Catalysis and Heterogeneous Catalysis Catalysis and Heterogeneous Catalysis








- Slides: 46

Catalysis and Heterogeneous Catalysis

Catalysis and Heterogeneous Catalysis : objectives Develop understanding of catalysts, reaction mechanisms and catalytic reactor design 1. Define the catalyst and its properties 2. Descript the steps of catalytic reactions 3. Develop a rate law and determine the rate law parameters

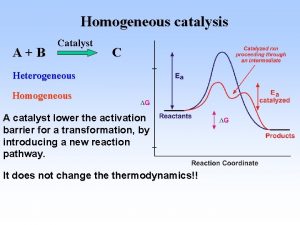
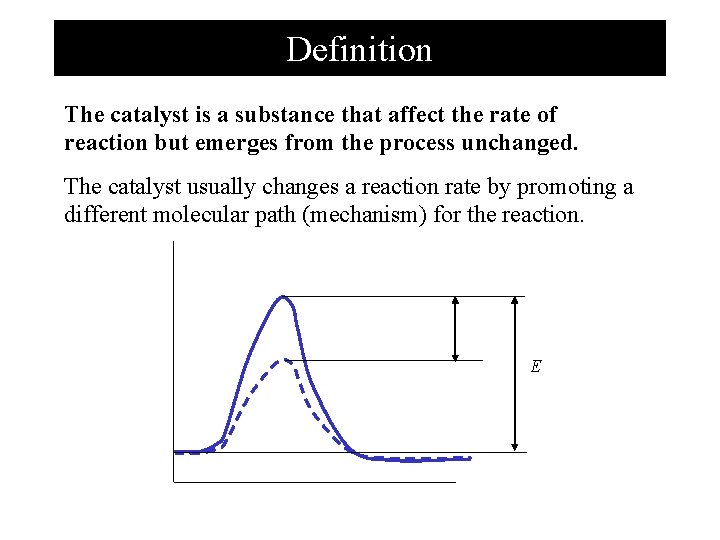
Definition The catalyst is a substance that affect the rate of reaction but emerges from the process unchanged. The catalyst usually changes a reaction rate by promoting a different molecular path (mechanism) for the reaction. E

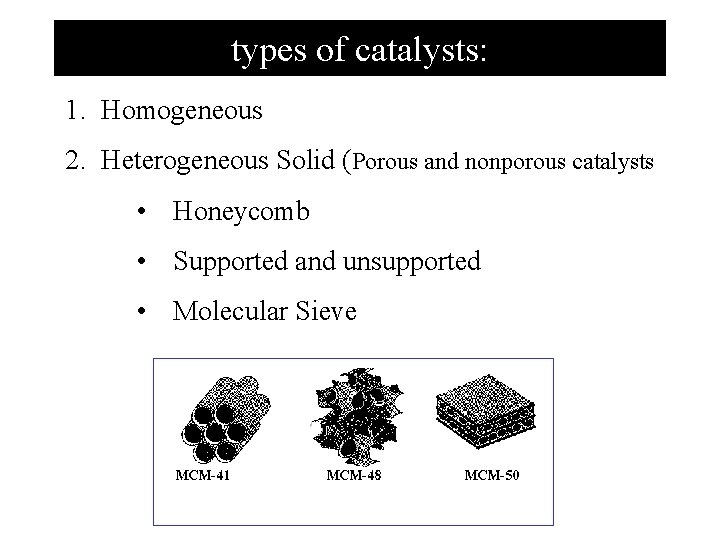
types of catalysts: 1. Homogeneous 2. Heterogeneous Solid (Porous and nonporous catalysts • Honeycomb • Supported and unsupported • Molecular Sieve

