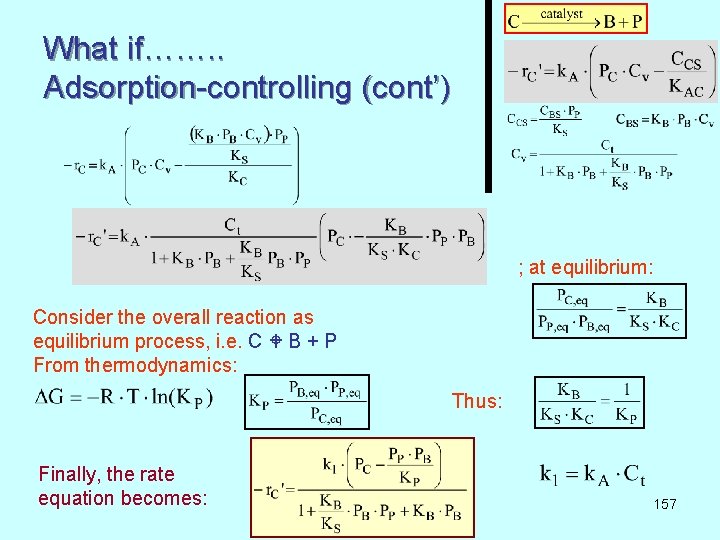
What if……. . Adsorption-controlling (cont’) ; at equilibrium: Consider the overall reaction as equilibrium process, i. e. C B + P From thermodynamics: Thus: Finally, the rate equation becomes: 157

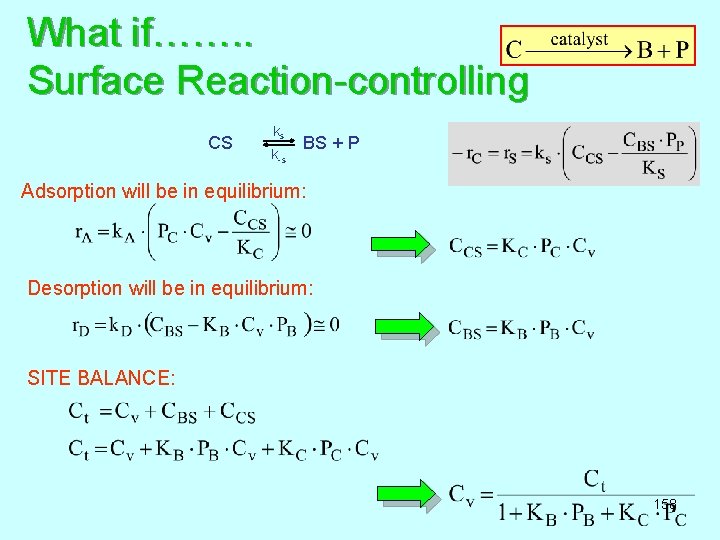
What if……. . Surface Reaction-controlling ks CS BS + P k-s Adsorption will be in equilibrium: Desorption will be in equilibrium: SITE BALANCE: 158

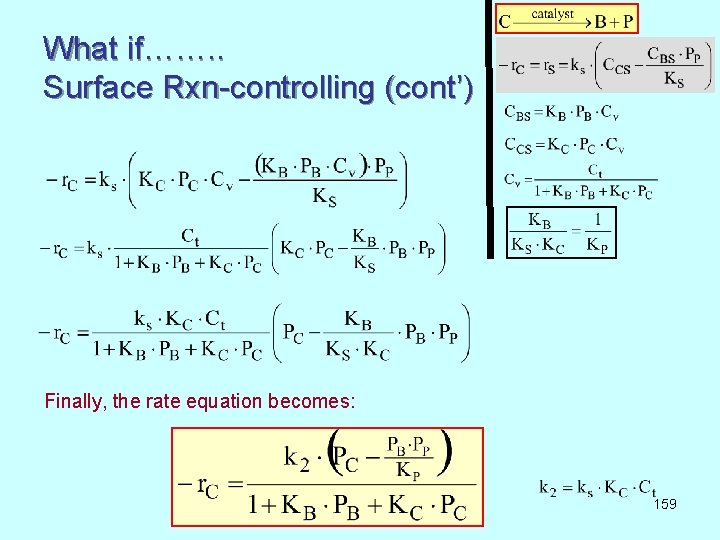
What if……. . Surface Rxn-controlling (cont’) Finally, the rate equation becomes: 159

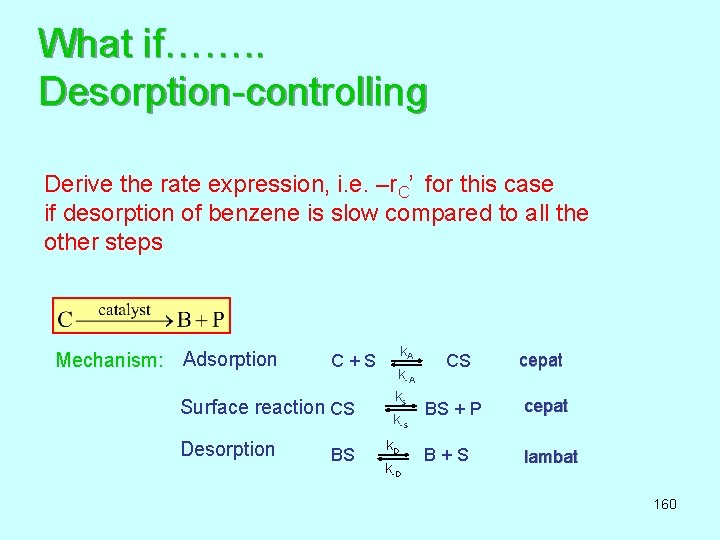
What if……. . Desorption-controlling Derive the rate expression, i. e. –r. C’ for this case if desorption of benzene is slow compared to all the other steps Mechanism: Adsorption k A C + S CS k-A k s Surface reaction CS BS + P k-s Desorption k D BS B + S k-D cepat lambat 160

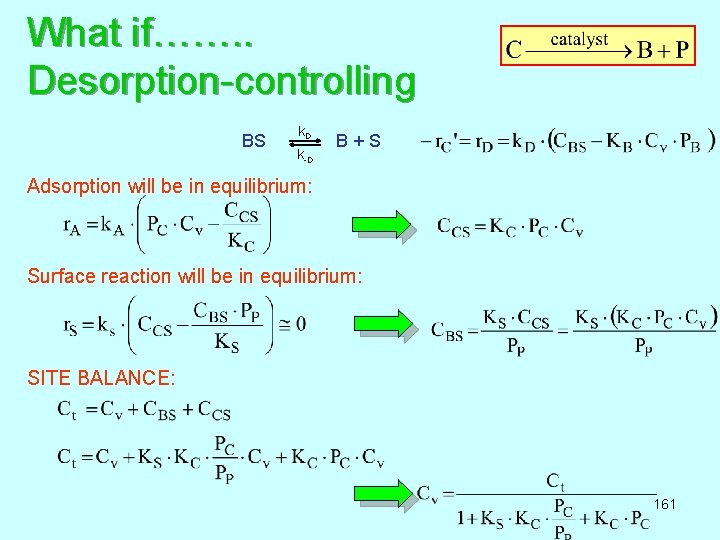
What if……. . Desorption-controlling k D BS B + S k-D Adsorption will be in equilibrium: Surface reaction will be in equilibrium: SITE BALANCE: 161

