Chapter 6 Reactions of Alkenes Addition Reactions Reactions









- Slides: 27

Chapter 6 Reactions of Alkenes: Addition Reactions

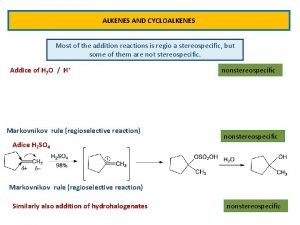
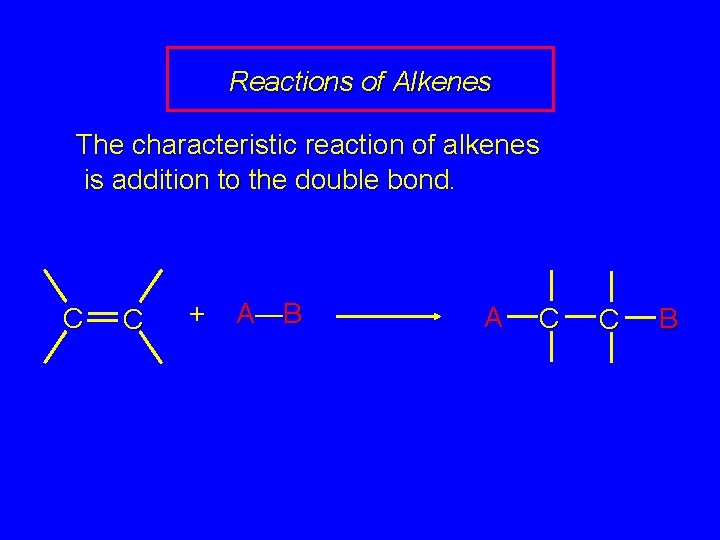
Reactions of Alkenes The characteristic reaction of alkenes is addition to the double bond. C C + A—B A C C B

6. 1 Hydrogenation of Alkenes

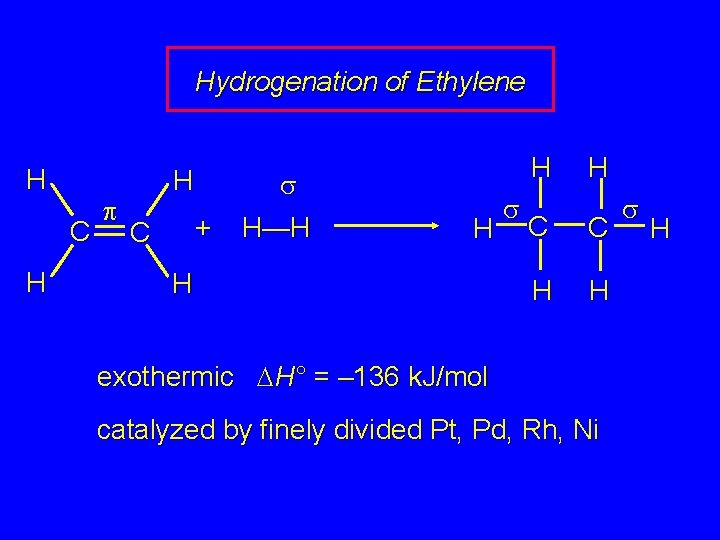
Hydrogenation of Ethylene H C H p H + C s H—H H s H C C H H exothermic DH° = – 136 k. J/mol catalyzed by finely divided Pt, Pd, Rh, Ni s H

