Reaction of alkenes Reactions of alkenes with hydrogen









- Slides: 21

Reaction of alkenes

Reactions of alkenes with hydrogen halides Objective: To know how alkenes react with hydrogen halides Success criteria: • understand the addition reactions of alkenes with hydrogen halides to produce halogenoalkanes • understand the mechanism of the electrophilic addition reactions for the above including using curly arrow notation • Explain why a mixture of products is obtained from unsymmetrical alkenes • Know the relative stability of primary, secondary and tertiary carbocation intermediates

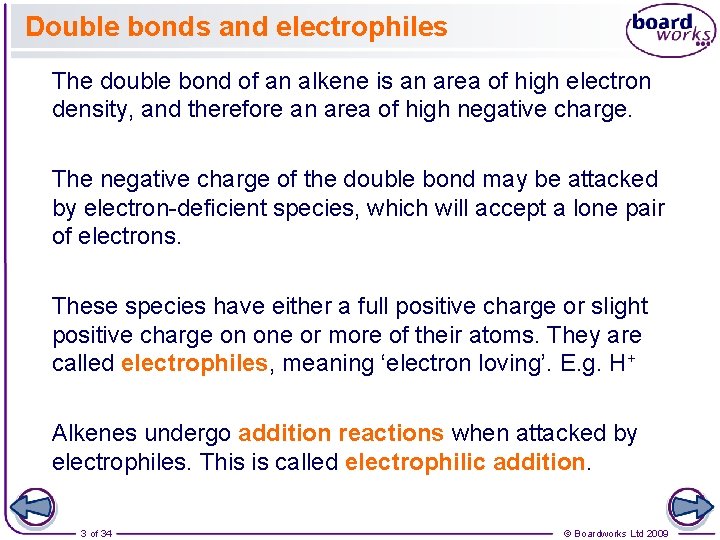
Double bonds and electrophiles The double bond of an alkene is an area of high electron density, and therefore an area of high negative charge. The negative charge of the double bond may be attacked by electron-deficient species, which will accept a lone pair of electrons. These species have either a full positive charge or slight positive charge on one or more of their atoms. They are called electrophiles, meaning ‘electron loving’. E. g. H+ Alkenes undergo addition reactions when attacked by electrophiles. This is called electrophilic addition. 3 of 34 © Boardworks Ltd 2009

Electrophilic addition mechanism: 1 In the first stage of electrophilic addition, the positive charge on the electrophile is attracted to the electron density in the double bond. δ+ δ- ® As the electrophile approaches the double bond, electrons in the A–B bond are repelled towards B. The pi bond breaks, and A bonds to the carbon, forming a carbocation – an ion with a positively-charge carbon atom. The two electrons in the A–B bond move to B forming a B- ion. curly arrows should start from either a bond or from a lone pair of electrons 4 of 34 © Boardworks Ltd 2009

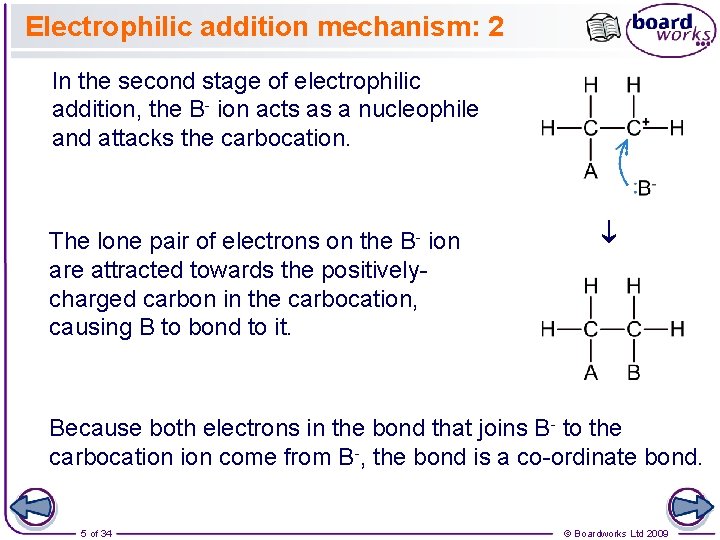
Electrophilic addition mechanism: 2 In the second stage of electrophilic addition, the B- ion acts as a nucleophile and attacks the carbocation. ® The lone pair of electrons on the B- ion are attracted towards the positivelycharged carbon in the carbocation, causing B to bond to it. Because both electrons in the bond that joins B- to the carbocation come from B-, the bond is a co-ordinate bond. 5 of 34 © Boardworks Ltd 2009