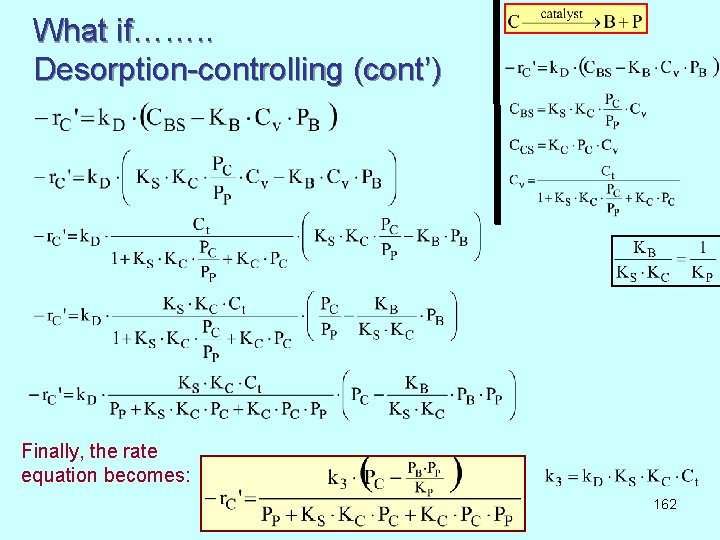
What if……. . Desorption-controlling (cont’) Finally, the rate equation becomes: 162

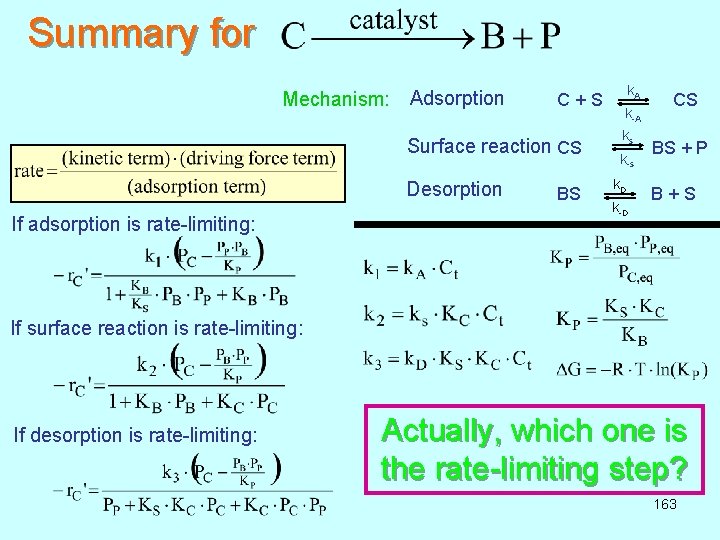
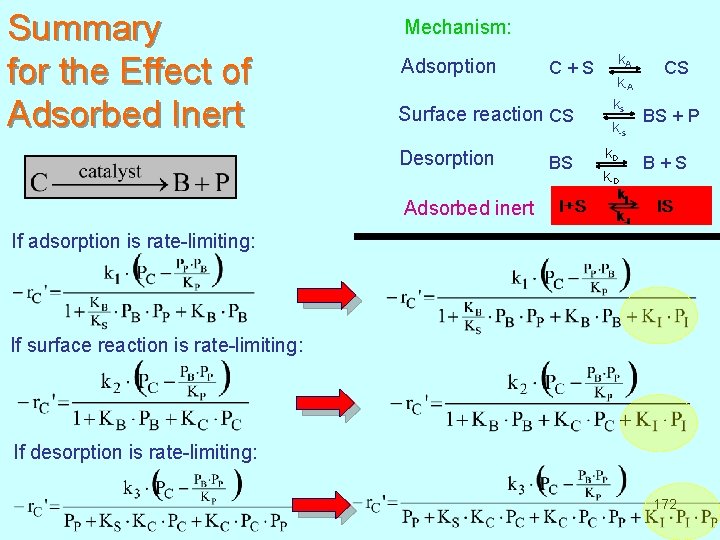
Summary for Mechanism: Adsorption k A C + S CS k-A k s Surface reaction CS BS + P k-s Desorption If adsorption is rate-limiting: k D BS B + S k-D If surface reaction is rate-limiting: If desorption is rate-limiting: Actually, which one is the rate-limiting step? 163

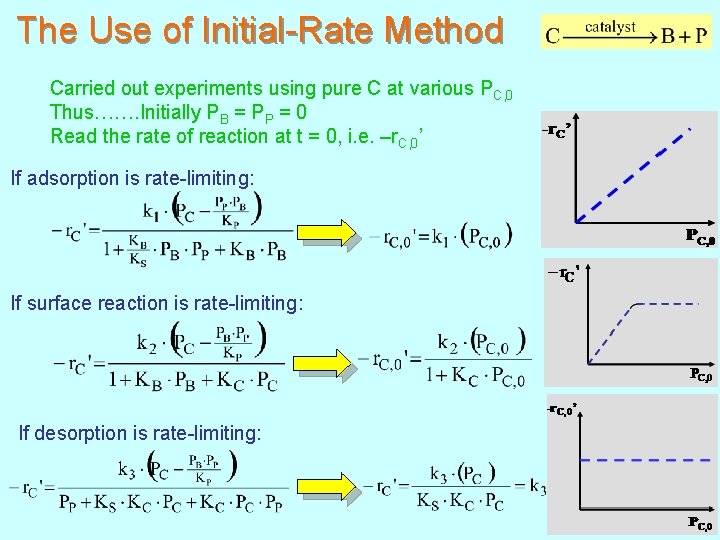
The Use of Initial-Rate Method Carried out experiments using pure C at various PC, 0 Thus……. Initially PB = PP = 0 Read the rate of reaction at t = 0, i. e. –r. C, 0’ If adsorption is rate-limiting: If surface reaction is rate-limiting: If desorption is rate-limiting: 164

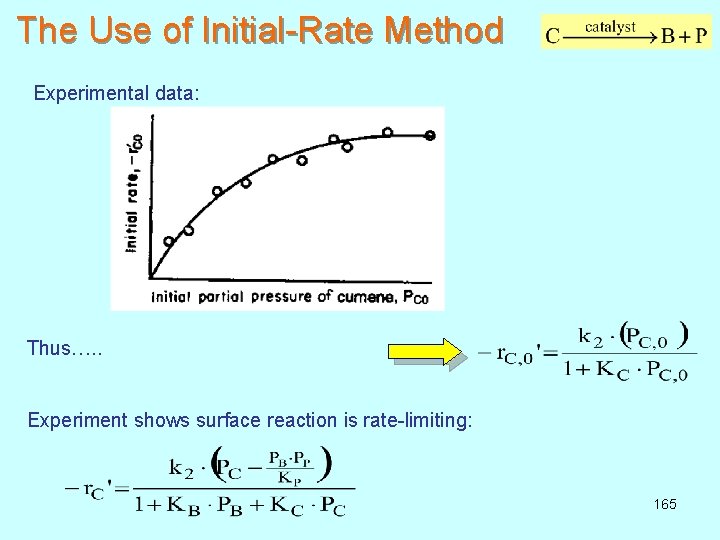
The Use of Initial-Rate Method Experimental data: Thus…. . Experiment shows surface reaction is rate-limiting: 165

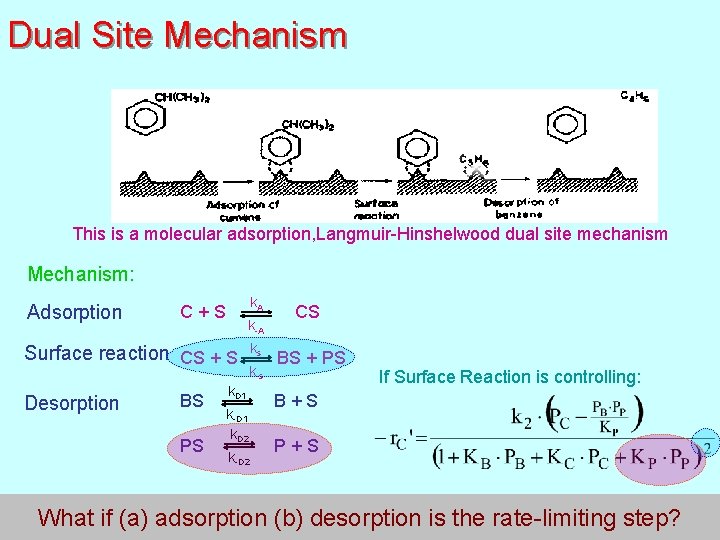
Dual Site Mechanism This is a molecular adsorption, Langmuir-Hinshelwood dual site mechanism Mechanism: Adsorption k A C + S CS k-A ks Surface reaction CS + S BS + PS k-s Desorption k. D 1 If Surface Reaction is controlling: BS B + S k-D 1 k. D 2 PS P + S k-D 2 166 What if (a) adsorption (b) desorption is the rate-limiting step?

