Chapter 23 Catalysis in Organic Reactions and in

- Slides: 50

Chapter 23 Catalysis in Organic Reactions and in Enzymatic Reactions Paula Yurkanis Bruice University of California, Santa Barbara © 2014 Pearson Education, Inc.

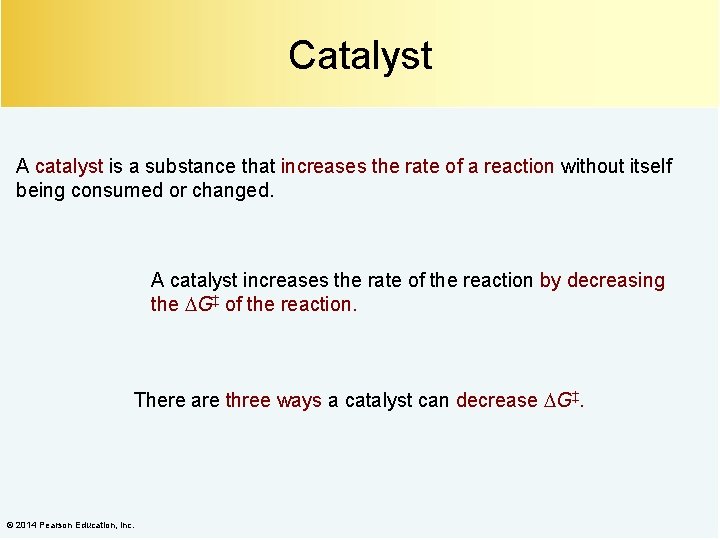
Catalyst A catalyst is a substance that increases the rate of a reaction without itself being consumed or changed. A catalyst increases the rate of the reaction by decreasing the G‡ of the reaction. There are three ways a catalyst can decrease G‡. © 2014 Pearson Education, Inc.

The Catalyst Converts the Reactant to a Less Stable (More Reactive) Species © 2014 Pearson Education, Inc.

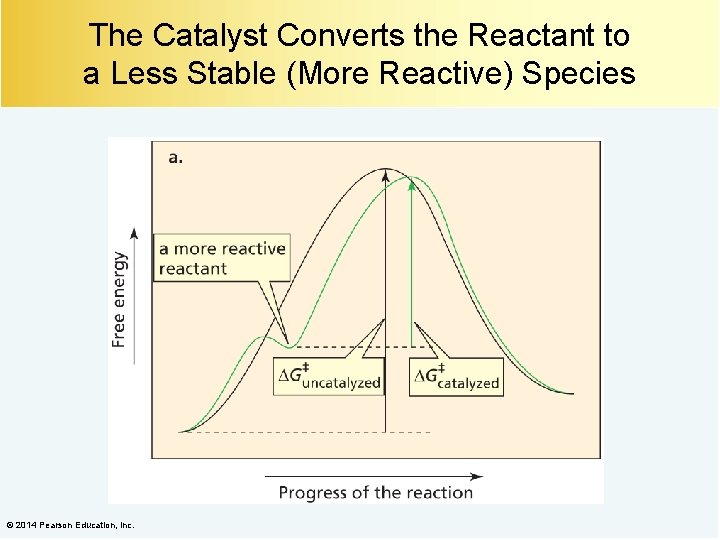
The Catalyst Stabilizes the Transition State © 2014 Pearson Education, Inc.

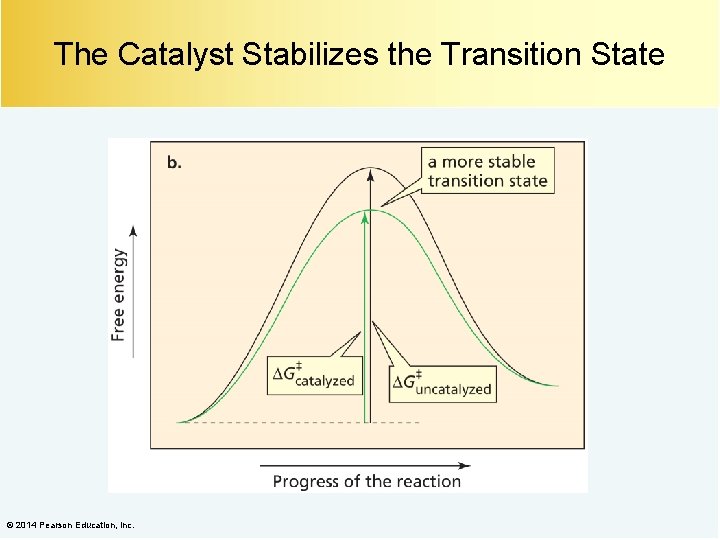
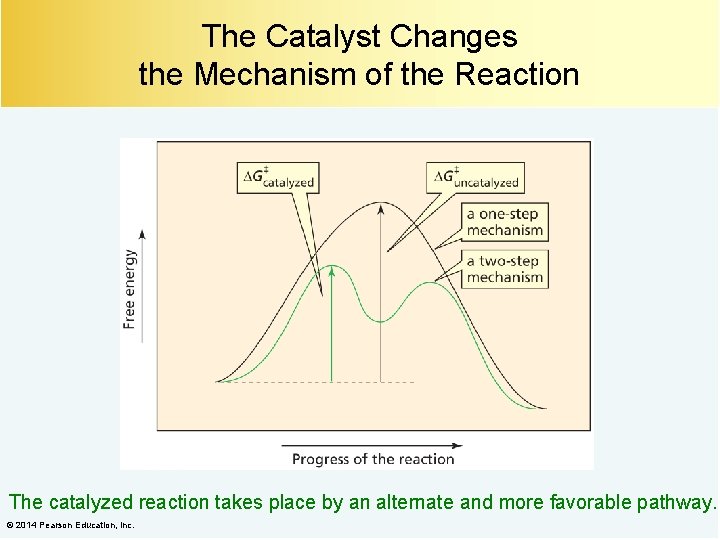
The Catalyst Changes the Mechanism of the Reaction The catalyzed reaction takes place by an alternate and more favorable pathway. © 2014 Pearson Education, Inc.

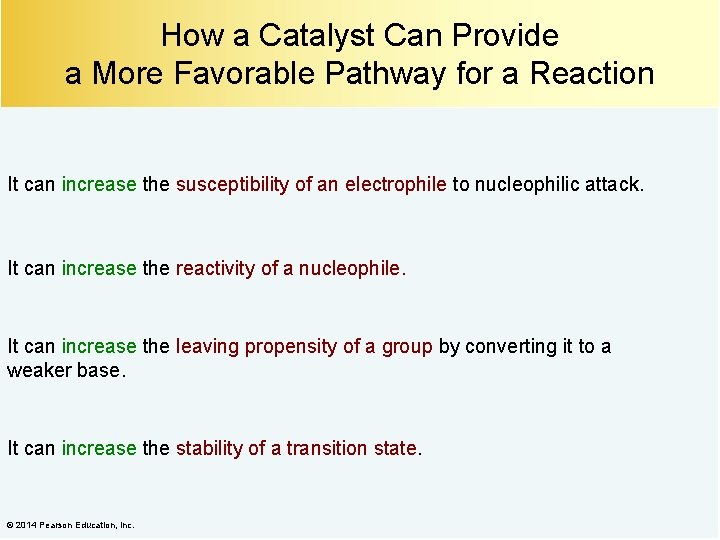
How a Catalyst Can Provide a More Favorable Pathway for a Reaction It can increase the susceptibility of an electrophile to nucleophilic attack. It can increase the reactivity of a nucleophile. It can increase the leaving propensity of a group by converting it to a weaker base. It can increase the stability of a transition state. © 2014 Pearson Education, Inc.

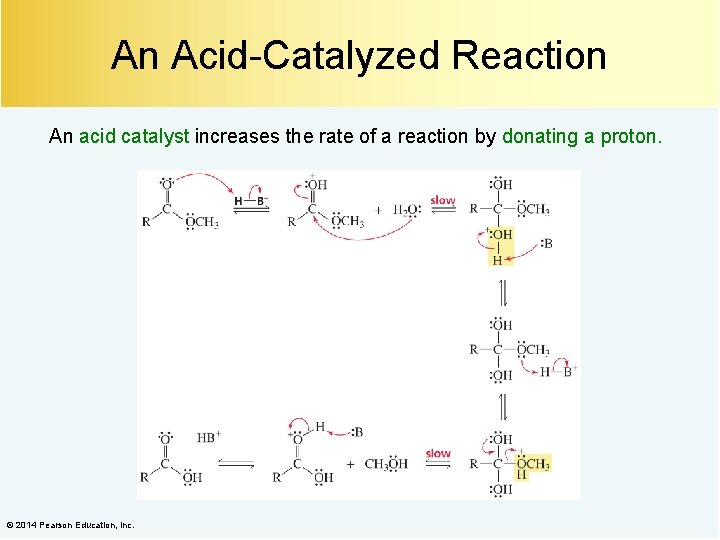
An Acid-Catalyzed Reaction An acid catalyst increases the rate of a reaction by donating a proton. © 2014 Pearson Education, Inc.