Alkenes react with hydrogen halides (e. g. HCl) to form halogenalkanes Halogenalkanes are alkanes with halogen atoms

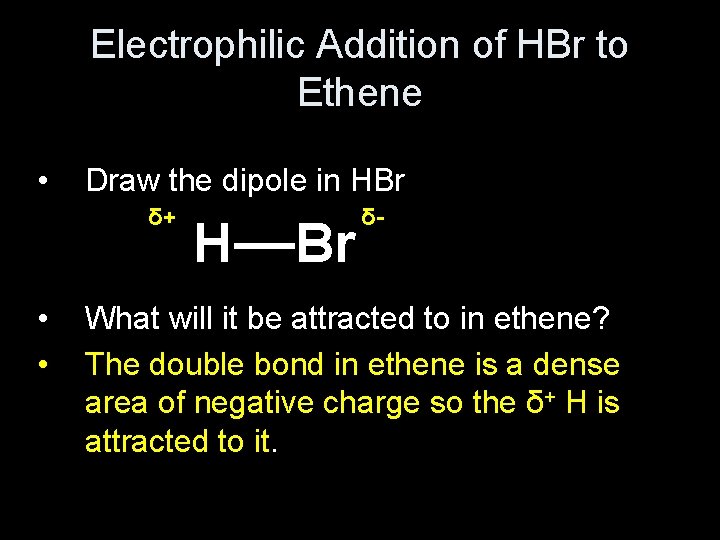
Electrophilic Addition of HBr to Ethene • Draw the dipole in HBr δ+ • • δ__ H Br What will it be attracted to in ethene? The double bond in ethene is a dense area of negative charge so the δ+ H is attracted to it.

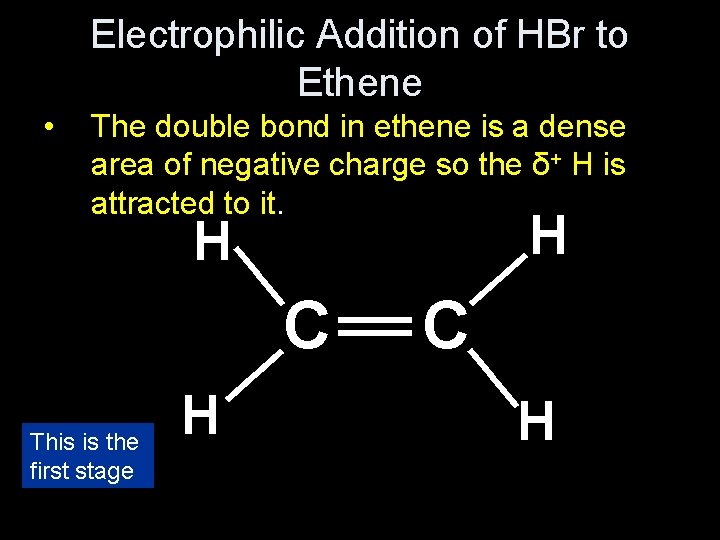
Electrophilic Addition of HBr to Ethene • The double bond in ethene is a dense area of negative charge so the δ+ H is attracted to it. __ This is the first stage H _ __ _ C C __ H H

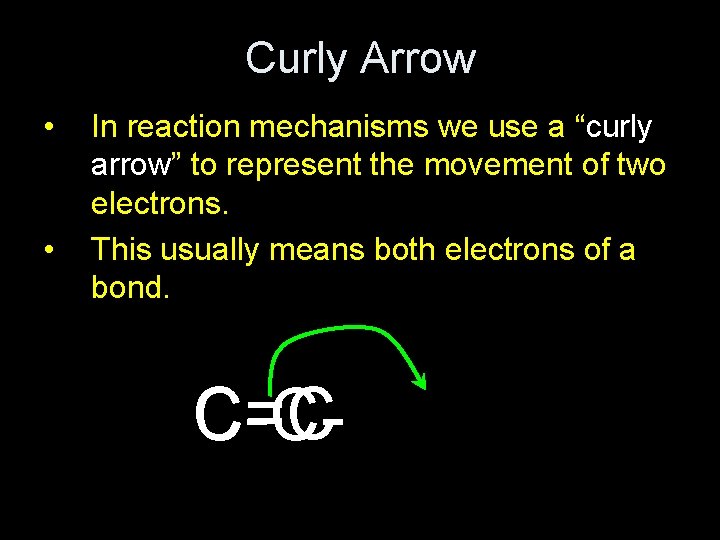
Curly Arrow • • In reaction mechanisms we use a “curly arrow” to represent the movement of two electrons. This usually means both electrons of a bond. C=C C-C-