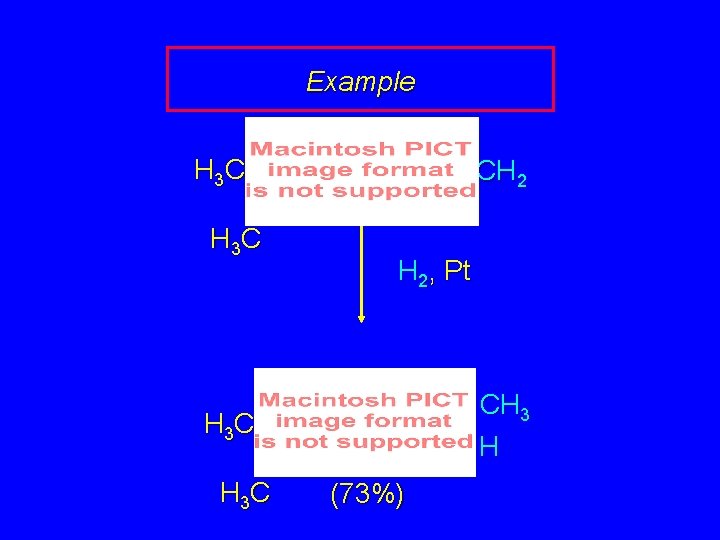
Example H 3 C CH 2 H 2, Pt CH 3 H H 3 C (73%)

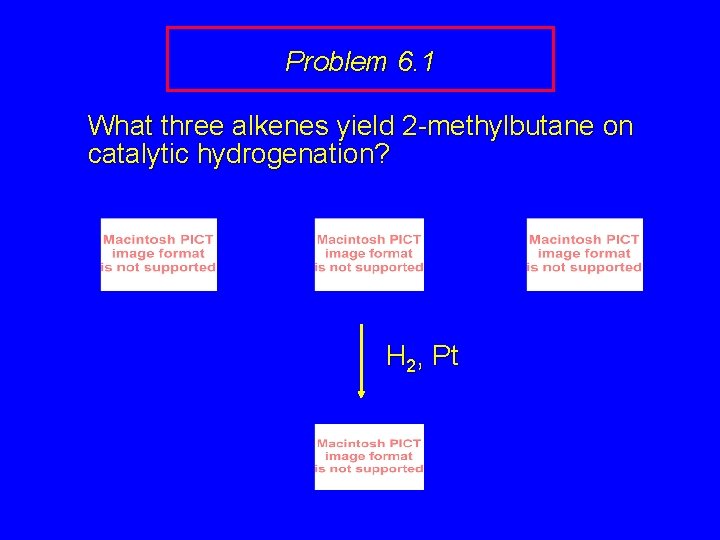
Problem 6. 1 What three alkenes yield 2 -methylbutane on catalytic hydrogenation?

Problem 6. 1 What three alkenes yield 2 -methylbutane on catalytic hydrogenation? H 2, Pt