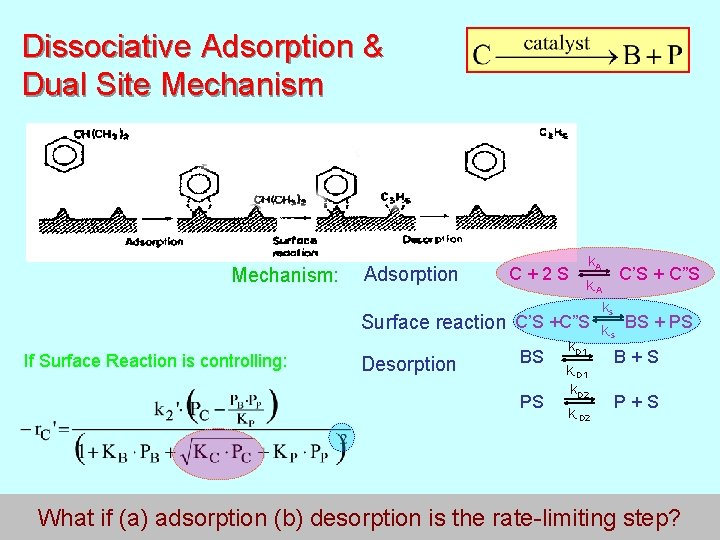
Dissociative Adsorption & Dual Site Mechanism: Adsorption k. A C + 2 S C’S + C”S k-A ks Surface reaction C’S +C”S BS + PS k-s If Surface Reaction is controlling: Desorption k D 1 BS B + S k-D 1 k. D 2 PS P + S k-D 2 167 What if (a) adsorption (b) desorption is the rate-limiting step?

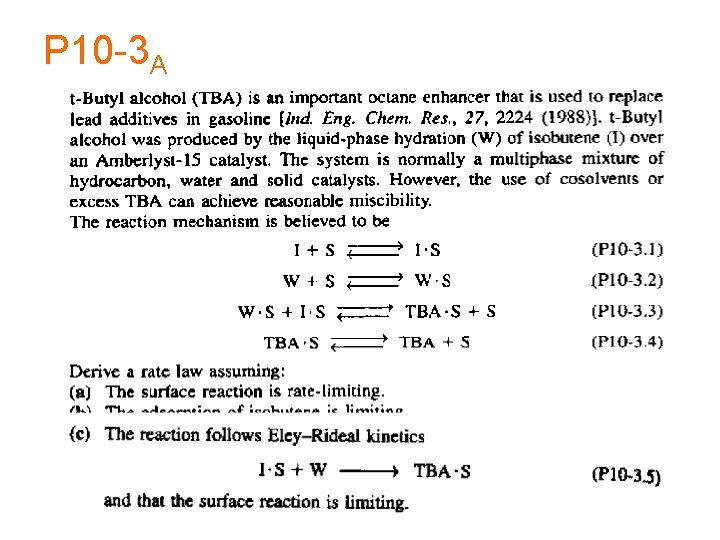
P 10 -3 A 168

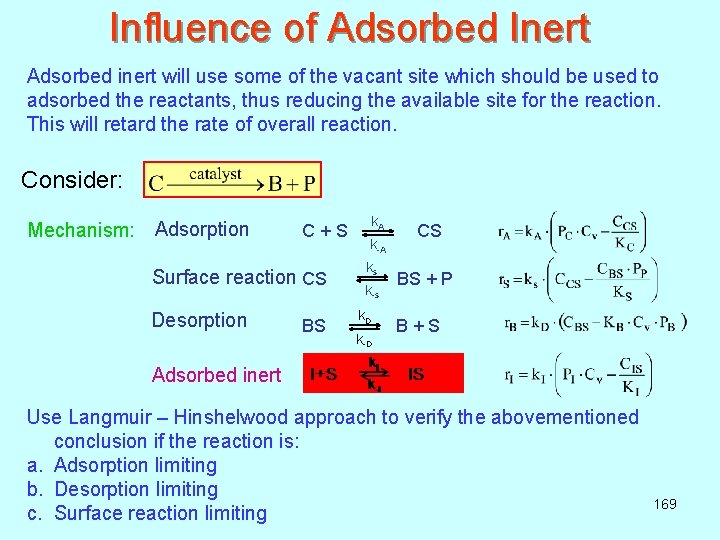
Influence of Adsorbed Inert Adsorbed inert will use some of the vacant site which should be used to adsorbed the reactants, thus reducing the available site for the reaction. This will retard the rate of overall reaction. Consider: Mechanism: Adsorption k A C + S CS k-A ks Surface reaction CS BS + P k-s Desorption k D BS B + S k-D Adsorbed inert Use Langmuir – Hinshelwood approach to verify the abovementioned conclusion if the reaction is: a. Adsorption limiting b. Desorption limiting c. Surface reaction limiting 169

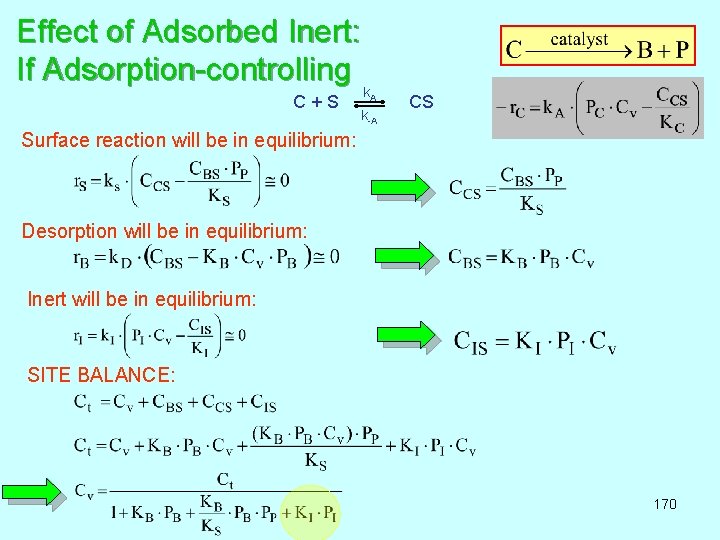
Effect of Adsorbed Inert: If Adsorption-controlling k A C + S CS k-A Surface reaction will be in equilibrium: Desorption will be in equilibrium: Inert will be in equilibrium: SITE BALANCE: 170

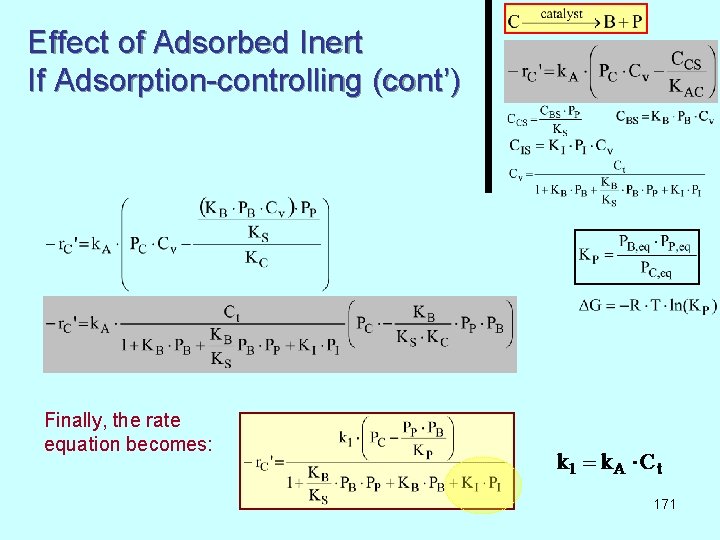
Effect of Adsorbed Inert If Adsorption-controlling (cont’) Finally, the rate equation becomes: 171