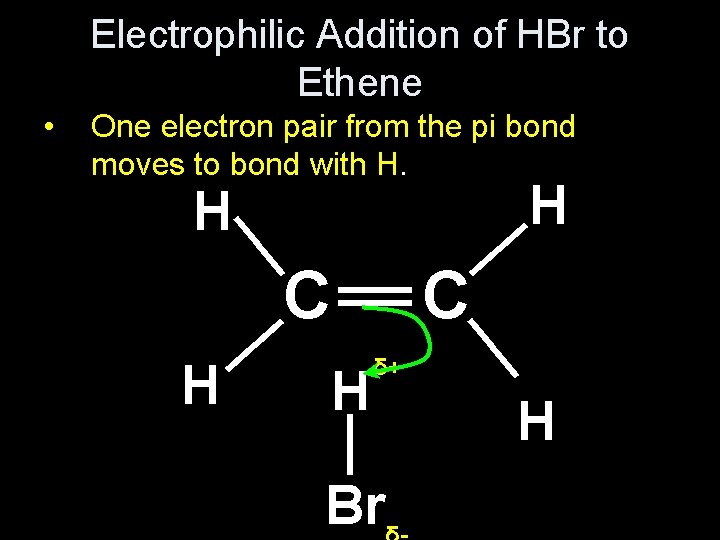
Electrophilic Addition of HBr to Ethene • One electron pair from the pi bond moves to bond with H. H _ H δ+ __ H _ _ Br __ __ _ C C __ H H

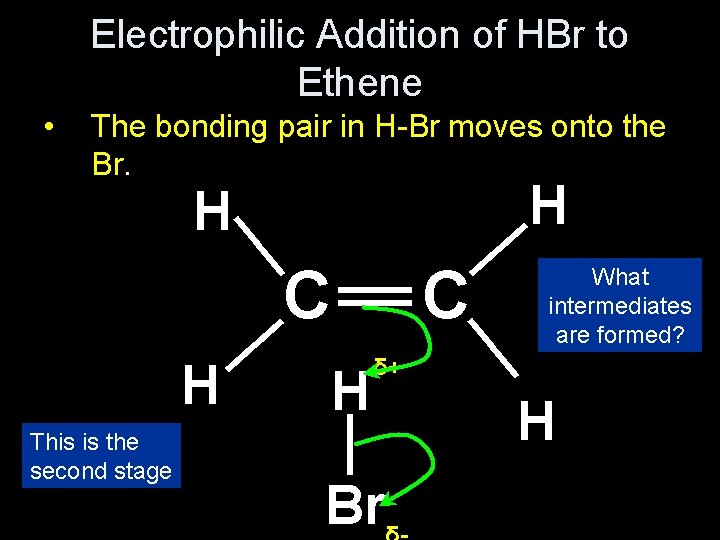
Electrophilic Addition of HBr to Ethene • The bonding pair in H-Br moves onto the Br. H _ This is the second stage H δ+ __ H _ _ Br What intermediates are formed? __ __ _ C C __ H H

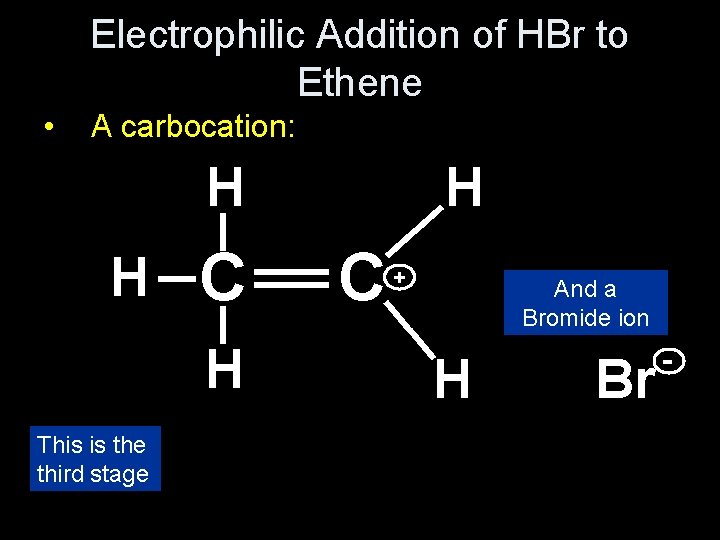
Electrophilic Addition of HBr to Ethene • A carbocation: H H _ H + __ _ This is the third stage _ _ __ _ H C C H And a Bromide ion Br -