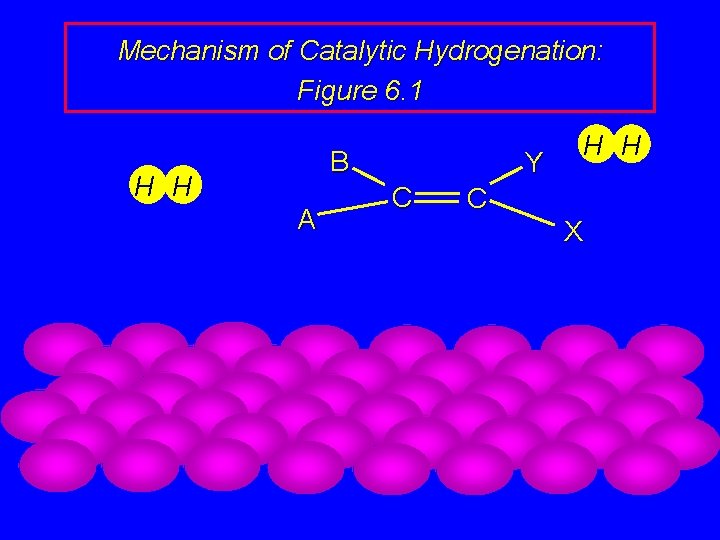
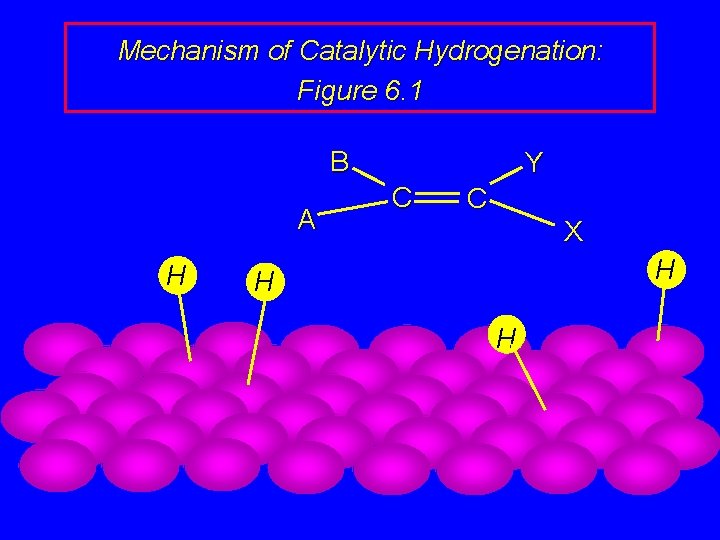
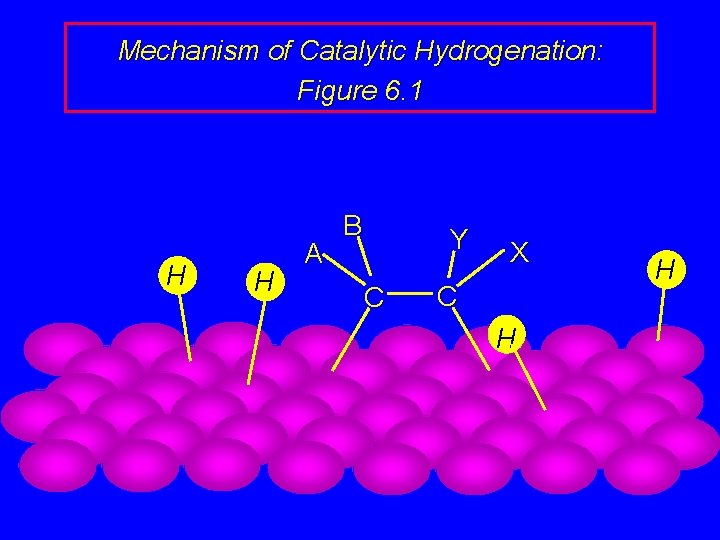
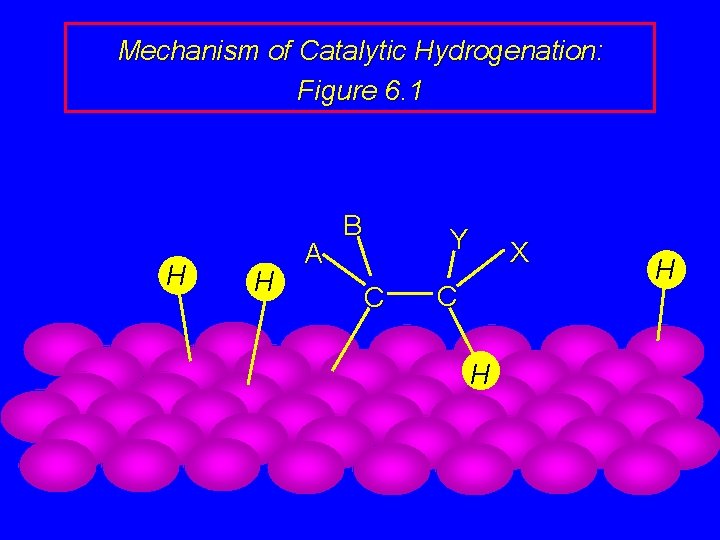
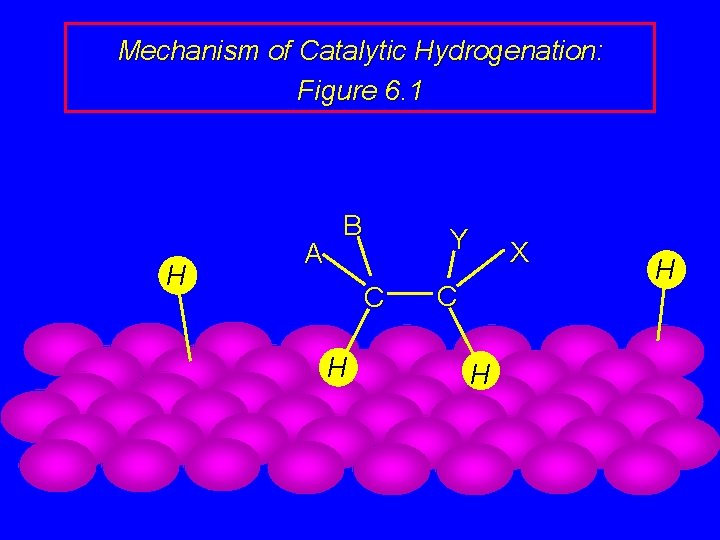
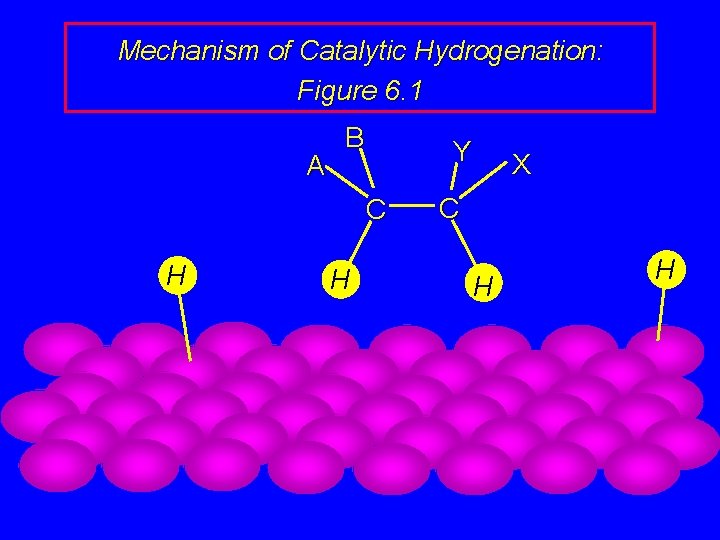
Mechanism of Catalytic Hydrogenation: Figure 6. 1 B H H A Y C H H C X