Summary for the Effect of Adsorbed Inert Mechanism: Adsorption k A C + S CS k-A k s Surface reaction CS BS + P k-s Desorption k D BS B + S k-D Adsorbed inert If adsorption is rate-limiting: If surface reaction is rate-limiting: If desorption is rate-limiting: 172

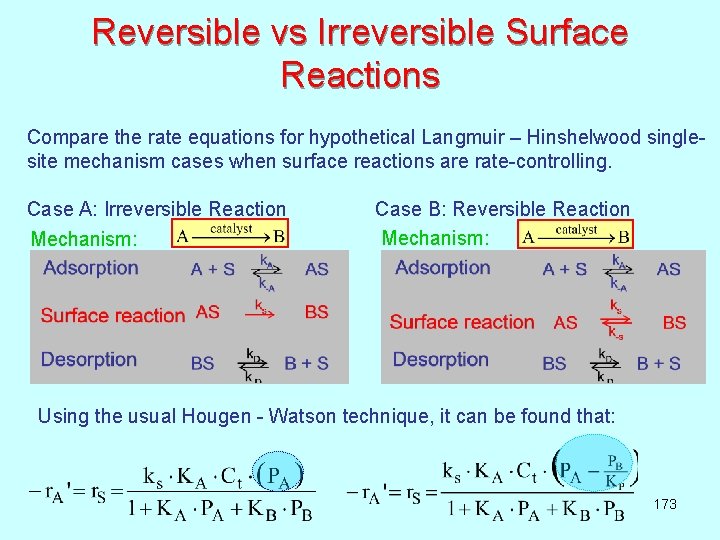
Reversible vs Irreversible Surface Reactions Compare the rate equations for hypothetical Langmuir – Hinshelwood singlesite mechanism cases when surface reactions are rate-controlling. Case A: Irreversible Reaction Case B: Reversible Reaction Mechanism: Using the usual Hougen - Watson technique, it can be found that: 173

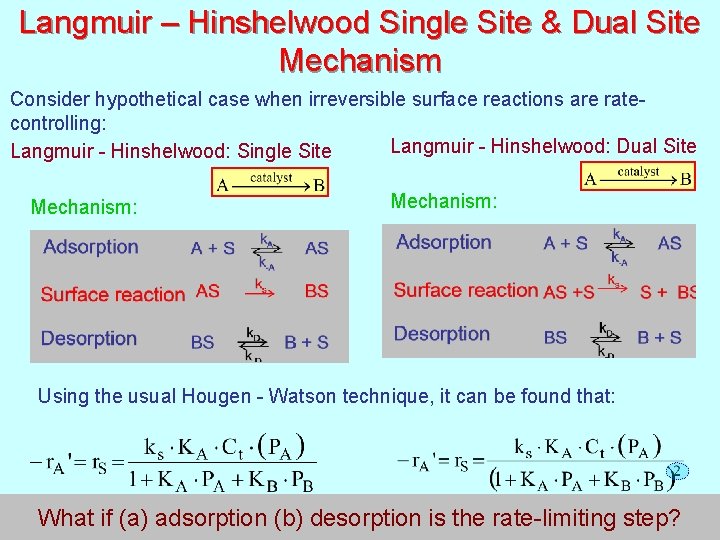
Langmuir – Hinshelwood Single Site & Dual Site Mechanism Consider hypothetical case when irreversible surface reactions are ratecontrolling: Langmuir - Hinshelwood: Dual Site Langmuir - Hinshelwood: Single Site Mechanism: Using the usual Hougen - Watson technique, it can be found that: 174 What if (a) adsorption (b) desorption is the rate-limiting step?

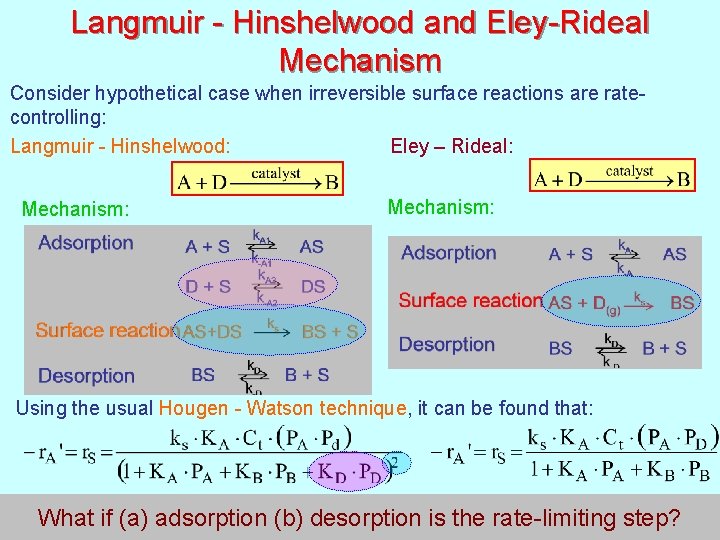
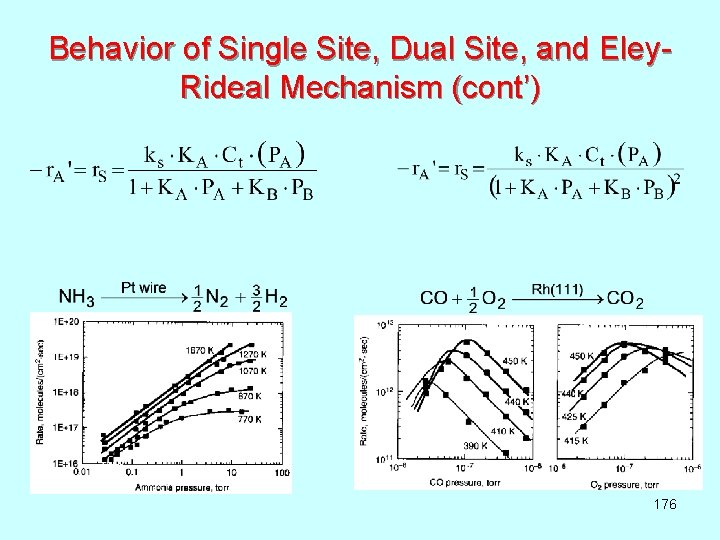
Langmuir - Hinshelwood and Eley-Rideal Mechanism Consider hypothetical case when irreversible surface reactions are ratecontrolling: Eley – Rideal: Langmuir - Hinshelwood: Mechanism: Using the usual Hougen - Watson technique, it can be found that: 175 What if (a) adsorption (b) desorption is the rate-limiting step?

Behavior of Single Site, Dual Site, and Eley. Rideal Mechanism (cont’) 176

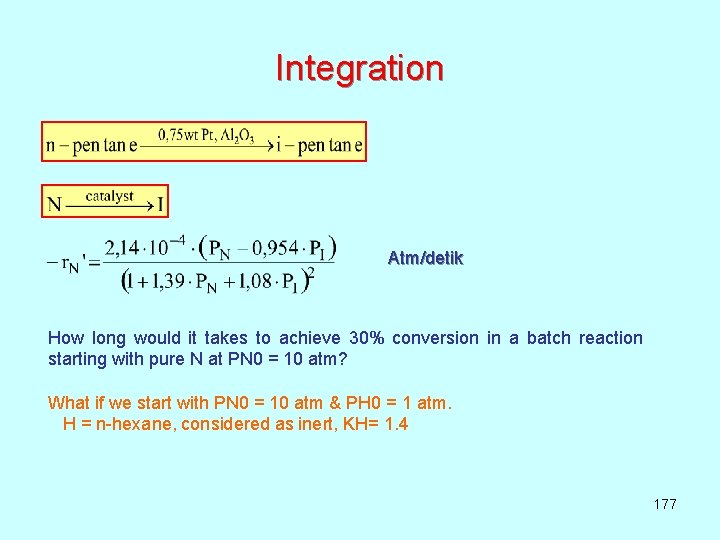
Integration Atm/detik How long would it takes to achieve 30% conversion in a batch reaction starting with pure N at PN 0 = 10 atm? What if we start with PN 0 = 10 atm & PH 0 = 1 atm. H = n-hexane, considered as inert, KH= 1. 4 177