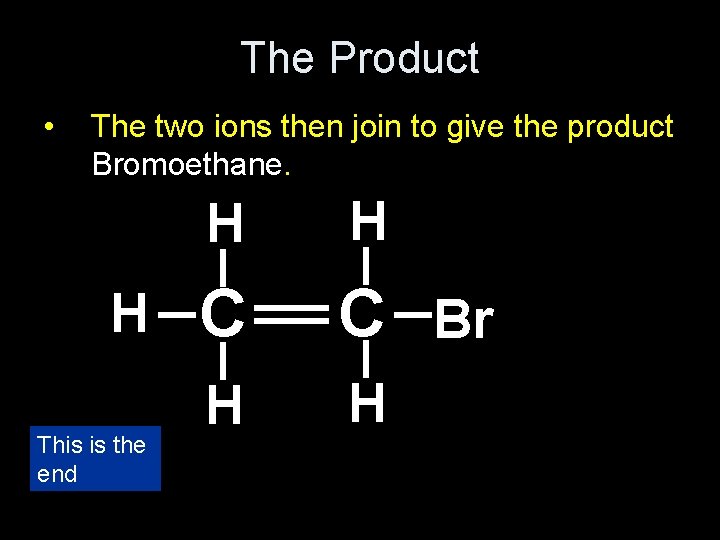
The Product • The two ions then join to give the product Bromoethane. H H _ _ This is the end _ _ __ H C C Br

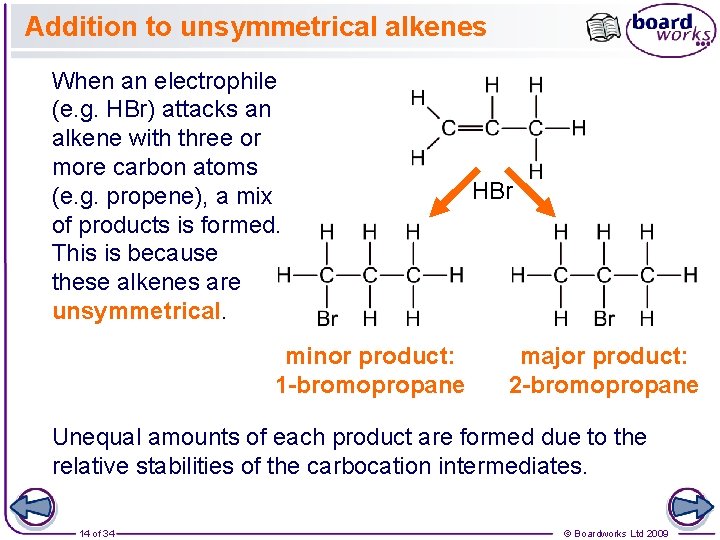
Addition to unsymmetrical alkenes When an electrophile (e. g. HBr) attacks an alkene with three or more carbon atoms (e. g. propene), a mix of products is formed. This is because these alkenes are unsymmetrical. minor product: 1 -bromopropane HBr major product: 2 -bromopropane Unequal amounts of each product are formed due to the relative stabilities of the carbocation intermediates. 14 of 34 © Boardworks Ltd 2009

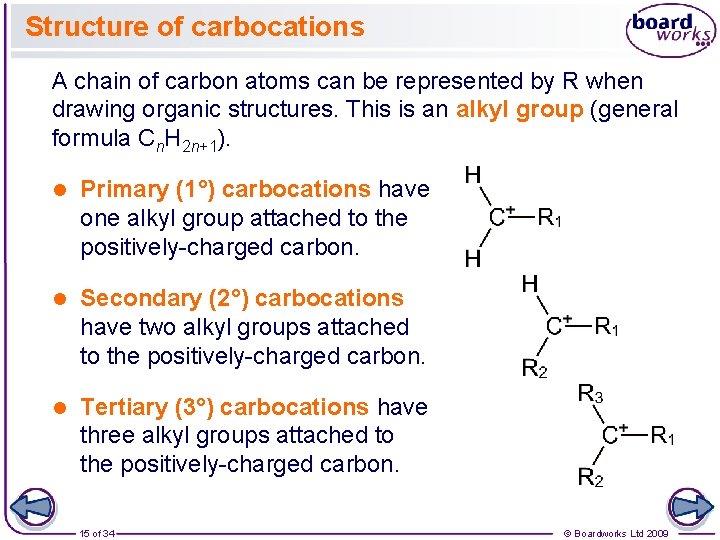
Structure of carbocations A chain of carbon atoms can be represented by R when drawing organic structures. This is an alkyl group (general formula Cn. H 2 n+1). l Primary (1°) carbocations have one alkyl group attached to the positively-charged carbon. l Secondary (2°) carbocations have two alkyl groups attached to the positively-charged carbon. l Tertiary (3°) carbocations have three alkyl groups attached to the positively-charged carbon. 15 of 34 © Boardworks Ltd 2009