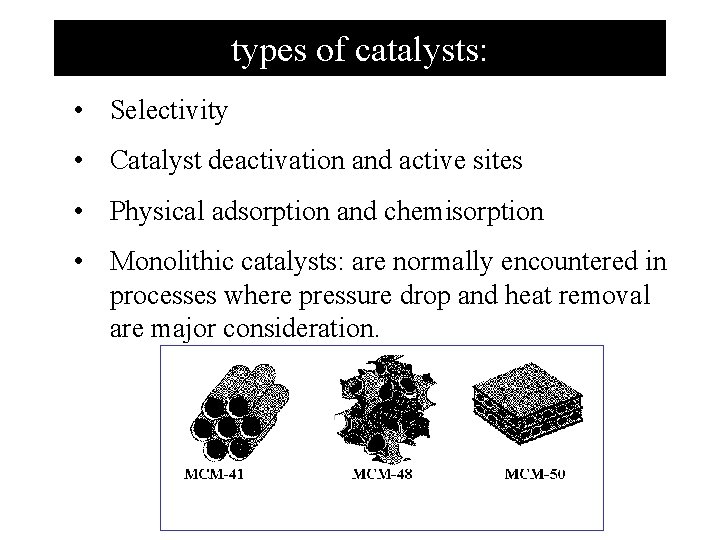
types of catalysts: • Selectivity • Catalyst deactivation and active sites • Physical adsorption and chemisorption • Monolithic catalysts: are normally encountered in processes where pressure drop and heat removal are major consideration.


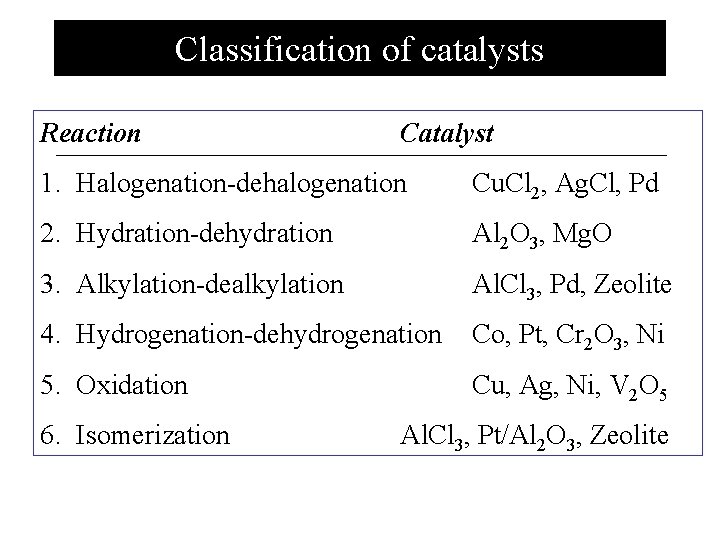
Classification of catalysts Reaction Catalyst 1. Halogenation-dehalogenation Cu. Cl 2, Ag. Cl, Pd 2. Hydration-dehydration Al 2 O 3, Mg. O 3. Alkylation-dealkylation Al. Cl 3, Pd, Zeolite 4. Hydrogenation-dehydrogenation Co, Pt, Cr 2 O 3, Ni 5. Oxidation Cu, Ag, Ni, V 2 O 5 6. Isomerization Al. Cl 3, Pt/Al 2 O 3, Zeolite

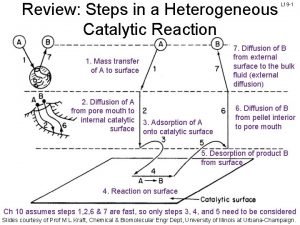
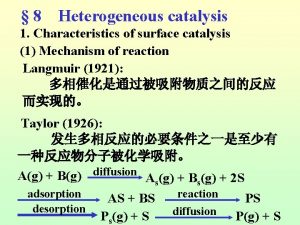
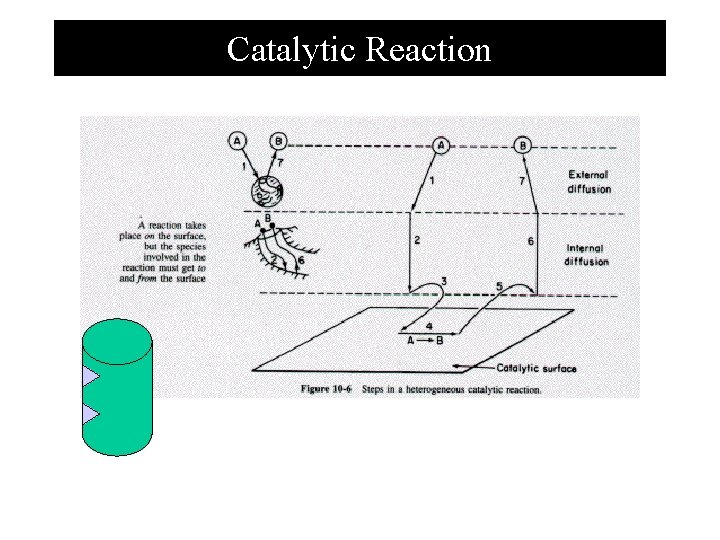
Steps in catalytic reaction 1. Mass transfer (diffusion) of the reactants from bulk fluid to the external surface of the catalyst. 2. Diffusion from pore mouth through the pores to the internal catalyst surface. 3. Adsorption onto the catalyst surface. 4. Reaction on the catalyst surface. 5. Desorption of the products 6. Diffusion of products from the interior to the pore mouth. 7. Mass transfer of products from external pellet surface to the bulk fluid.