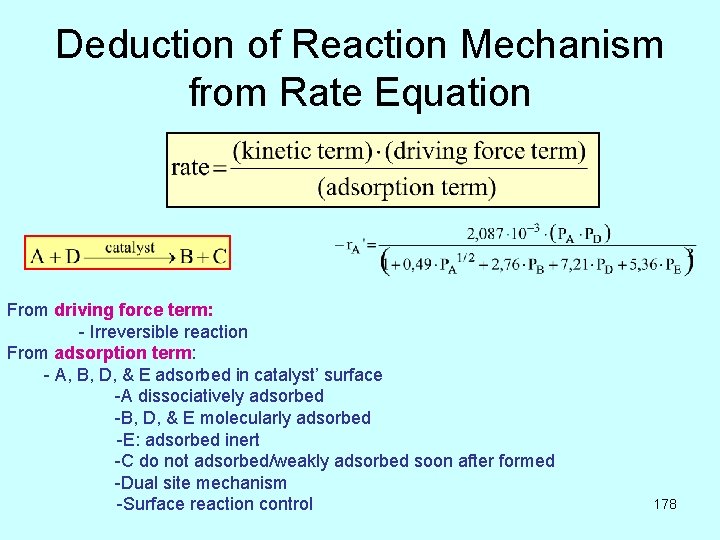
Deduction of Reaction Mechanism from Rate Equation From driving force term: - Irreversible reaction From adsorption term: - A, B, D, & E adsorbed in catalyst’ surface -A dissociatively adsorbed -B, D, & E molecularly adsorbed -E: adsorbed inert -C do not adsorbed/weakly adsorbed soon after formed -Dual site mechanism -Surface reaction control 178

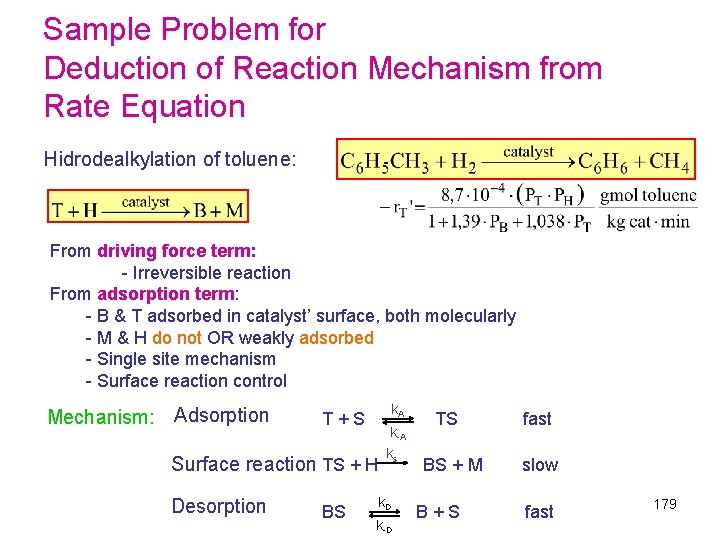
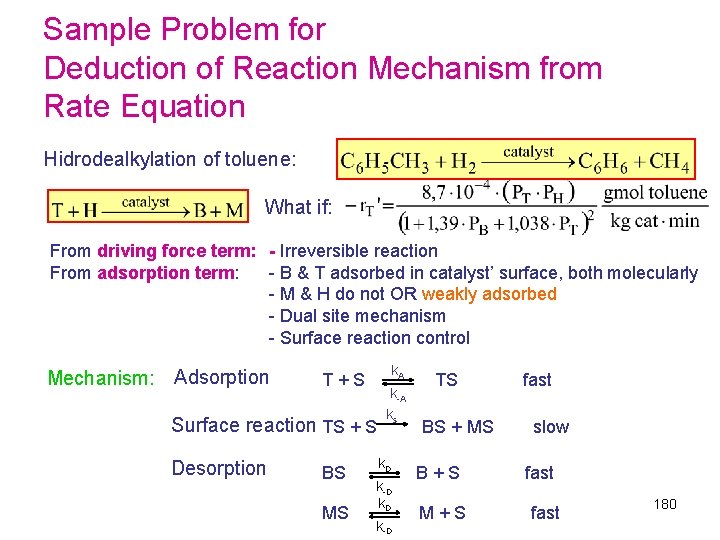
Sample Problem for Deduction of Reaction Mechanism from Rate Equation Hidrodealkylation of toluene: From driving force term: - Irreversible reaction From adsorption term: - B & T adsorbed in catalyst’ surface, both molecularly - M & H do not OR weakly adsorbed - Single site mechanism - Surface reaction control Mechanism: Adsorption k A T + S TS fast k-A k s Surface reaction TS + H BS + M slow Desorption k D BS B + S fast k-D 179

Sample Problem for Deduction of Reaction Mechanism from Rate Equation Hidrodealkylation of toluene: What if: From driving force term: - Irreversible reaction From adsorption term: - B & T adsorbed in catalyst’ surface, both molecularly - M & H do not OR weakly adsorbed - Dual site mechanism - Surface reaction control Mechanism: Adsorption k A T + S TS fast k-A k s Surface reaction TS + S BS + MS slow Desorption k D BS B + S fast k-D k. D MS M + S fast k-D 180

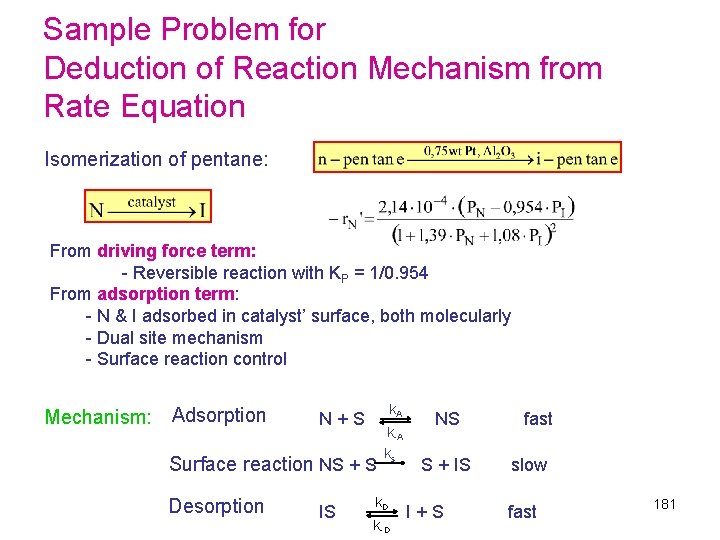
Sample Problem for Deduction of Reaction Mechanism from Rate Equation Isomerization of pentane: From driving force term: - Reversible reaction with KP = 1/0. 954 From adsorption term: - N & I adsorbed in catalyst’ surface, both molecularly - Dual site mechanism - Surface reaction control Mechanism: Adsorption k A N + S NS fast k-A k s Surface reaction NS + S S + IS slow Desorption k D IS I + S fast k-D 181

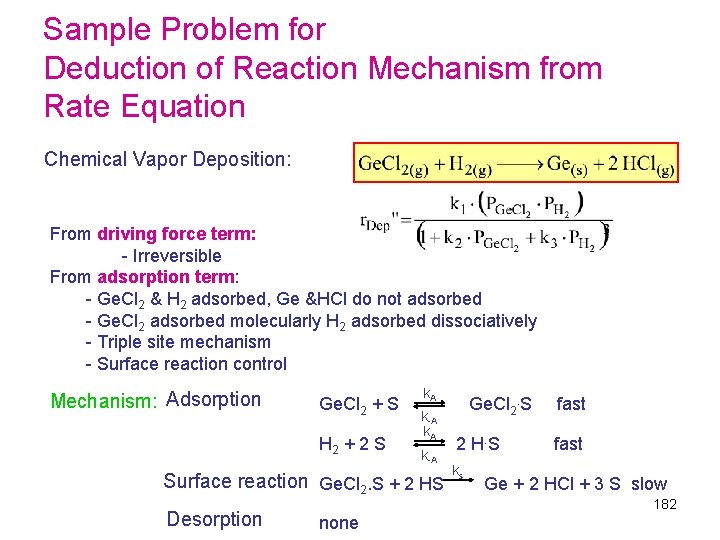
Sample Problem for Deduction of Reaction Mechanism from Rate Equation Chemical Vapor Deposition: From driving force term: - Irreversible From adsorption term: - Ge. Cl 2 & H 2 adsorbed, Ge &HCl do not adsorbed - Ge. Cl 2 adsorbed molecularly H 2 adsorbed dissociatively - Triple site mechanism - Surface reaction control Mechanism: Adsorption k. A Ge. Cl 2 + S Ge. Cl 2. S fast k-A k. A H 2 + 2 S 2 H. S fast k-A k s Surface reaction Ge. Cl 2. S + 2 HS Ge + 2 HCl + 3 S slow Desorption 182 none