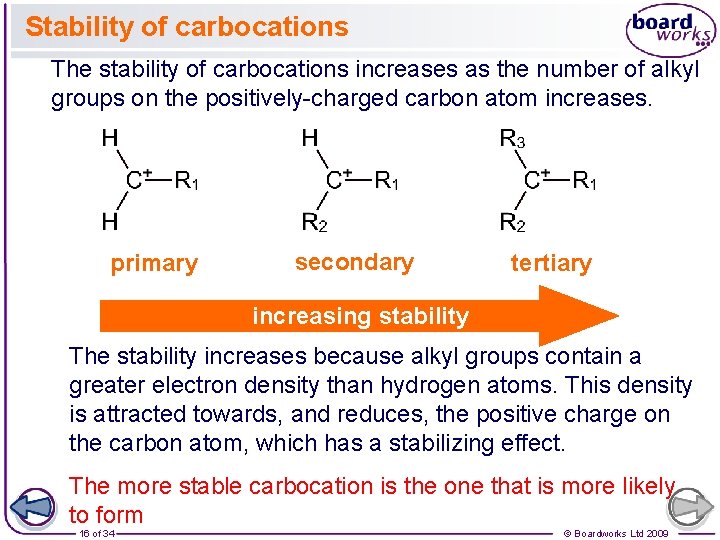
Stability of carbocations The stability of carbocations increases as the number of alkyl groups on the positively-charged carbon atom increases. primary secondary tertiary increasing stability The stability increases because alkyl groups contain a greater electron density than hydrogen atoms. This density is attracted towards, and reduces, the positive charge on the carbon atom, which has a stabilizing effect. The more stable carbocation is the one that is more likely to form 16 of 34 © Boardworks Ltd 2009

The more stable carbocation is the one that is more likely to form Markownikoff’s rule The major product from the addition of a hydrogen halide (HX) to an unsymmetrical alkene is the one where hydrogen adds to the carbon with the most hydrogens already attached.

Example hydrogen bromide reacting with propene Can you do this yourselves?

Hydrogen bromide reacts with but-2 -ene producing 2 – bromobutane, CH 3 CH 2 CHBr. CH 3. Name the mechanism involved Outline the mechanism for the reaction

Hydrogen bromide reacts with but-1 -ene producing two isomeric products. Draw the displayed formulae of these two isomers and explain which will be the major product.

Reactions of alkenes with hydrogen halides Objective: To know how alkenes react with hydrogen halides Success criteria: • understand the addition reactions of alkenes with hydrogen halides to produce halogenoalkanes • understand the mechanism of the electrophilic addition reactions for the above including using curly arrow notation • Explain why a mixture of products is obtained from unsymmetrical alkenes • Know the relative stability of primary, secondary and tertiary carbocation intermediates
 Diol formation from alkene
Diol formation from alkene Chemsheets as 1128 answers
Chemsheets as 1128 answers 2 methyl propene + hbr
2 methyl propene + hbr Half redox reaction
Half redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Dissolved oxygen and chlorine molecules act as
Dissolved oxygen and chlorine molecules act as Magnesium and copper oxide equation
Magnesium and copper oxide equation Sodium carbonate and hcl reaction
Sodium carbonate and hcl reaction Examples of isomers in chemistry
Examples of isomers in chemistry Properties of alkenes
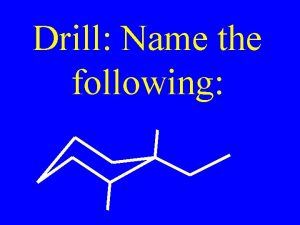
Properties of alkenes Name the following alkene:
Name the following alkene: Incomplete combustion of ethene
Incomplete combustion of ethene What is ozonolysis give an example
What is ozonolysis give an example Alkenes general formula
Alkenes general formula Alkyne prefix
Alkyne prefix Alkane chemical formula
Alkane chemical formula Alkenes introduction
Alkenes introduction Alkanes alkenes alkynes
Alkanes alkenes alkynes Bromine water test
Bromine water test