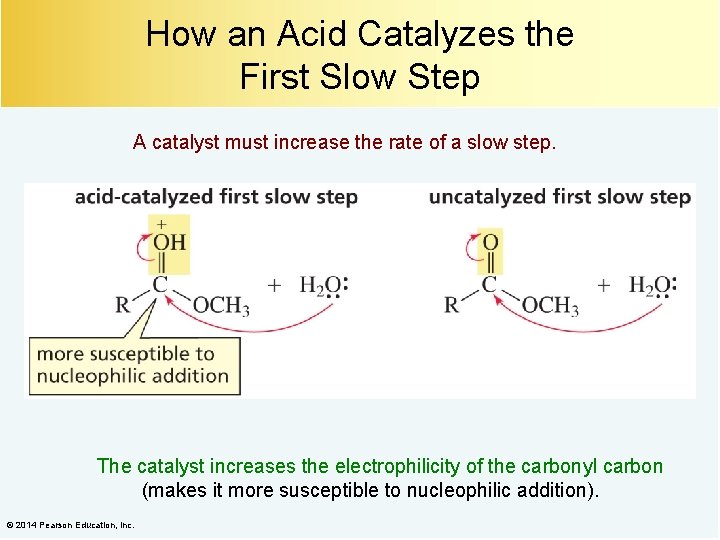
How an Acid Catalyzes the First Slow Step A catalyst must increase the rate of a slow step. The catalyst increases the electrophilicity of the carbonyl carbon (makes it more susceptible to nucleophilic addition). © 2014 Pearson Education, Inc.

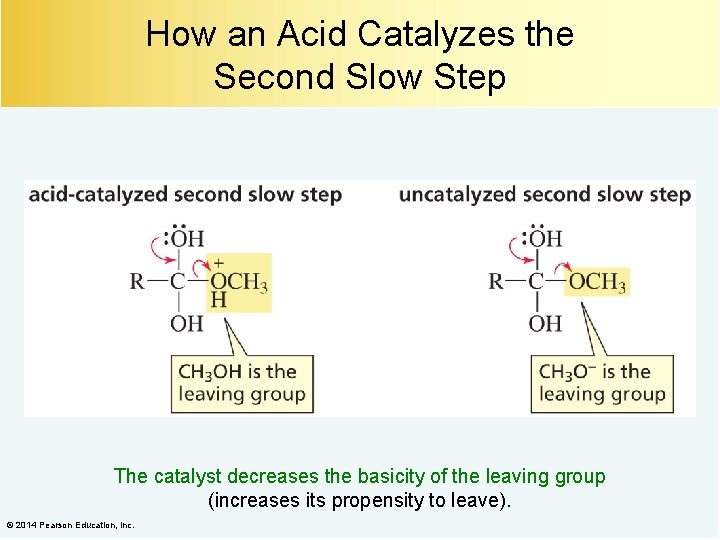
How an Acid Catalyzes the Second Slow Step The catalyst decreases the basicity of the leaving group (increases its propensity to leave). © 2014 Pearson Education, Inc.

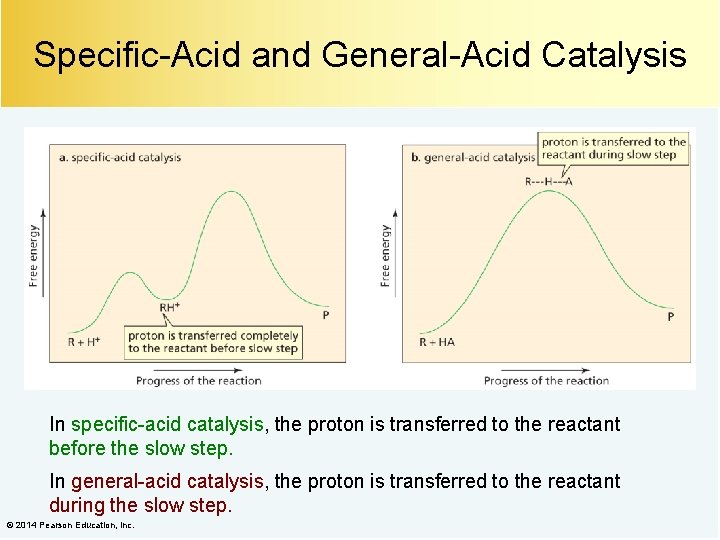
Specific-Acid and General-Acid Catalysis In specific-acid catalysis, the proton is transferred to the reactant before the slow step. In general-acid catalysis, the proton is transferred to the reactant during the slow step. © 2014 Pearson Education, Inc.

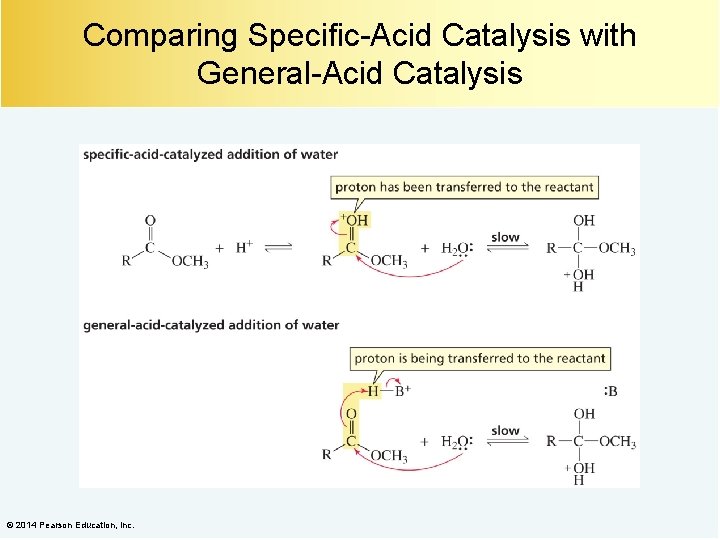
Comparing Specific-Acid Catalysis with General-Acid Catalysis © 2014 Pearson Education, Inc.

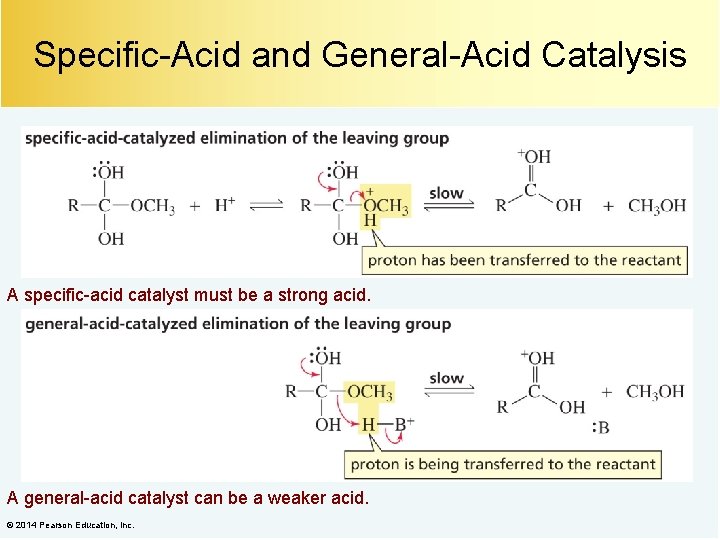
Specific-Acid and General-Acid Catalysis A specific-acid catalyst must be a strong acid. A general-acid catalyst can be a weaker acid. © 2014 Pearson Education, Inc.