Catalytic Reaction

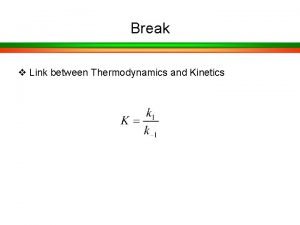
Rate Law The rate law in hetrogeneous catalysis seldom follow the power law discussed in Ch. 5 for homogeneous reactions. To form reaction rate law we need to formulate or propose catalytic mechanism then derive the rate law. 1. Adsorption step 2. Surface reaction 3. Desorption step One is the rate limiting

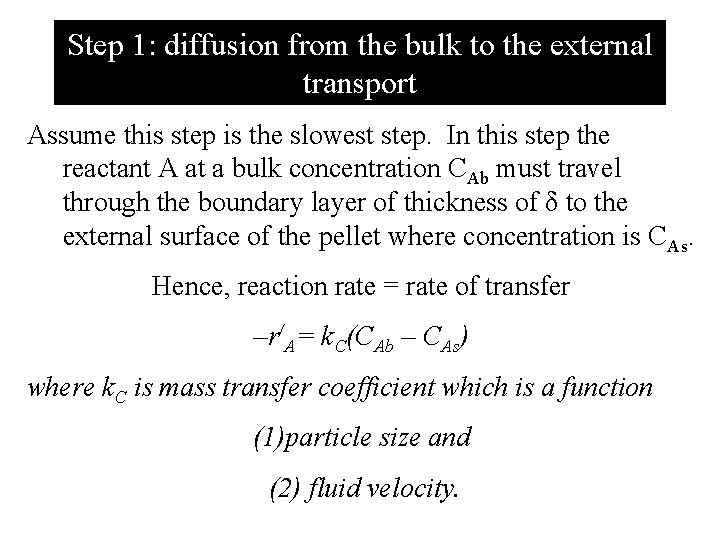
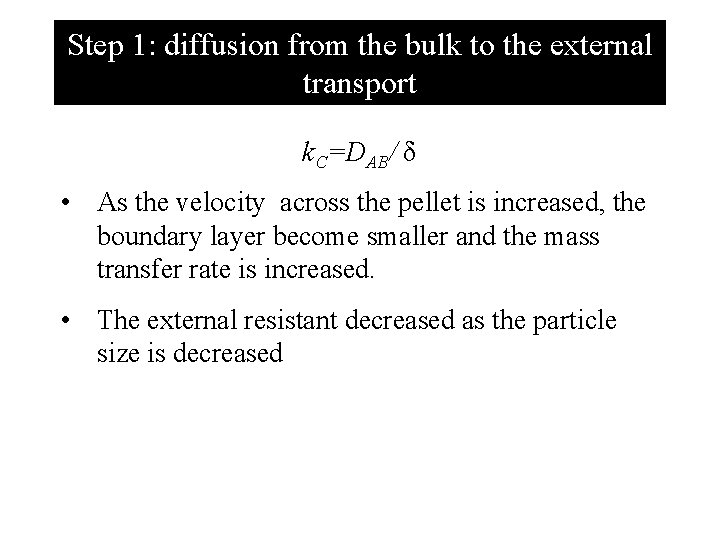
Step 1: diffusion from the bulk to the external transport Assume this step is the slowest step. In this step the reactant A at a bulk concentration CAb must travel through the boundary layer of thickness of δ to the external surface of the pellet where concentration is CAs. Hence, reaction rate = rate of transfer –r/A= k. C(CAb – CAs) where k. C is mass transfer coefficient which is a function (1)particle size and (2) fluid velocity.