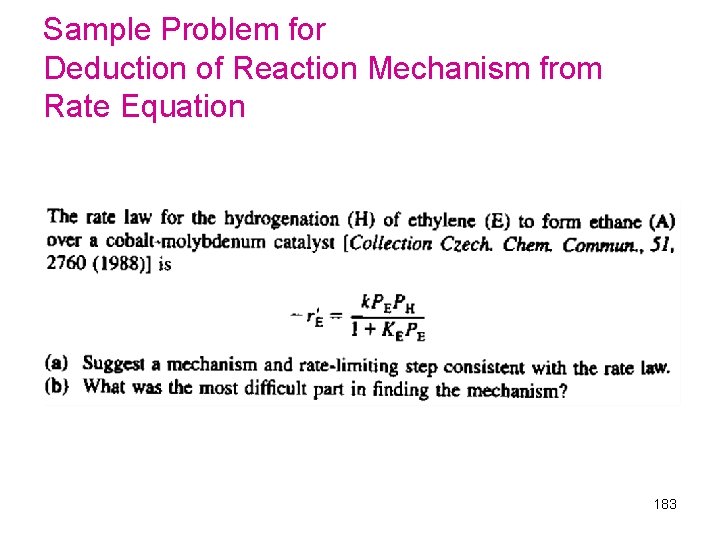
Sample Problem for Deduction of Reaction Mechanism from Rate Equation 183

Sample Problem for Deduction of Reaction Mechanism from Rate Equation 184

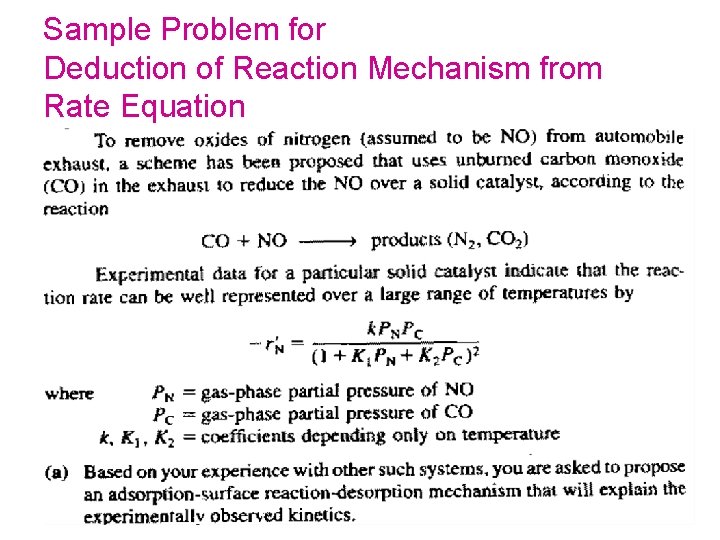
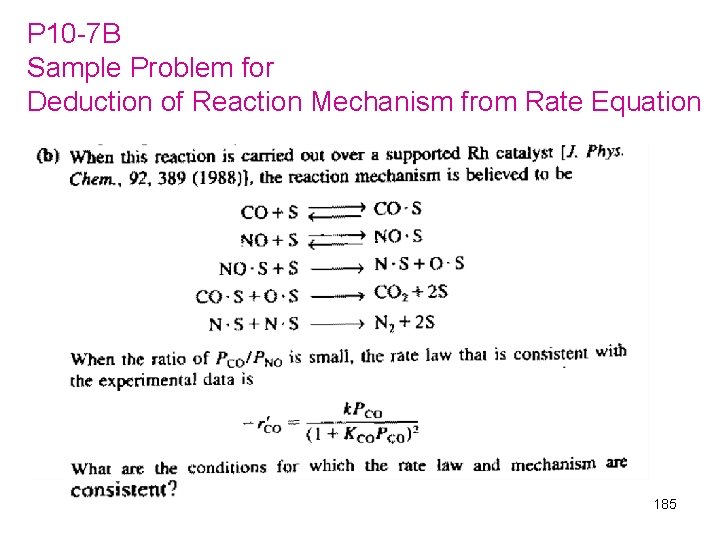
P 10 -7 B Sample Problem for Deduction of Reaction Mechanism from Rate Equation 185

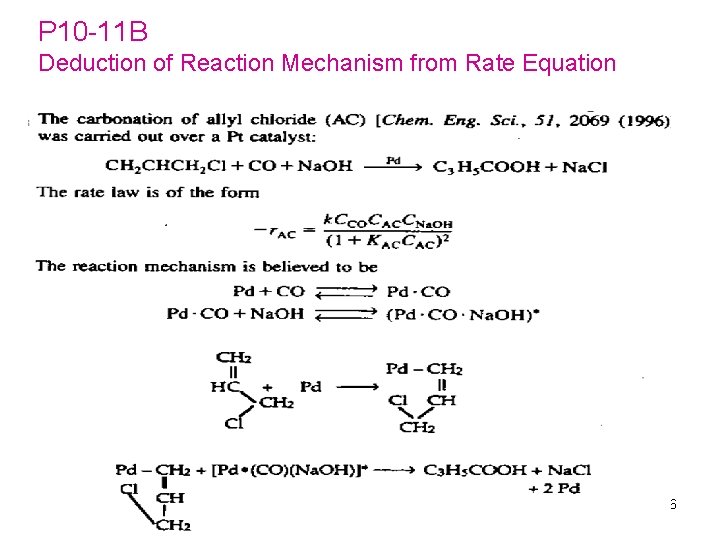
P 10 -11 B Deduction of Reaction Mechanism from Rate Equation 186

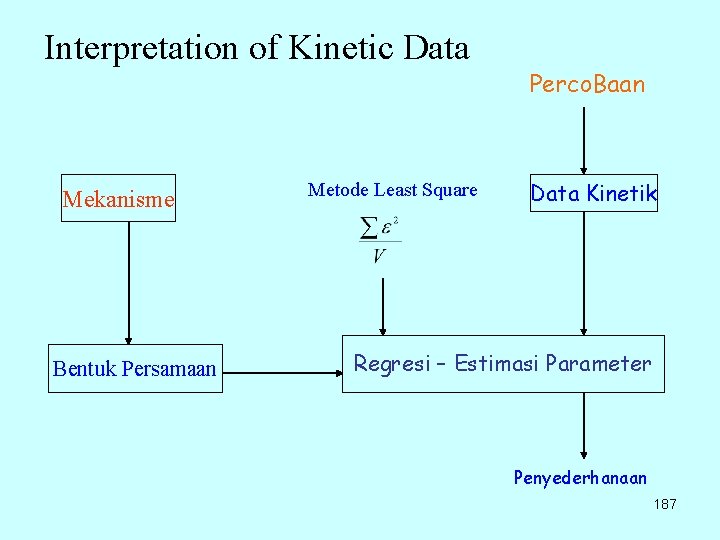
Interpretation of Kinetic Data Mekanisme Bentuk Persamaan Metode Least Square Perco. Baan Data Kinetik Regresi – Estimasi Parameter Penyederhanaan 187