Mechanism of Catalytic Hydrogenation: Figure 6. 1 B A H Y C C X H H H

Mechanism of Catalytic Hydrogenation: Figure 6. 1 H H A B Y C X C H H

Mechanism of Catalytic Hydrogenation: Figure 6. 1 H H A B Y C X C H H

Mechanism of Catalytic Hydrogenation: Figure 6. 1 H A B Y C H X C H H

Mechanism of Catalytic Hydrogenation: Figure 6. 1 A B Y C H H X C H H

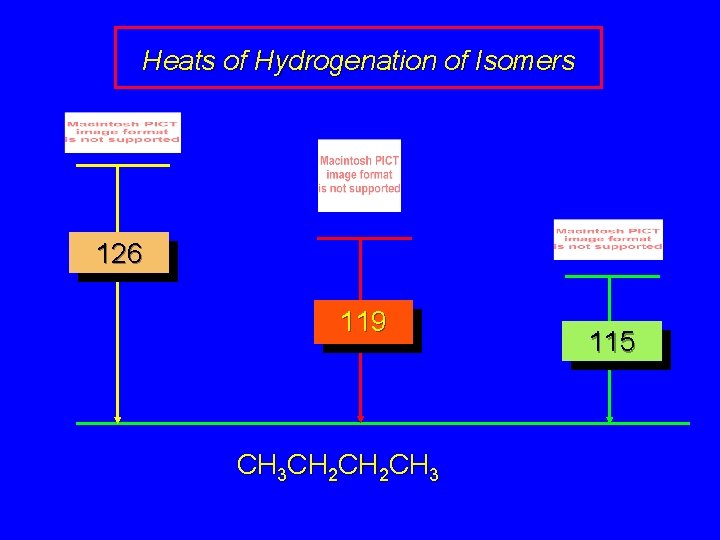
6. 2 Heats of Hydrogenation can be used to measure relative stability of isomeric alkenes correlation with structure is same as when heats of combustion are measured