Step 1: diffusion from the bulk to the external transport k. C=DAB/ δ • As the velocity across the pellet is increased, the boundary layer become smaller and the mass transfer rate is increased. • The external resistant decreased as the particle size is decreased

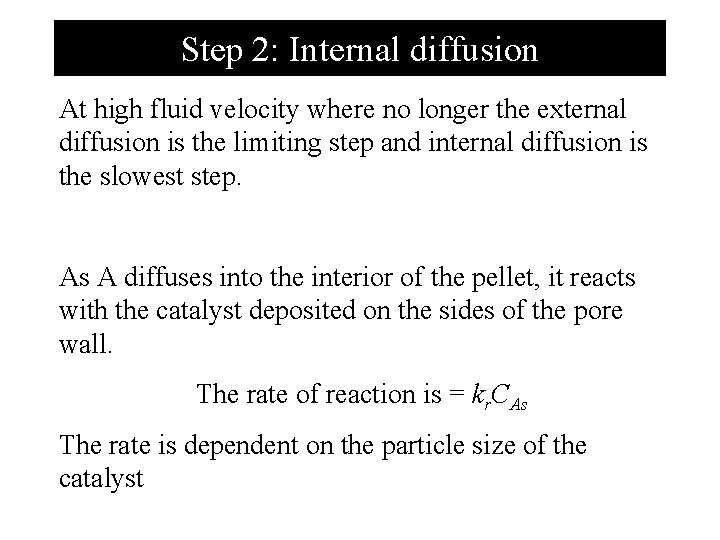
Step 2: Internal diffusion At high fluid velocity where no longer the external diffusion is the limiting step and internal diffusion is the slowest step. As A diffuses into the interior of the pellet, it reacts with the catalyst deposited on the sides of the pore wall. The rate of reaction is = kr. CAs The rate is dependent on the particle size of the catalyst

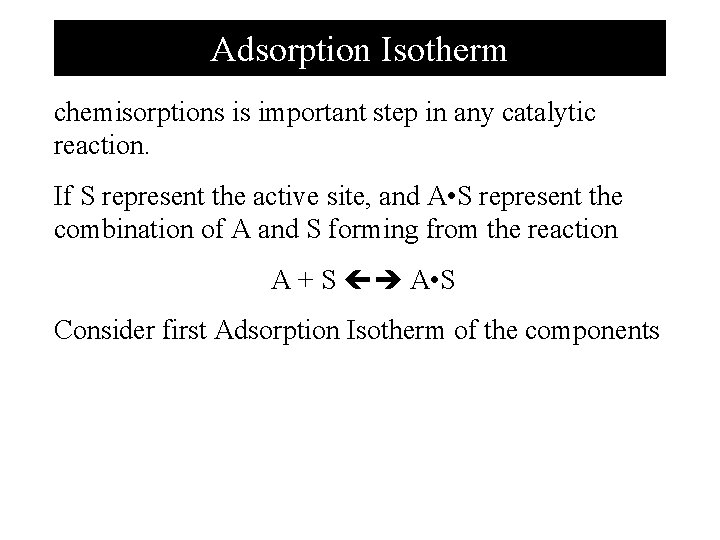
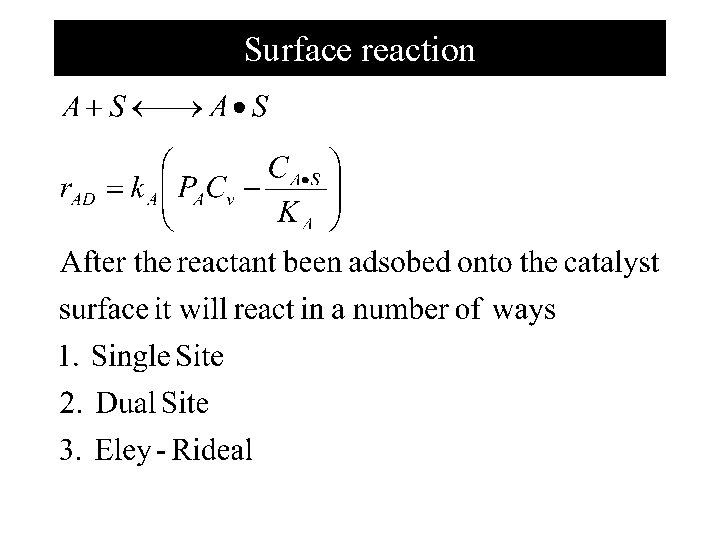
Adsorption Isotherm chemisorptions is important step in any catalytic reaction. If S represent the active site, and A • S represent the combination of A and S forming from the reaction A + S A • S Consider first Adsorption Isotherm of the components