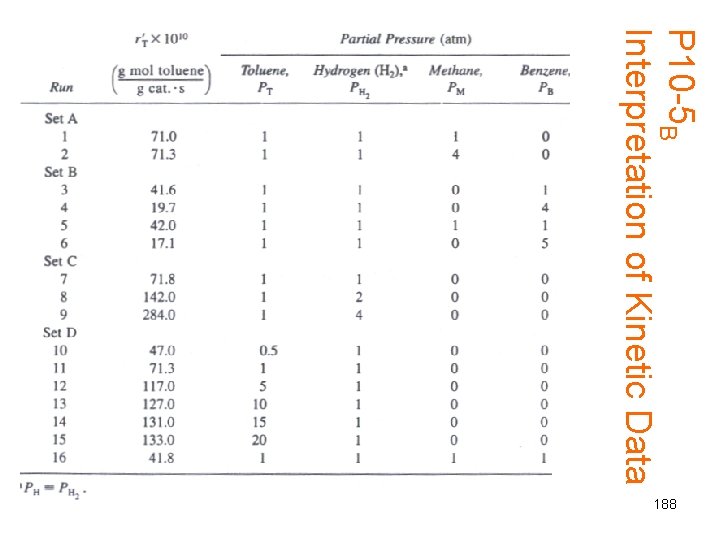
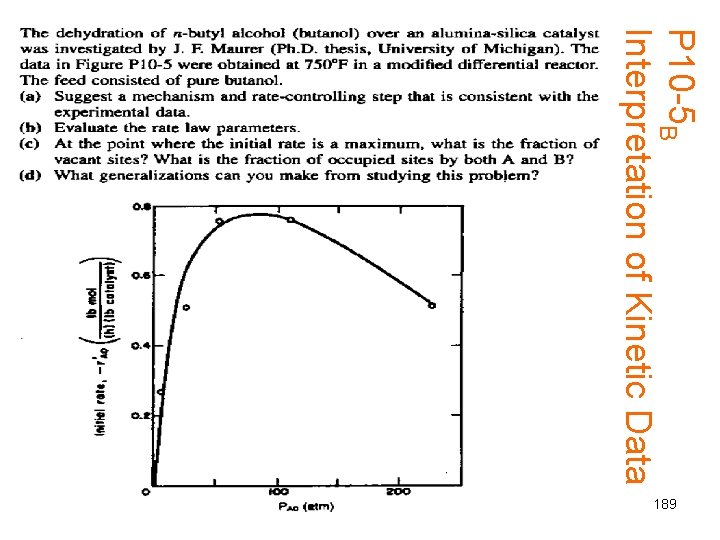
P 10 -5 B Interpretation of Kinetic Data 188

P 10 -5 B Interpretation of Kinetic Data 189

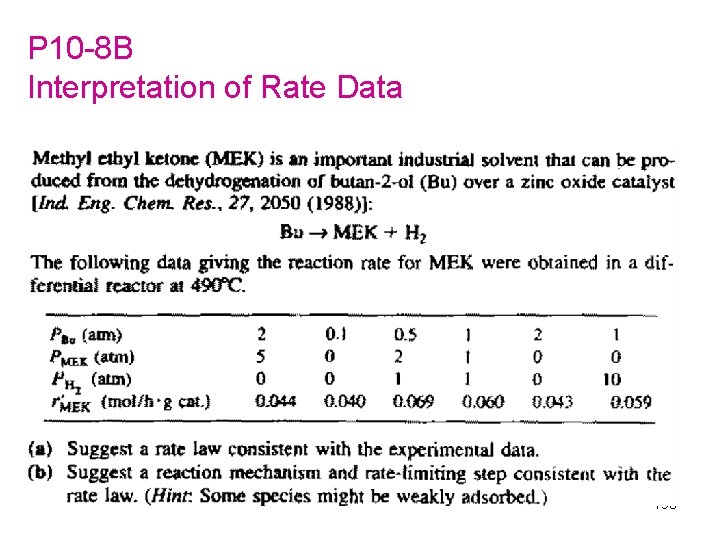
P 10 -8 B Interpretation of Rate Data 190

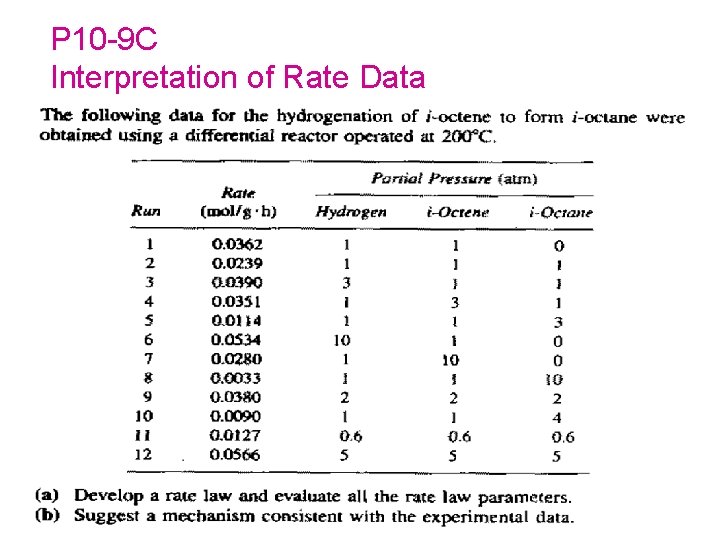
P 10 -9 C Interpretation of Rate Data 191

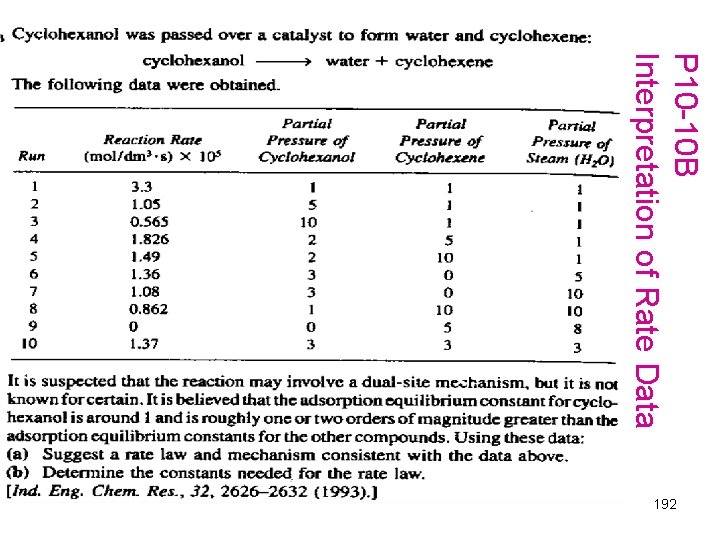
P 10 -10 B Interpretation of Rate Data 192

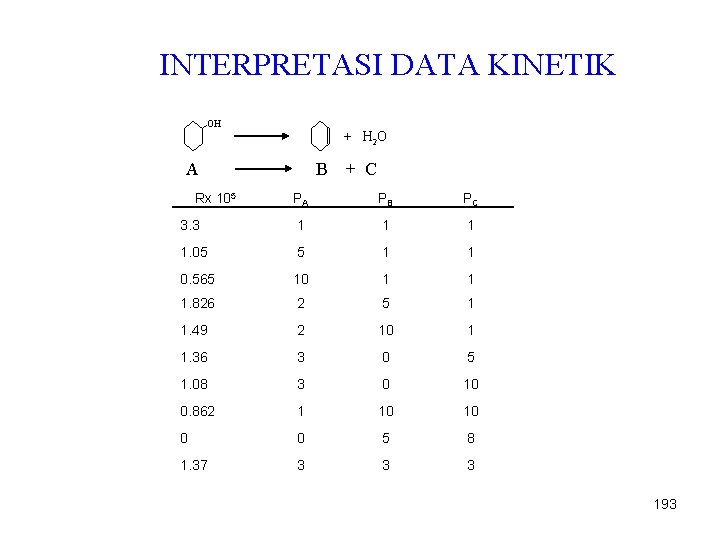
INTERPRETASI DATA KINETIK OH + H 2 O A Rx 105 B + C PA PB PC 3. 3 1 1. 05 5 1 1 0. 565 10 1 1 1. 826 2 5 1 1. 49 2 10 1 1. 36 3 0 5 1. 08 3 0 10 0. 862 1 10 10 0 0 5 8 1. 37 3 3 3 193