Heats of Hydrogenation of Isomers 126 119 CH 3 CH 2 CH 3 115

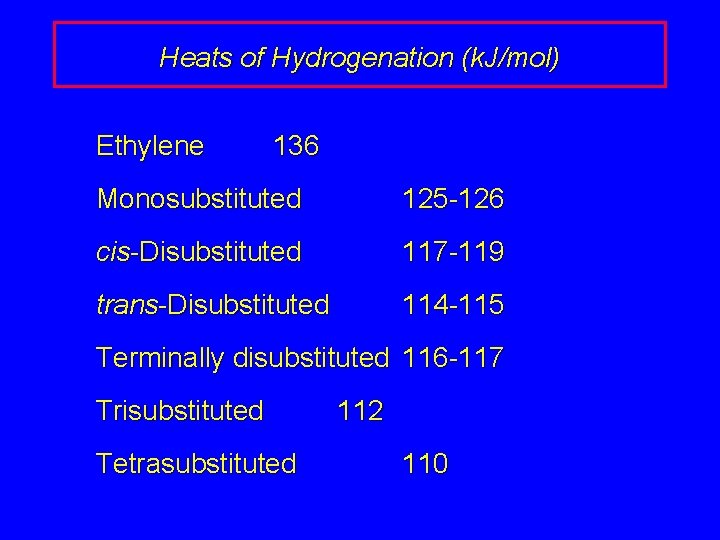
Heats of Hydrogenation (k. J/mol) Ethylene 136 Monosubstituted 125 -126 cis-Disubstituted 117 -119 trans-Disubstituted 114 -115 Terminally disubstituted 116 -117 Trisubstituted Tetrasubstituted 112 110

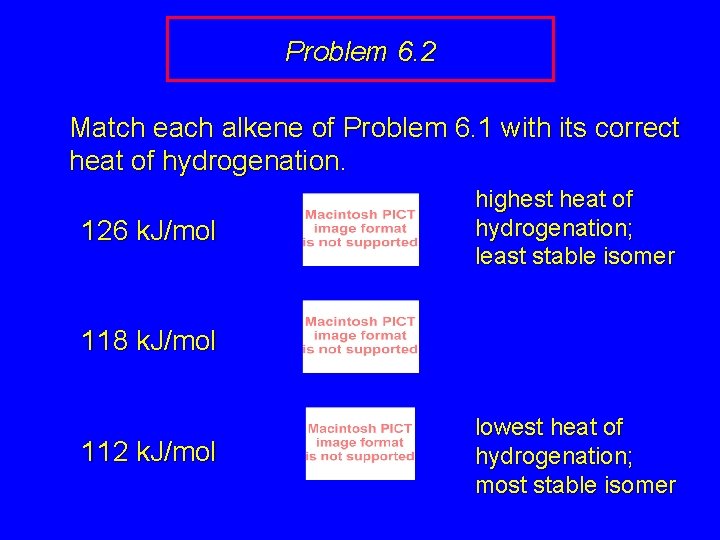
Problem 6. 2 Match each alkene of Problem 6. 1 with its correct heat of hydrogenation. 126 k. J/mol 118 k. J/mol 112 k. J/mol

Problem 6. 2 Match each alkene of Problem 6. 1 with its correct heat of hydrogenation. 126 k. J/mol highest heat of hydrogenation; least stable isomer 118 k. J/mol 112 k. J/mol lowest heat of hydrogenation; most stable isomer