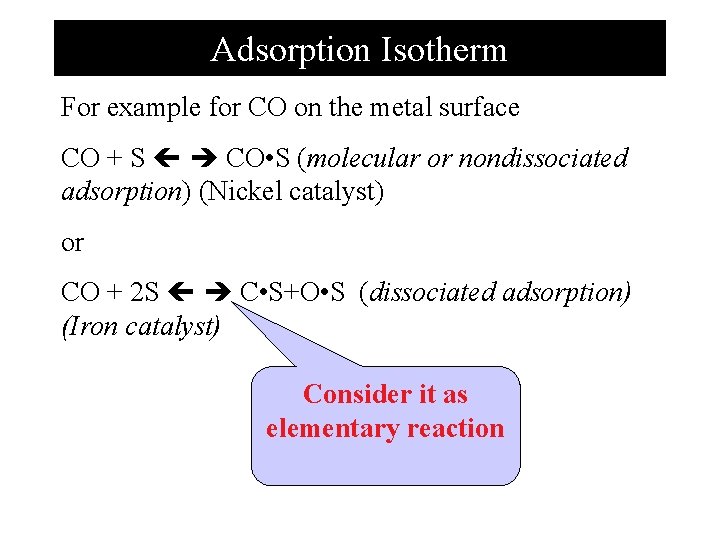
Adsorption Isotherm For example for CO on the metal surface CO + S CO • S (molecular or nondissociated adsorption) (Nickel catalyst) or CO + 2 S C • S+O • S (dissociated adsorption) (Iron catalyst) Consider it as elementary reaction

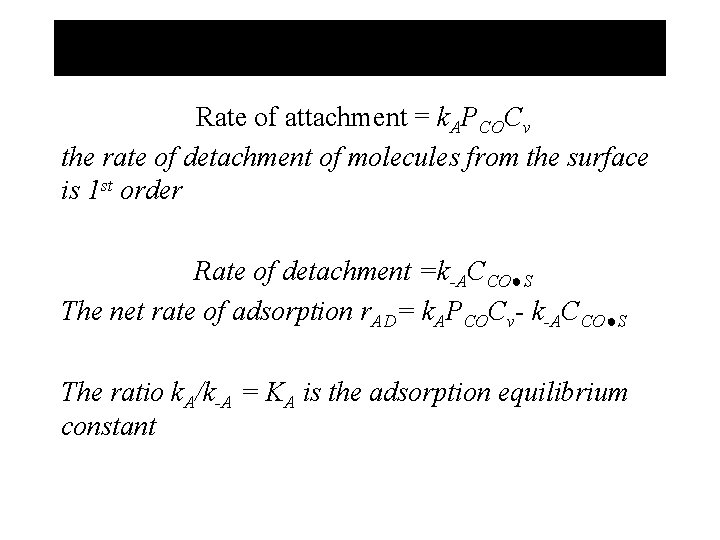
Rate of attachment = k. APCOCv the rate of detachment of molecules from the surface is 1 st order Rate of detachment =k-ACCO●S The net rate of adsorption r. AD= k. APCOCv- k-ACCO●S The ratio k. A/k-A = KA is the adsorption equilibrium constant

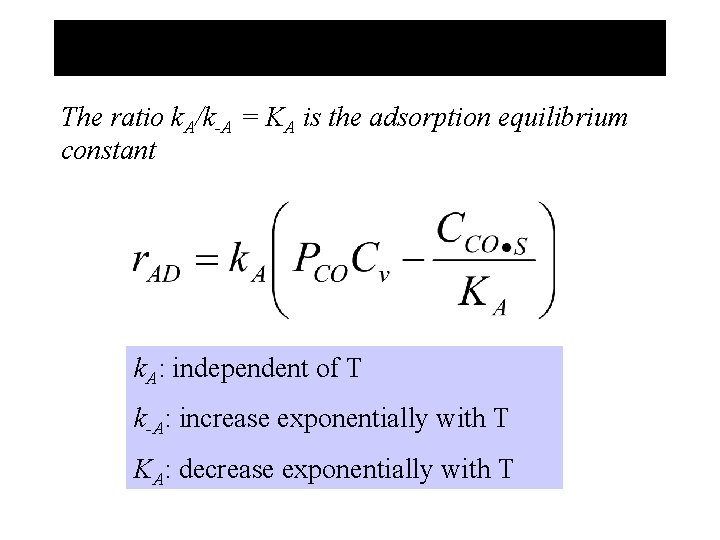
The ratio k. A/k-A = KA is the adsorption equilibrium constant k. A: independent of T k-A: increase exponentially with T KA: decrease exponentially with T