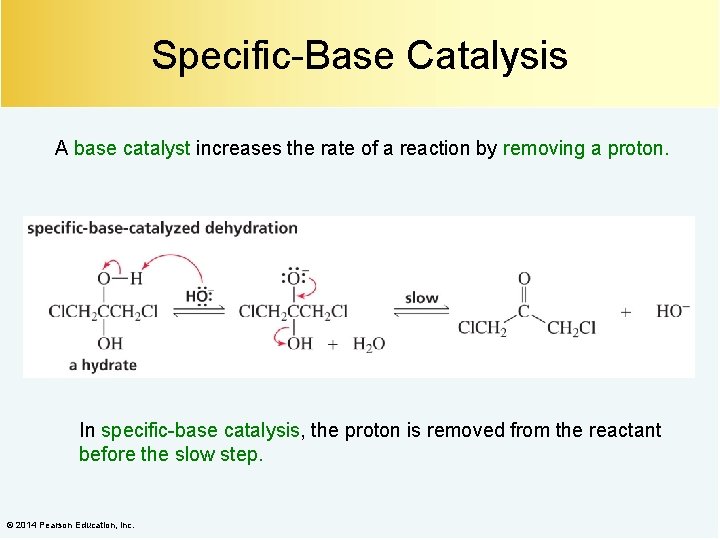
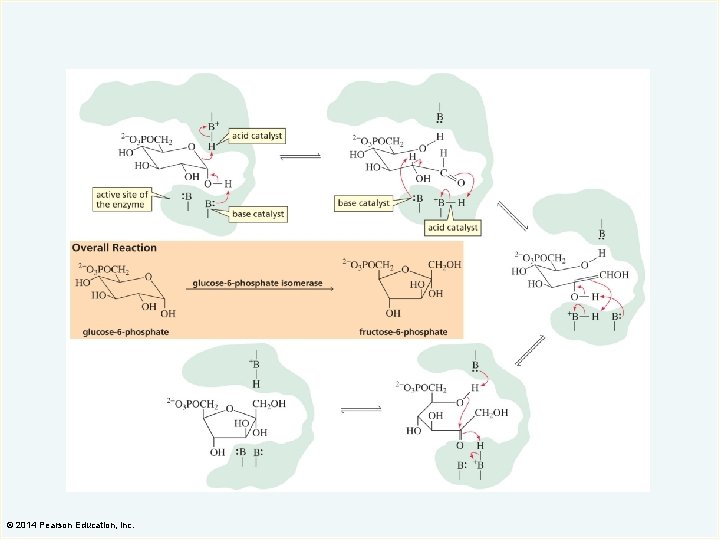
Specific-Base Catalysis A base catalyst increases the rate of a reaction by removing a proton. In specific-base catalysis, the proton is removed from the reactant before the slow step. © 2014 Pearson Education, Inc.

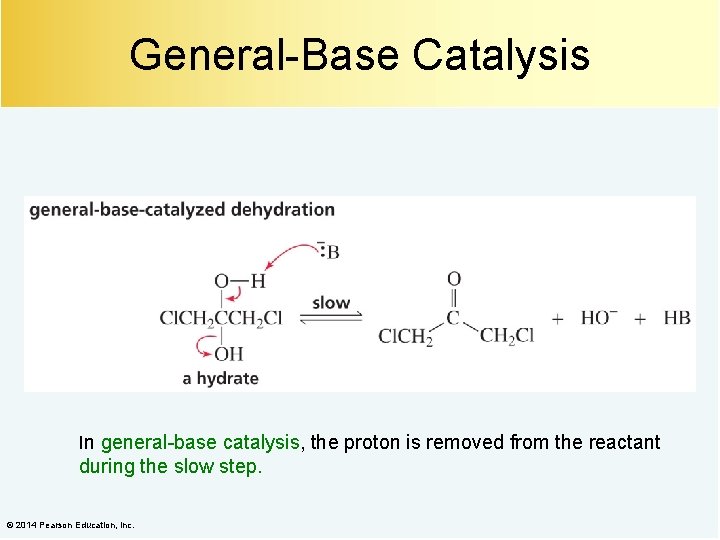
General-Base Catalysis In general-base catalysis, the proton is removed from the reactant during the slow step. © 2014 Pearson Education, Inc.

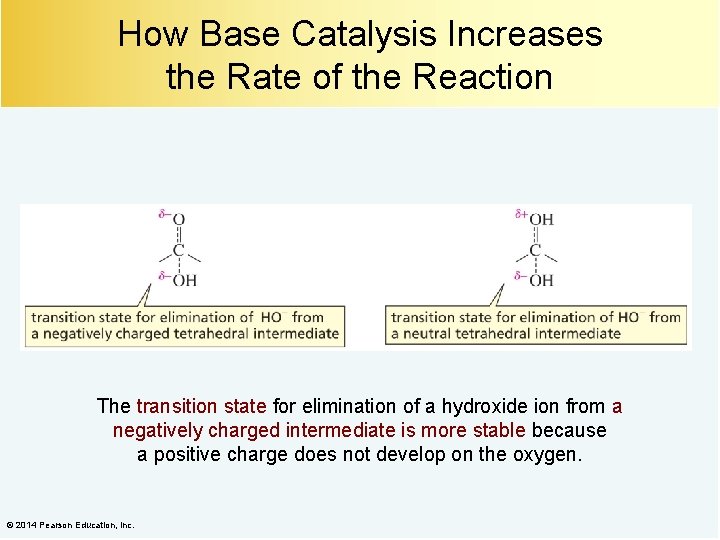
How Base Catalysis Increases the Rate of the Reaction The transition state for elimination of a hydroxide ion from a negatively charged intermediate is more stable because a positive charge does not develop on the oxygen. © 2014 Pearson Education, Inc.

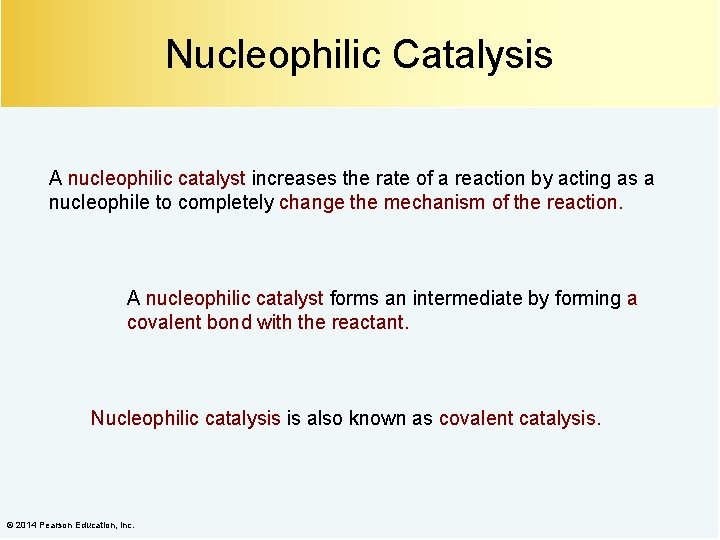
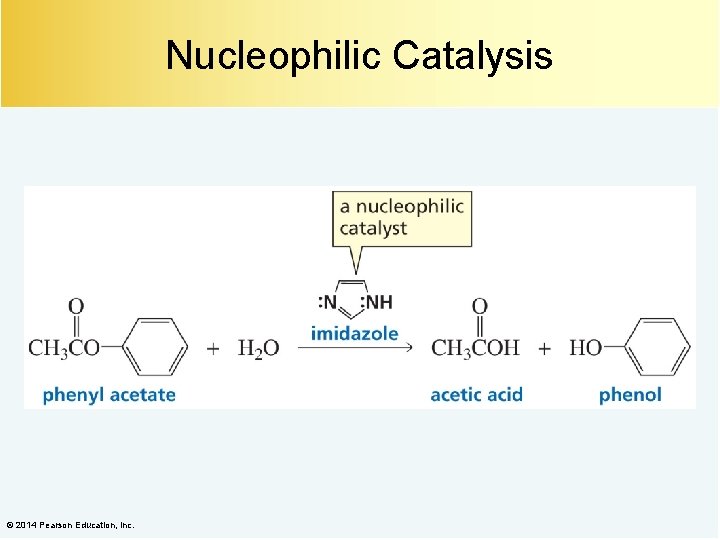
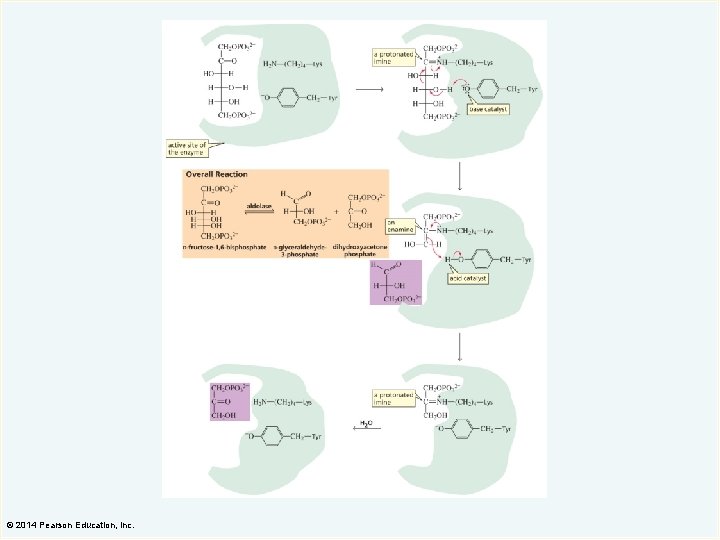
Nucleophilic Catalysis A nucleophilic catalyst increases the rate of a reaction by acting as a nucleophile to completely change the mechanism of the reaction. A nucleophilic catalyst forms an intermediate by forming a covalent bond with the reactant. Nucleophilic catalysis is also known as covalent catalysis. © 2014 Pearson Education, Inc.