6. 3 Stereochemistry of Alkene Hydrogenation

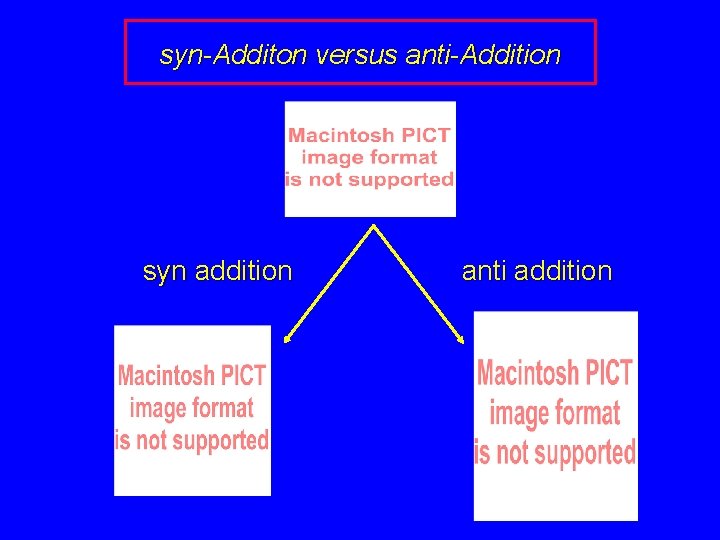
Two spatial (stereochemical) aspects of alkene hydrogenation: syn addition of both H atoms to double bond hydrogenation is stereoselective, corresponding to addition to less crowded face of double bond

syn-Additon versus anti-Addition syn addition anti addition

Example of Syn Addition H CO 2 CH 3 H 2, Pt H CO 2 CH 3 (100%)

Stereoselectivity A reaction in which a single starting material can give two or more stereoisomeric products but yields one of them in greater amounts than the other (or even to the exclusion of the other) is said to be stereoselective.

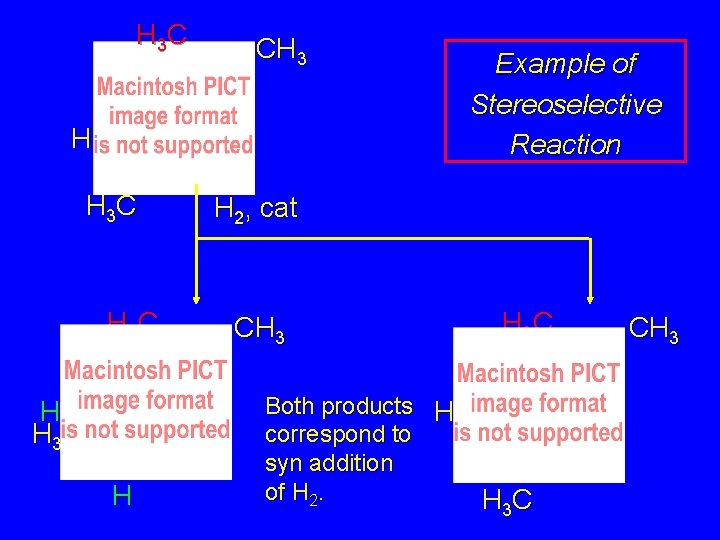
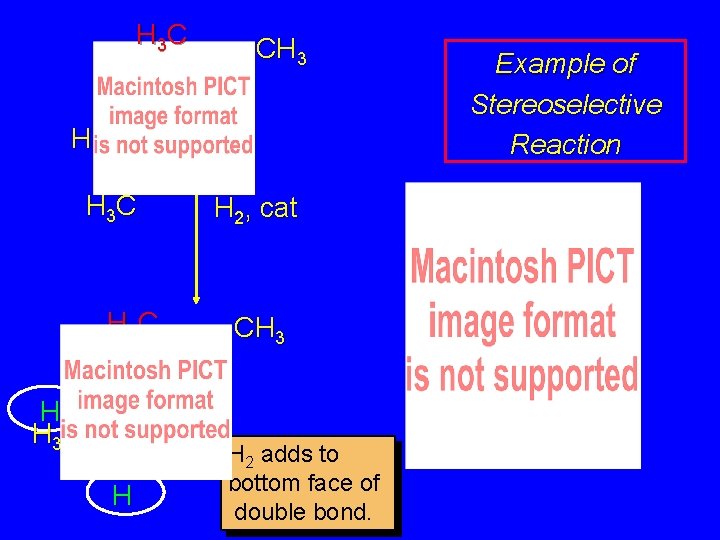
H 3 C CH 3 H H 3 C H Example of Stereoselective Reaction H 2, cat CH 3 H 3 C H Both products H correspond to H syn addition of H 2. H 3 C CH 3