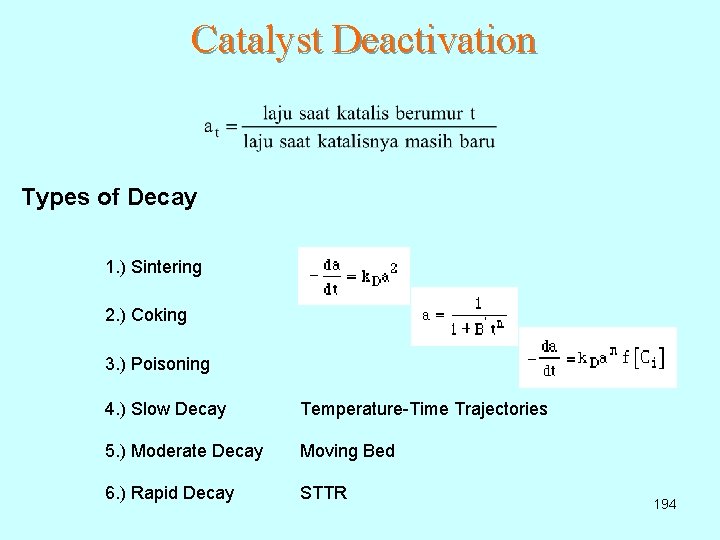
Catalyst Deactivation Types of Decay 1. ) Sintering 2. ) Coking 3. ) Poisoning 4. ) Slow Decay Temperature-Time Trajectories 5. ) Moderate Decay Moving Bed 6. ) Rapid Decay STTR 194

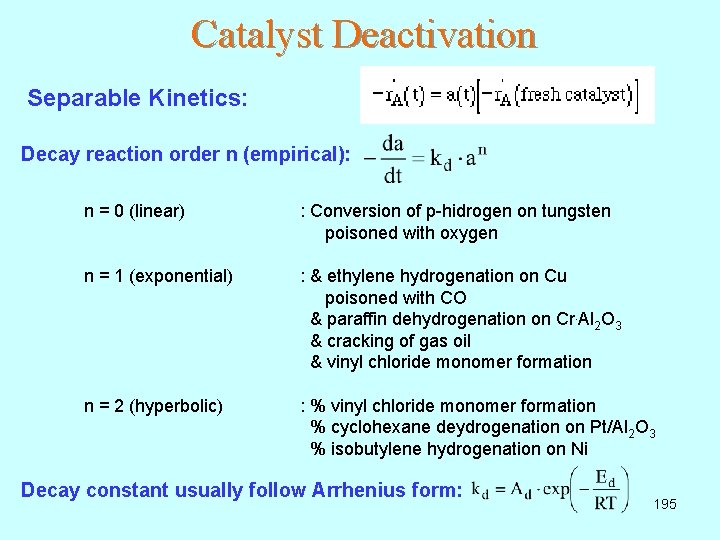
Catalyst Deactivation Separable Kinetics: Decay reaction order n (empirical): n = 0 (linear) : Conversion of p-hidrogen on tungsten poisoned with oxygen n = 1 (exponential) : & ethylene hydrogenation on Cu poisoned with CO & paraffin dehydrogenation on Cr. Al 2 O 3 & cracking of gas oil & vinyl chloride monomer formation n = 2 (hyperbolic) : % vinyl chloride monomer formation % cyclohexane deydrogenation on Pt/Al 2 O 3 % isobutylene hydrogenation on Ni Decay constant usually follow Arrhenius form: 195

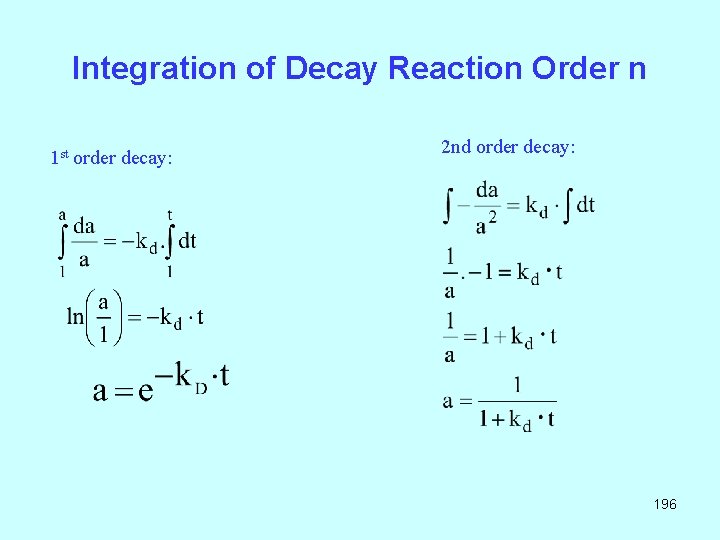
Integration of Decay Reaction Order n 1 st order decay: 2 nd order decay: 196

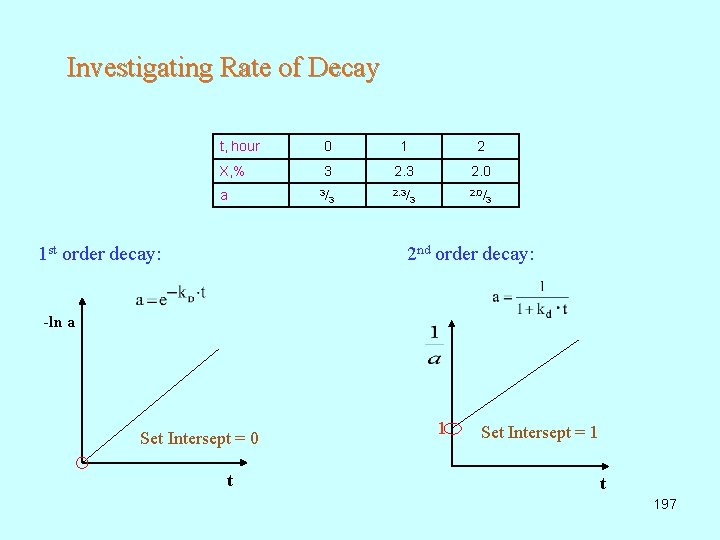
Investigating Rate of Decay t, hour 0 1 2 X, % 3 2. 0 3/ 2. 0/ a 1 st order decay: 3 3 3 2 nd order decay: -ln a Set Intersept = 0 t 1 Set Intersept = 1 t 197

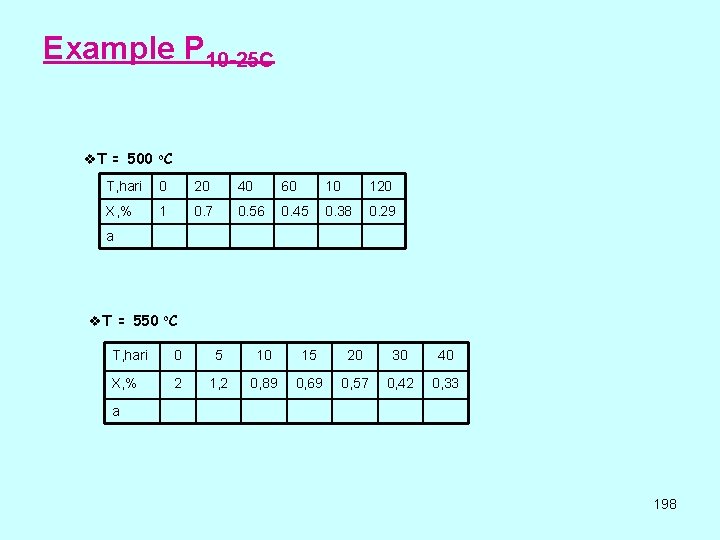
Example P 10 -25 C v. T = 500 o. C T, hari 0 20 40 60 10 120 X, % 1 0. 7 0. 56 0. 45 0. 38 0. 29 a v. T = 550 o. C T, hari 0 5 10 15 20 30 40 X, % 2 1, 2 0, 89 0, 69 0, 57 0, 42 0, 33 a 198