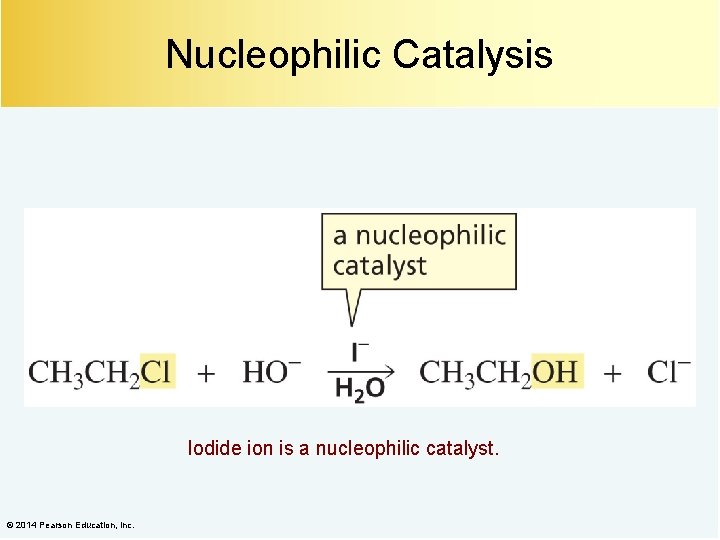
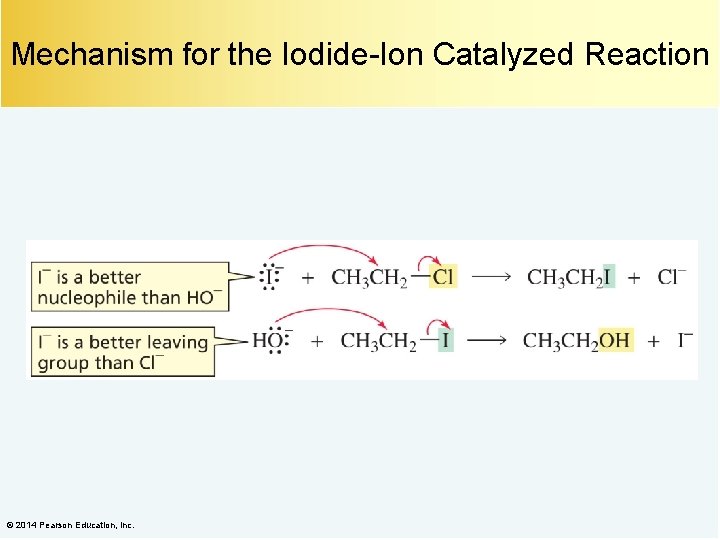
Nucleophilic Catalysis Iodide ion is a nucleophilic catalyst. © 2014 Pearson Education, Inc.

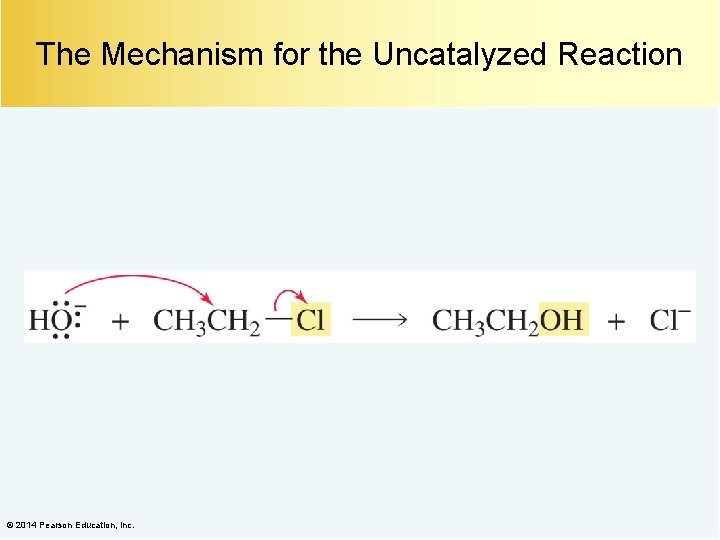
The Mechanism for the Uncatalyzed Reaction © 2014 Pearson Education, Inc.

Mechanism for the Iodide-Ion Catalyzed Reaction © 2014 Pearson Education, Inc.

Nucleophilic Catalysis © 2014 Pearson Education, Inc.

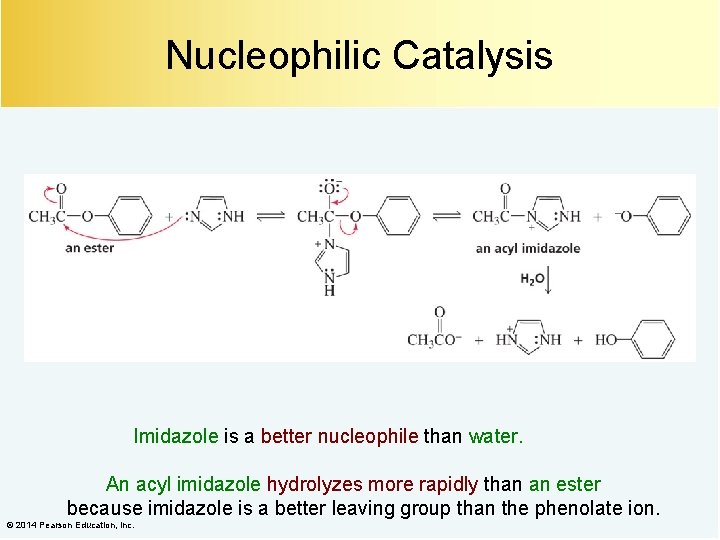
Nucleophilic Catalysis Imidazole is a better nucleophile than water. An acyl imidazole hydrolyzes more rapidly than an ester because imidazole is a better leaving group than the phenolate ion. © 2014 Pearson Education, Inc.

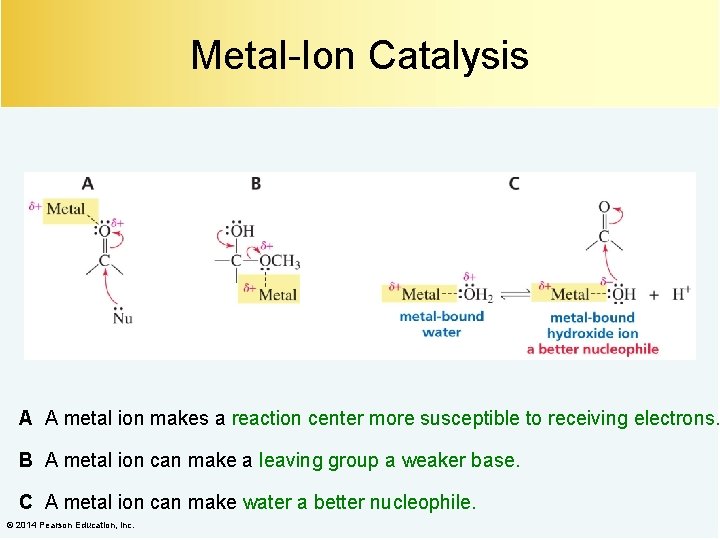
Metal-Ion Catalysis A A metal ion makes a reaction center more susceptible to receiving electrons. B A metal ion can make a leaving group a weaker base. C A metal ion can make water a better nucleophile. © 2014 Pearson Education, Inc.

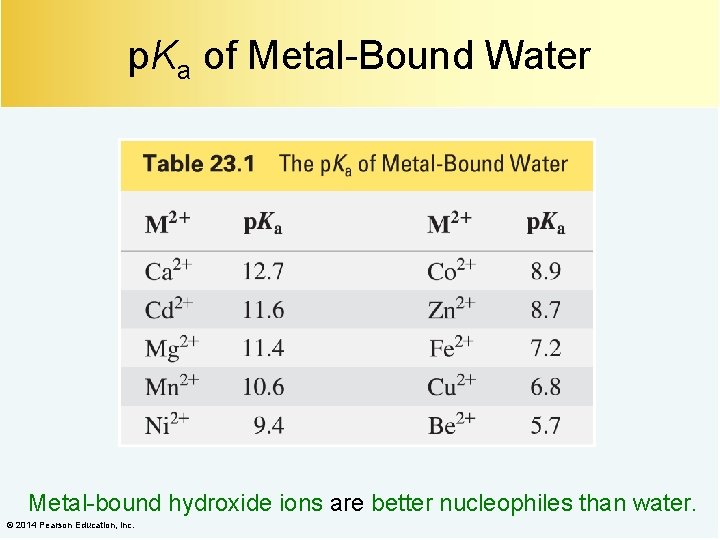
p. Ka of Metal-Bound Water Metal-bound hydroxide ions are better nucleophiles than water. © 2014 Pearson Education, Inc.