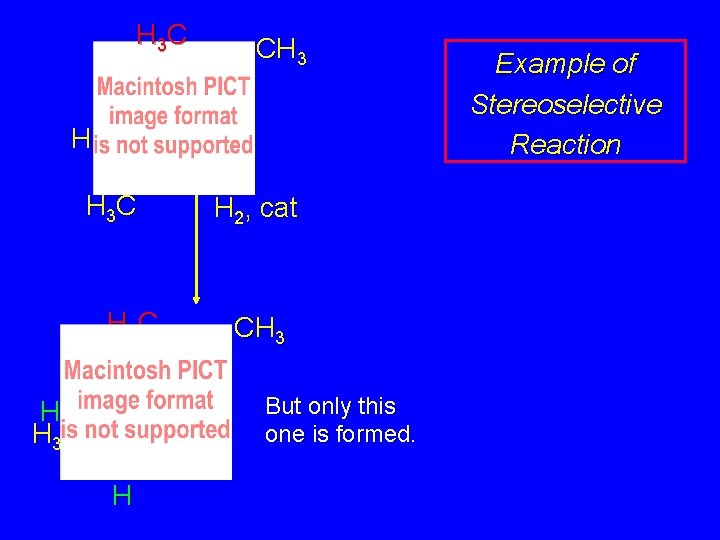
H 3 C CH 3 H H 3 C H H 2, cat CH 3 But only this one is formed. H H 3 C H Example of Stereoselective Reaction

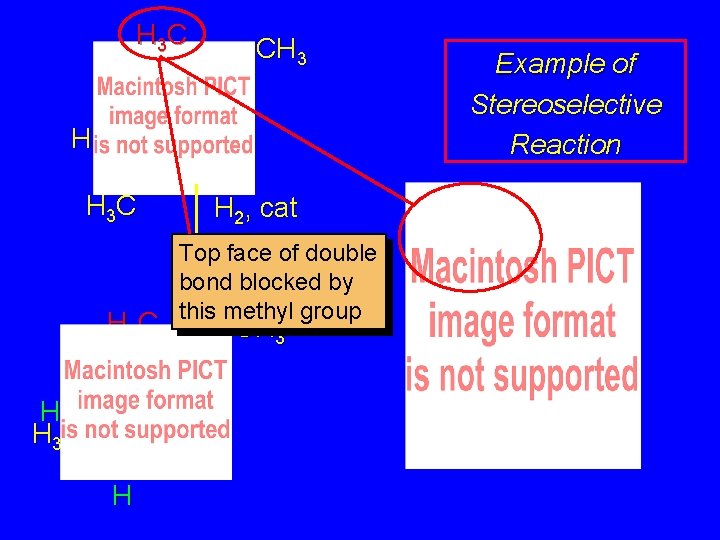
H 3 C CH 3 H H 3 C H H 2, cat Top face of double bond blocked by this methyl group CH 3 Example of Stereoselective Reaction

H 3 C CH 3 H H 3 C H H 2, cat CH 3 H 2 adds to bottom face of double bond. Example of Stereoselective Reaction
 2 methyl propene with hbr
2 methyl propene with hbr Addition of halogens to alkenes
Addition of halogens to alkenes Chemsheets
Chemsheets Diol formation from alkene
Diol formation from alkene Bromine test saturated or unsaturated
Bromine test saturated or unsaturated Propene undergoes addition reaction
Propene undergoes addition reaction 7 carbon alkene
7 carbon alkene Hobr addition to alkene
Hobr addition to alkene Properties of alkenes
Properties of alkenes Alkene general formula
Alkene general formula Alkanes alkenes alkynes
Alkanes alkenes alkynes Sp2 hybridization in alkenes
Sp2 hybridization in alkenes Name the following alkenes
Name the following alkenes Alkene prefix
Alkene prefix Bromine water test
Bromine water test What is the displayed formula
What is the displayed formula Define ozonolysis with example
Define ozonolysis with example Alkenes introduction
Alkenes introduction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Balancing redox reactions
Balancing redox reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Chapter 19 review oxidation-reduction reactions
Chapter 19 review oxidation-reduction reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions