Nomenclature of Alkenes 1 Nomenclature of Alkenes Figure











- Slides: 13

Nomenclature of Alkenes 1

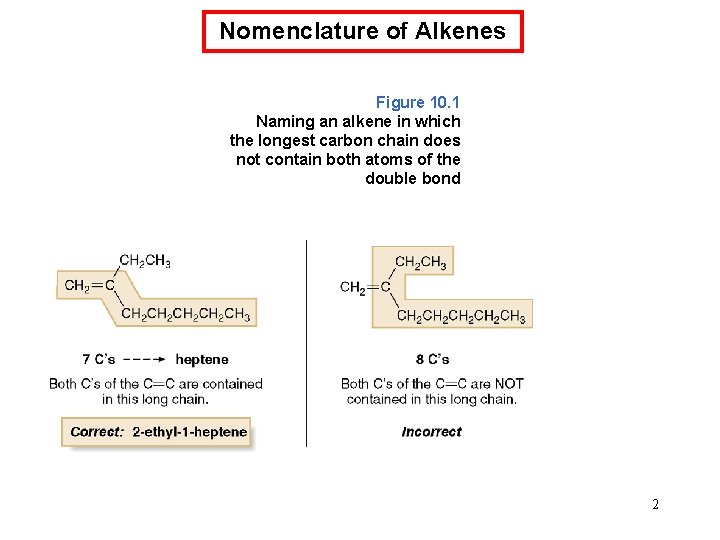
Nomenclature of Alkenes Figure 10. 1 Naming an alkene in which the longest carbon chain does not contain both atoms of the double bond 2

Cis-Trans (Z-E) Isomerism in Alkenes If each end of the double bond is attached to two different groups, then the compound exists in two different forms called (Diastereomers; These are non mirror image stereoisomers). If the two groups are identical we distinguish the two isomers by adding the prefix cis (same side) or trans (opposite sides) Example

If the groups attached to the double bond are different, we distinguish the two isomers by adding the prefix Z (same side) or E (opposite sides) depending on the atomic number of the atoms attached to each end of the double bond

Isomers and common names of simple alkenes


• Saytzeff orientation: • In dehydrohalogenation the preferred product is the alkene that has the greater number of alkyl groups attached to the doubly bonded carbon atoms • (the more substituted alkene will form) • Ease of formation of alkenes: • R 2 C=CR 2 > R 2 C=CHR > R 2 C=CH 2, RCH=CHR > RCH=CH 2 > CH 2=CH 2 • Stability of alkenes: • R 2 C=CR 2 > R 2 C=CHR > R 2 C=CH 2, RCH=CHR > RCH=CH 2 > CH 2=CH 2 • • • CH 3 CH 2 CHCH 3 + Br sec-butyl bromide KOH(alc) CH 3 CH 2 CH=CH 2 1 -butene 19% + CH 3 CH=CHCH 3 2 -butene RCH=CH 2 RCH=CHR 81% 7

8

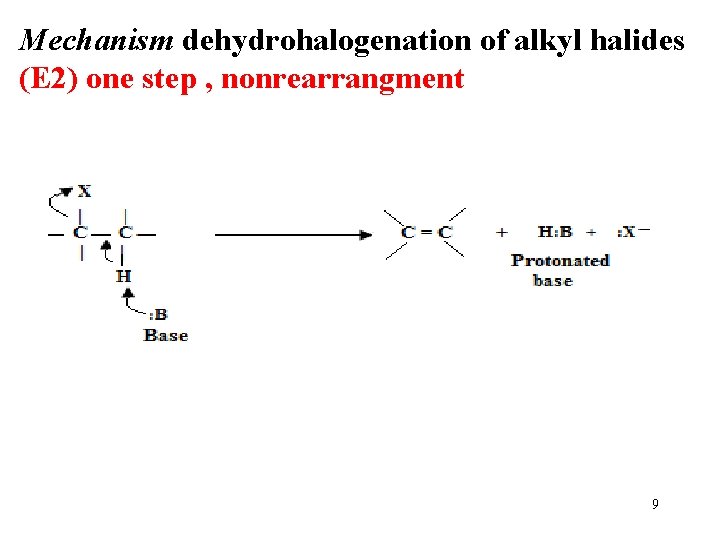
Mechanism dehydrohalogenation of alkyl halides (E 2) one step , nonrearrangment 9

Mechanism of Dehydration (E 1) two step carbocation form, rearrangment


Dehydration (Rearrangement)

 Sp2 hybridization in alkenes
Sp2 hybridization in alkenes Chemsheets as 1139
Chemsheets as 1139 Functional group vs homologous series
Functional group vs homologous series Bromine water test
Bromine water test Physical properties of alkenes
Physical properties of alkenes Chemical properties of alkenes
Chemical properties of alkenes Name the following alkene:
Name the following alkene: Ozonolysis of alkyne
Ozonolysis of alkyne Ozonolysis
Ozonolysis Alkenes general formula
Alkenes general formula Bromine water test
Bromine water test Alkyne prefix
Alkyne prefix Addition of hydrogen halides to alkynes
Addition of hydrogen halides to alkynes Alkenes introduction
Alkenes introduction