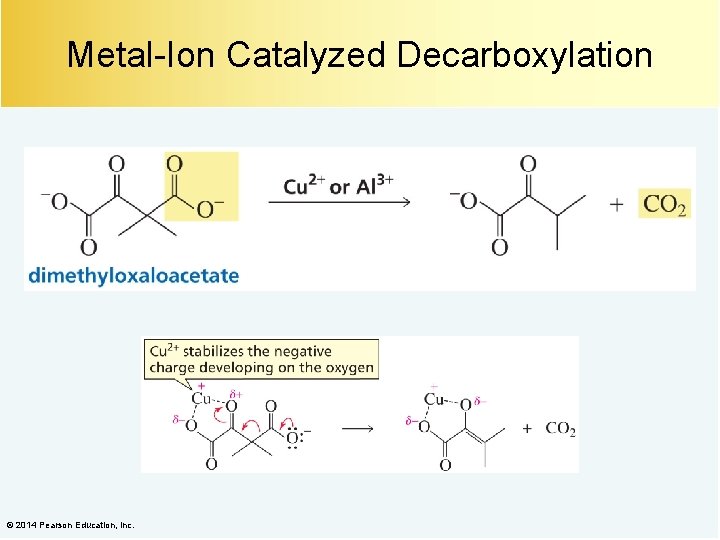
Metal-Ion Catalyzed Decarboxylation © 2014 Pearson Education, Inc.

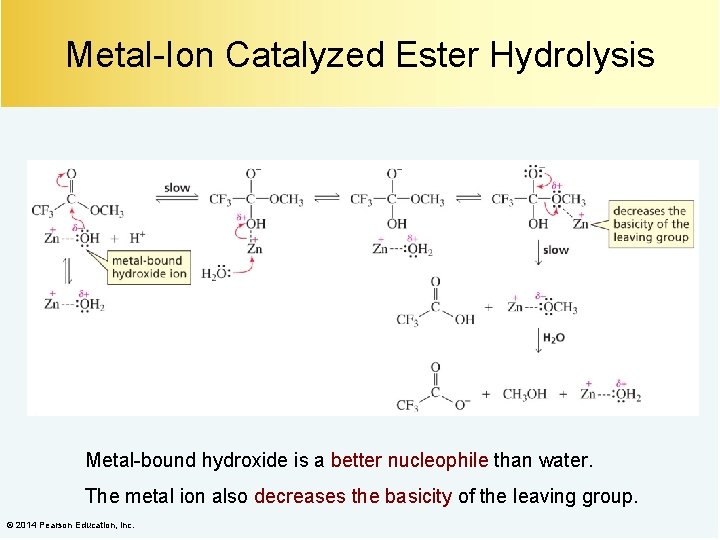
Metal-Ion Catalyzed Ester Hydrolysis Metal-bound hydroxide is a better nucleophile than water. The metal ion also decreases the basicity of the leaving group. © 2014 Pearson Education, Inc.

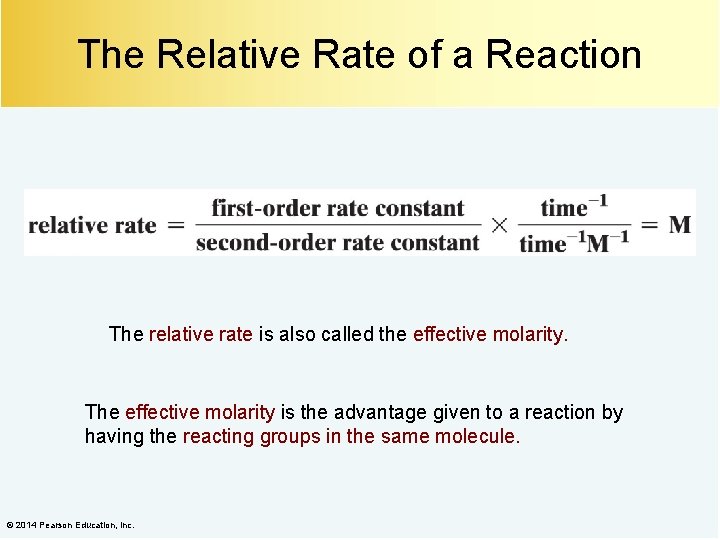
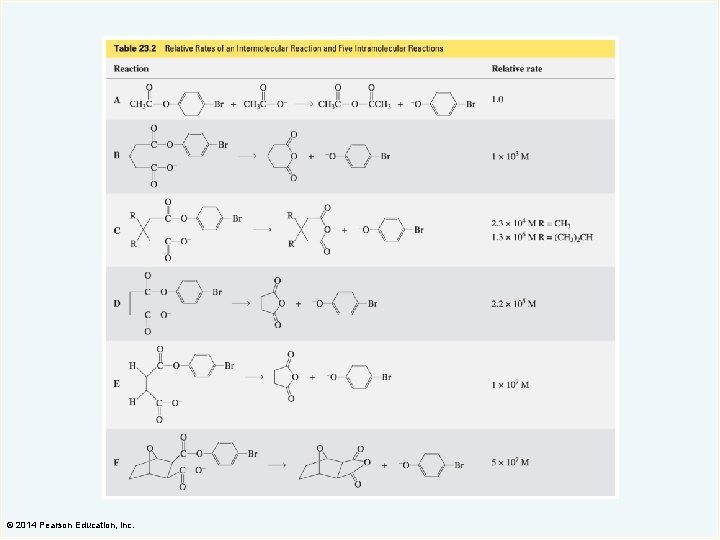
The Relative Rate of a Reaction The relative rate is also called the effective molarity. The effective molarity is the advantage given to a reaction by having the reacting groups in the same molecule. © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

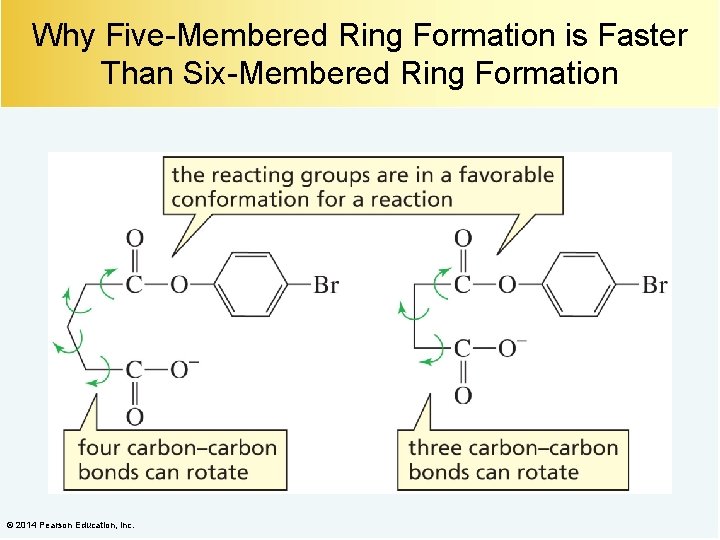
Why Five-Membered Ring Formation is Faster Than Six-Membered Ring Formation © 2014 Pearson Education, Inc.

Intramolecular Catalysis Putting a reacting group and a catalyst in the same molecule increases the rate of the reaction. © 2014 Pearson Education, Inc.

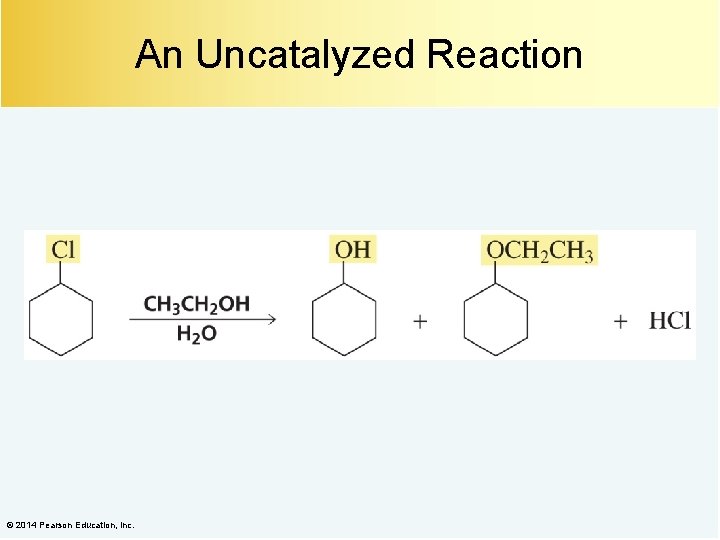
An Uncatalyzed Reaction © 2014 Pearson Education, Inc.

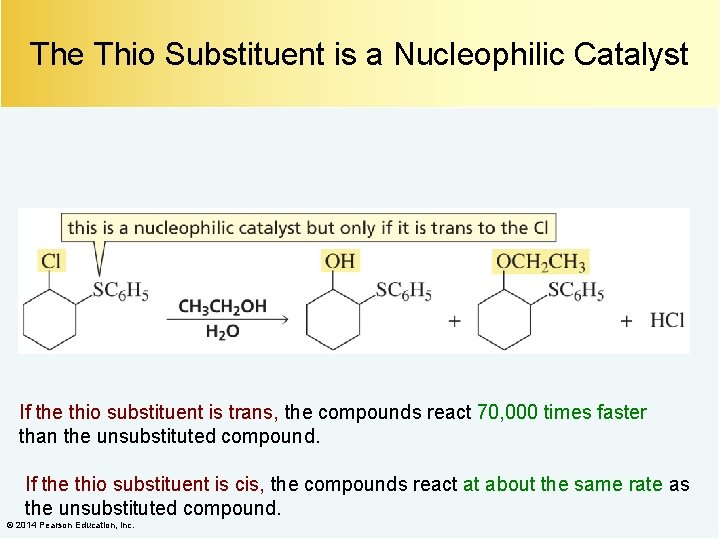
The Thio Substituent is a Nucleophilic Catalyst If the thio substituent is trans, the compounds react 70, 000 times faster than the unsubstituted compound. If the thio substituent is cis, the compounds react at about the same rate as the unsubstituted compound. © 2014 Pearson Education, Inc.

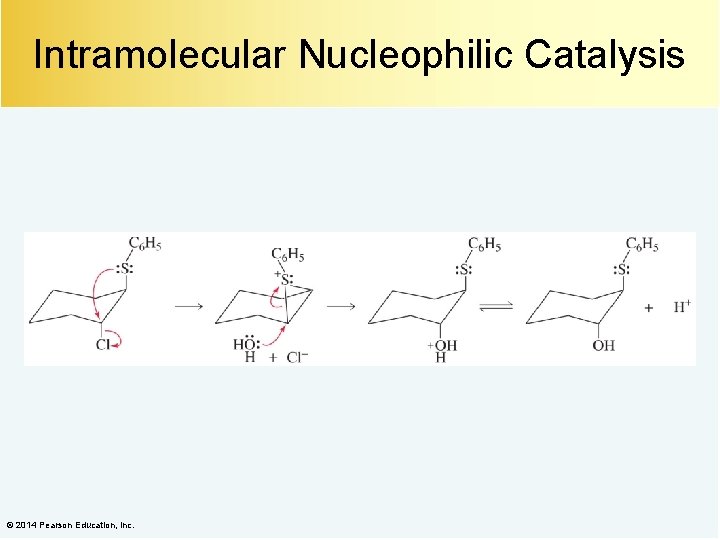
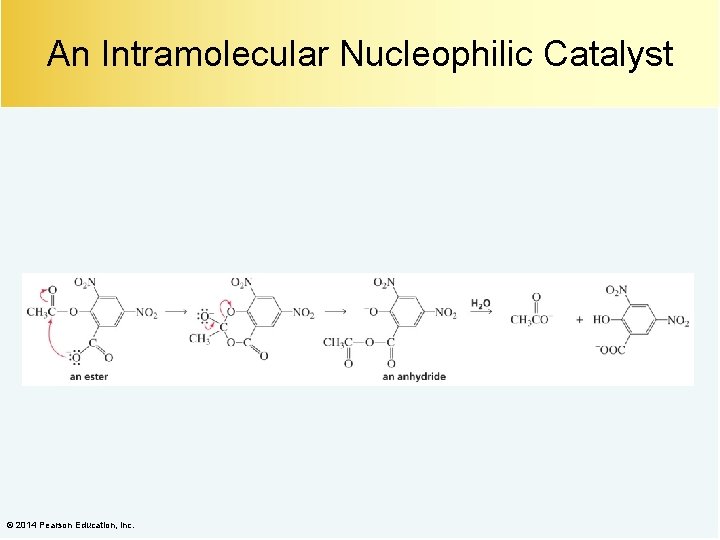
Intramolecular Nucleophilic Catalysis © 2014 Pearson Education, Inc.

The Carboxyl Group is a Catalyst © 2014 Pearson Education, Inc.

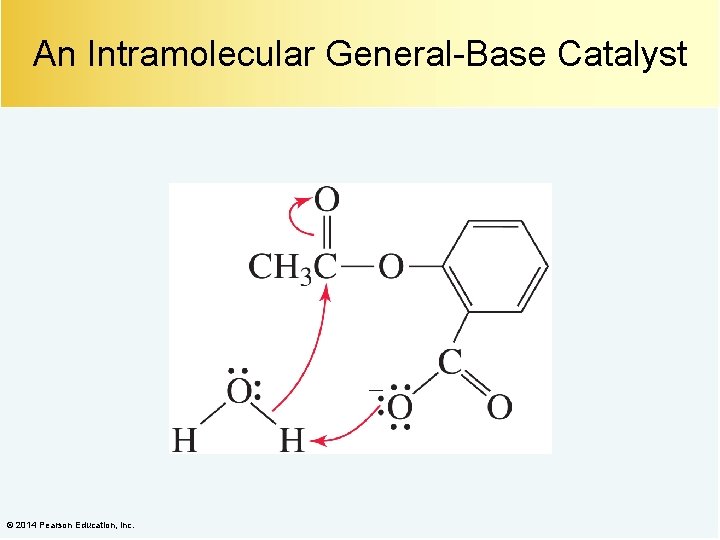
An Intramolecular General-Base Catalyst © 2014 Pearson Education, Inc.

An Intramolecular Nucleophilic Catalyst © 2014 Pearson Education, Inc.

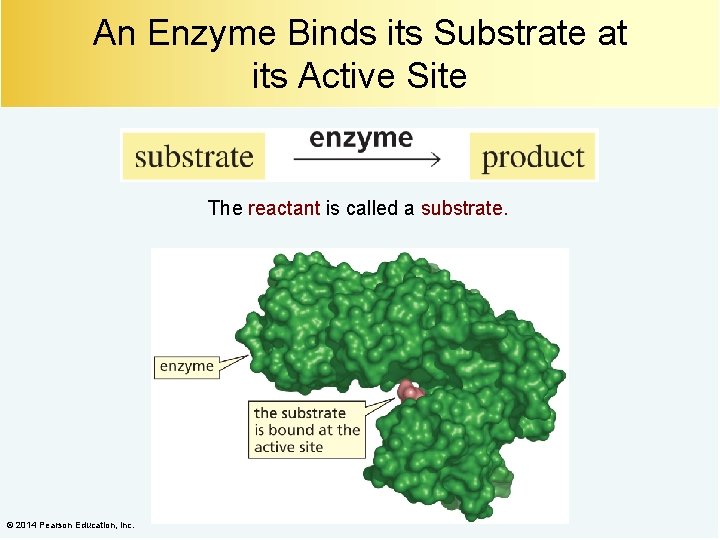
An Enzyme Binds its Substrate at its Active Site The reactant is called a substrate. © 2014 Pearson Education, Inc.

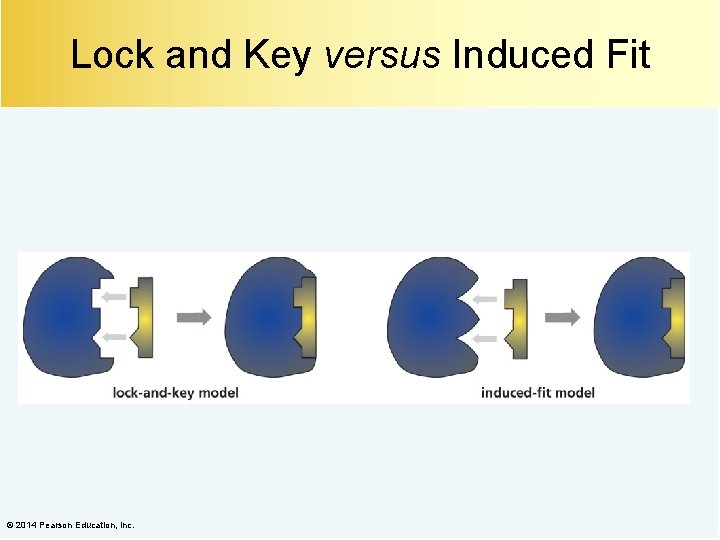
Lock and Key versus Induced Fit © 2014 Pearson Education, Inc.

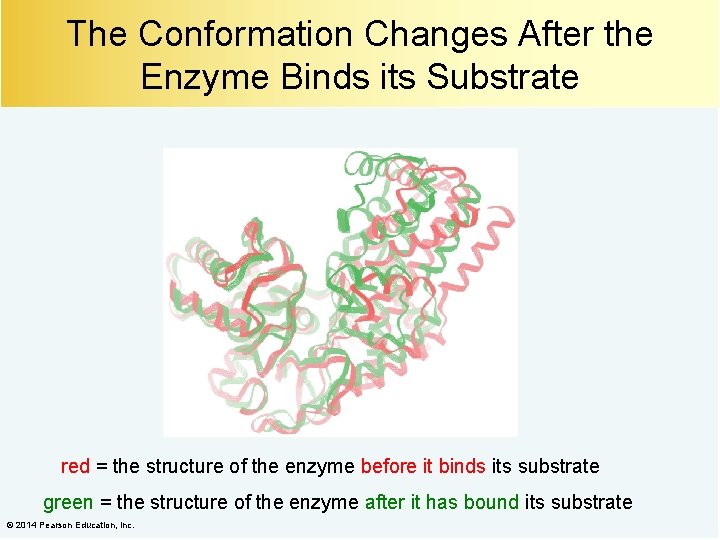
The Conformation Changes After the Enzyme Binds its Substrate red = the structure of the enzyme before it binds its substrate green = the structure of the enzyme after it has bound its substrate © 2014 Pearson Education, Inc.

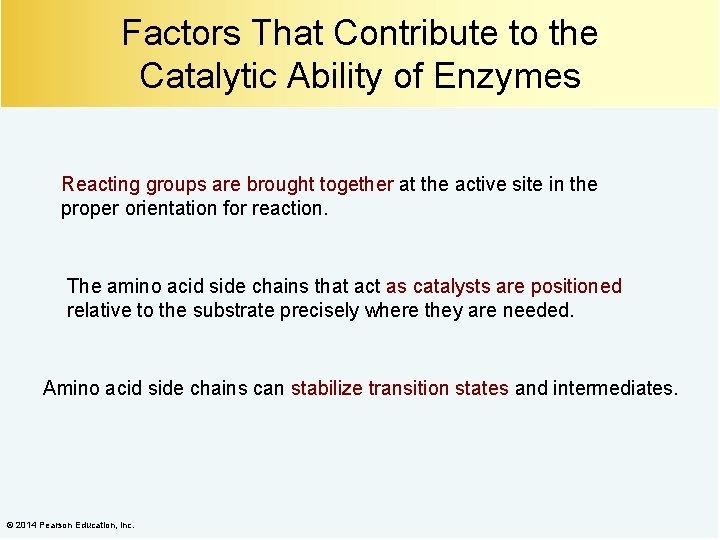
Factors That Contribute to the Catalytic Ability of Enzymes Reacting groups are brought together at the active site in the proper orientation for reaction. The amino acid side chains that act as catalysts are positioned relative to the substrate precisely where they are needed. Amino acid side chains can stabilize transition states and intermediates. © 2014 Pearson Education, Inc.

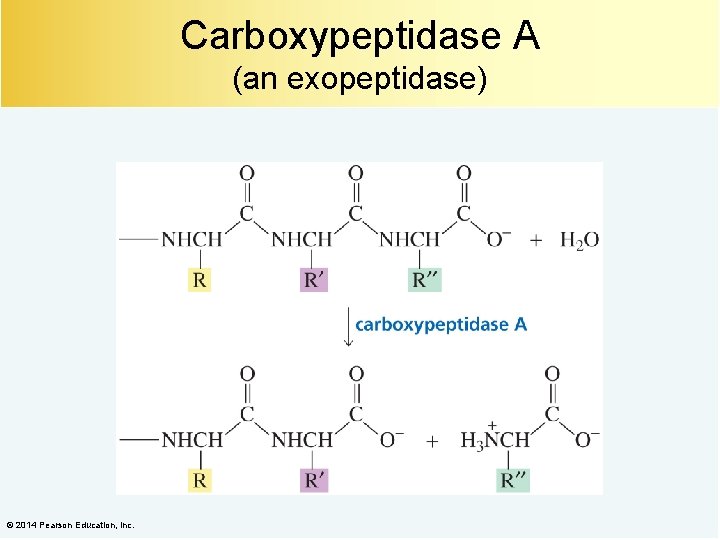
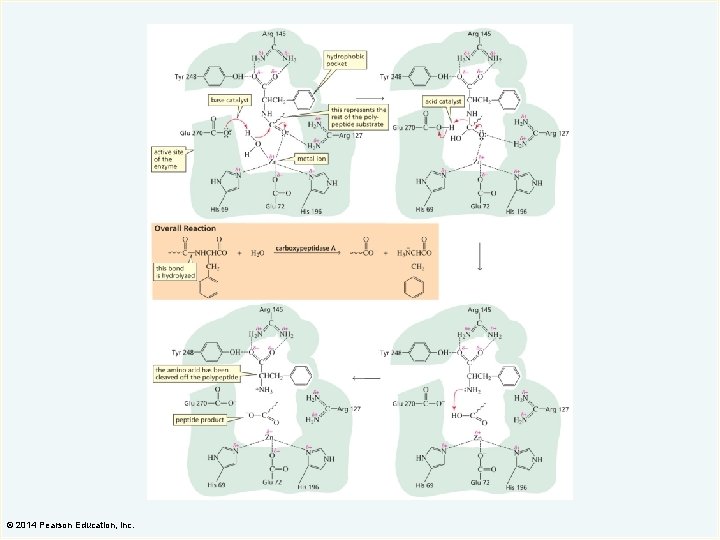
Carboxypeptidase A (an exopeptidase) © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

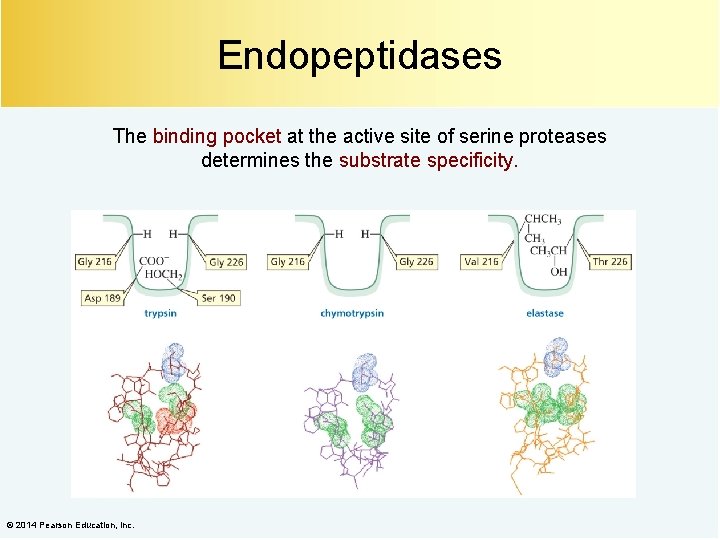
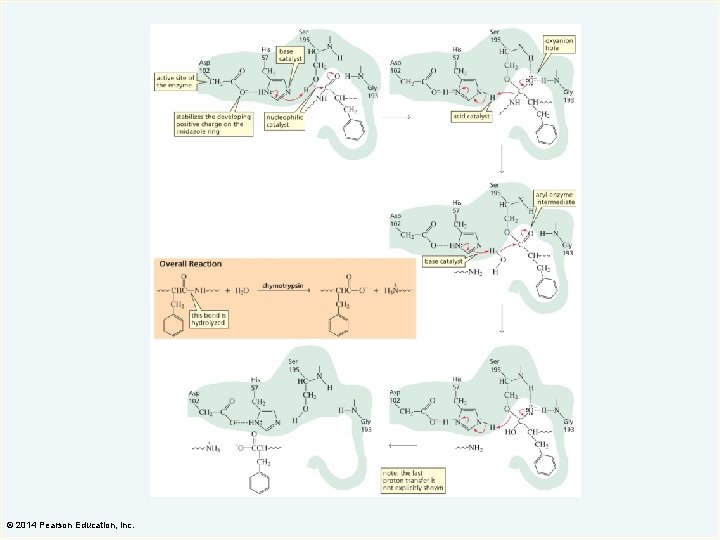
Endopeptidases The binding pocket at the active site of serine proteases determines the substrate specificity. © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

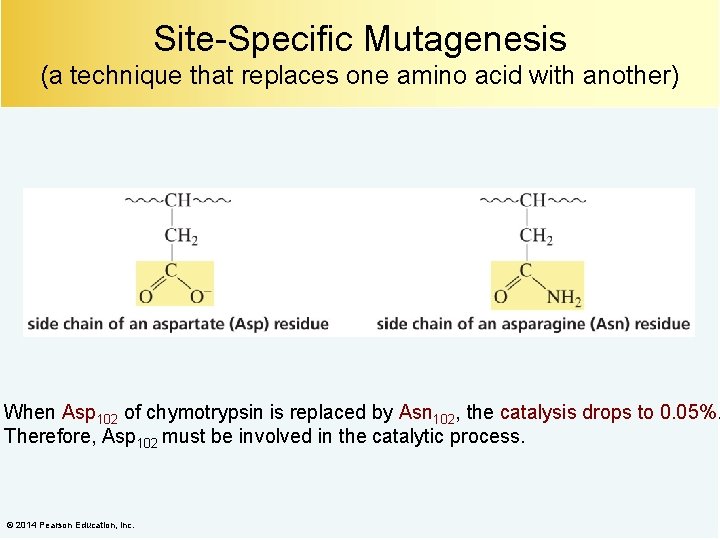
Site-Specific Mutagenesis (a technique that replaces one amino acid with another) When Asp 102 of chymotrypsin is replaced by Asn 102, the catalysis drops to 0. 05%. Therefore, Asp 102 must be involved in the catalytic process. © 2014 Pearson Education, Inc.

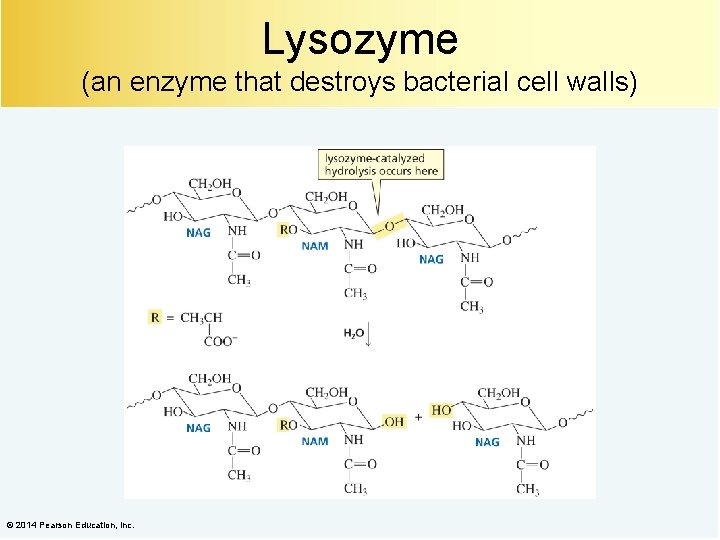
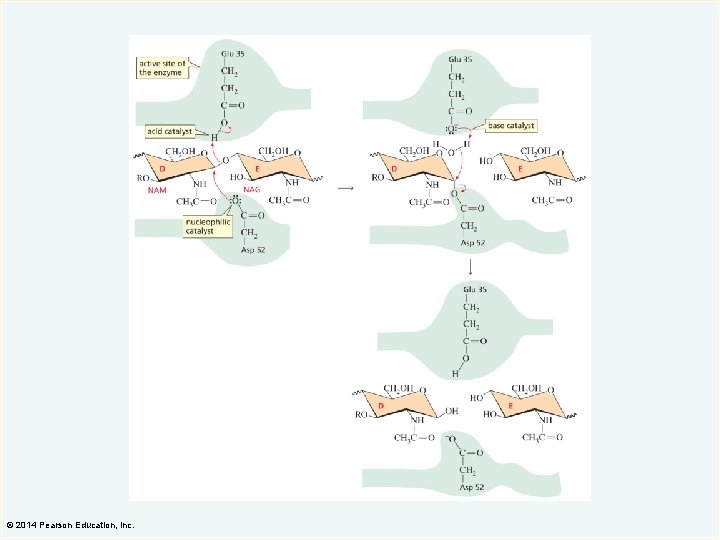
Lysozyme (an enzyme that destroys bacterial cell walls) © 2014 Pearson Education, Inc.

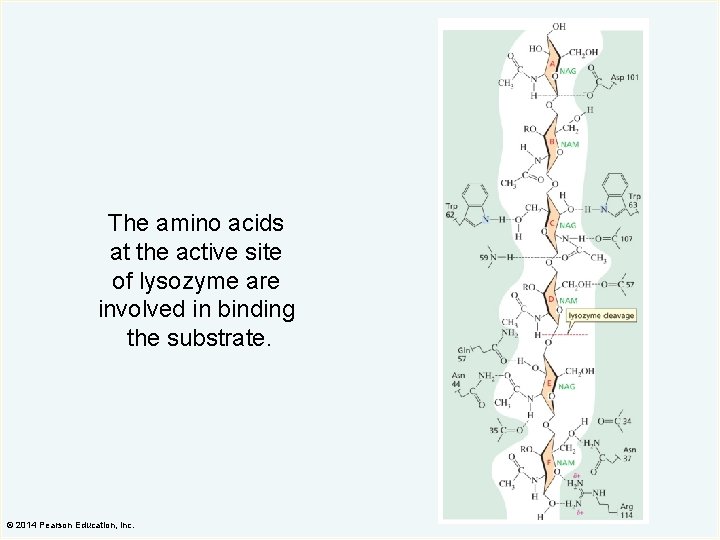
The amino acids at the active site of lysozyme are involved in binding the substrate. © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

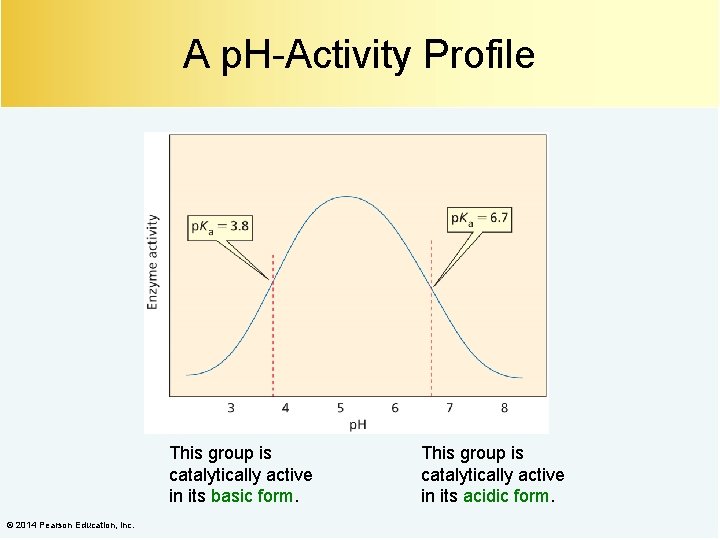
A p. H-Activity Profile This group is catalytically active in its basic form. © 2014 Pearson Education, Inc. This group is catalytically active in its acidic form.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.
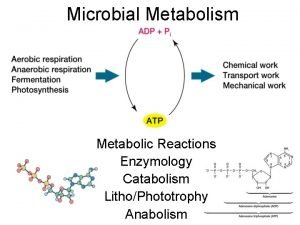
 Energy catalysis and biosynthesis
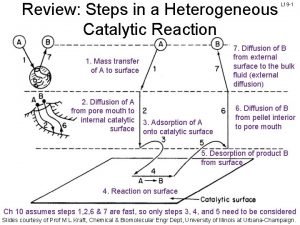
Energy catalysis and biosynthesis 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Catabolism
Catabolism Catalysis by approximation
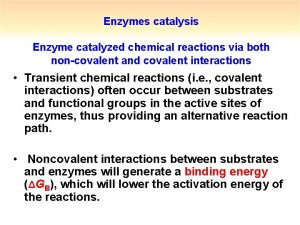
Catalysis by approximation What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis General principles of catalysis
General principles of catalysis Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Erzeng
Erzeng Site:slidetodoc.com
Site:slidetodoc.com Sodium hydroxide iupac id sodium oxidanide
Sodium hydroxide iupac id sodium oxidanide Reactions of organic compounds
Reactions of organic compounds Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Redox reaction
Redox reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
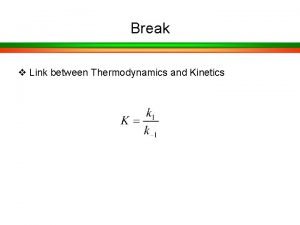
Chemical equations and reactions chapter 8 Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Importance of organic chemistry
Importance of organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Acid chloride + grignard reagent
Acid chloride + grignard reagent Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 chemistry review
Chapter 7 chemistry review Entane
Entane Analytical chemistry chapter 1
Analytical chemistry chapter 1 Halohydrin
Halohydrin What is this?
What is this? Chapter 19 redox reactions study guide answers
Chapter 19 redox reactions study guide answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 20 oxidation-reduction reactions answer key
Chapter 20 oxidation-reduction reactions answer key Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Are kc and kp equal
Are kc and kp equal How to write reduction half reactions
How to write reduction half reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key 5 type of reactions
5 type of reactions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Concentrated solution
Concentrated solution Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Which solution
Which solution What are active metals
What are active metals The rate of weathering depends upon the area's ____
The rate of weathering depends upon the area's ____