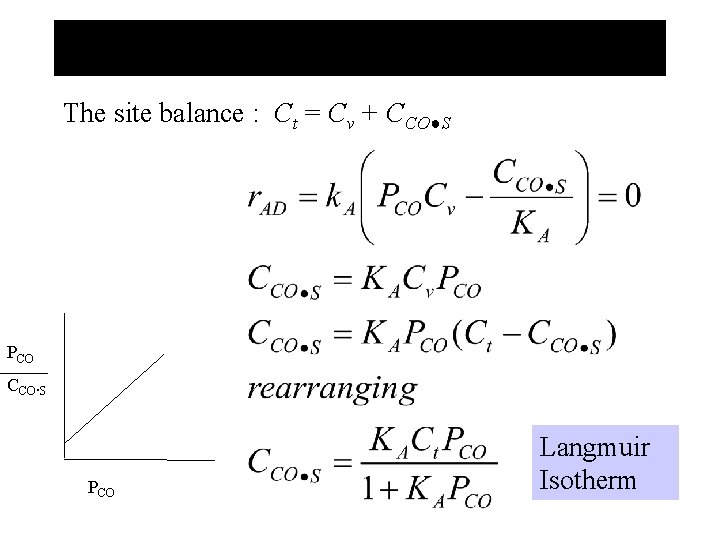
The site balance : Ct = Cv + CCO●S PCO CCO. S PCO Langmuir Isotherm

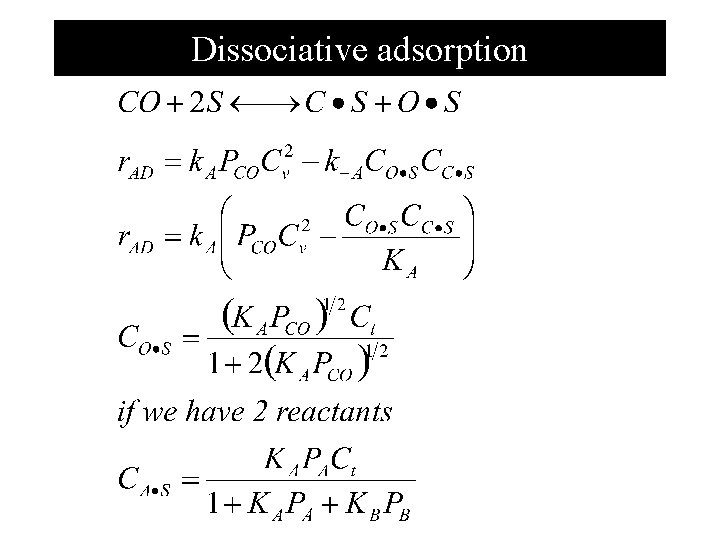
Dissociative adsorption

Surface reaction

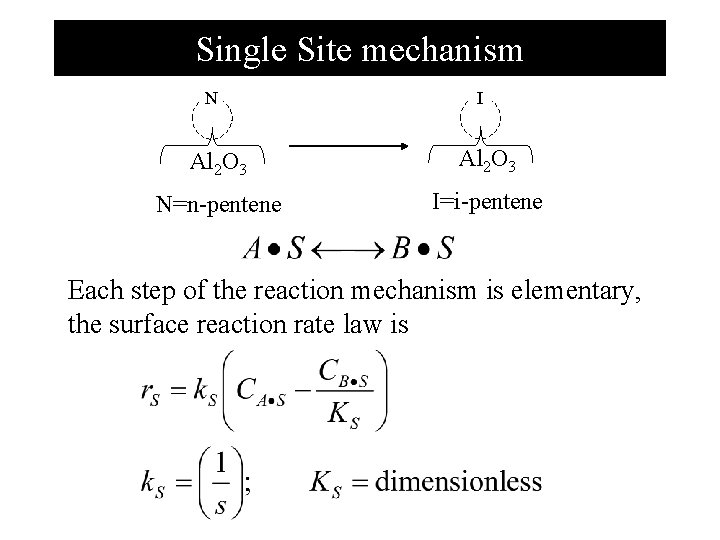
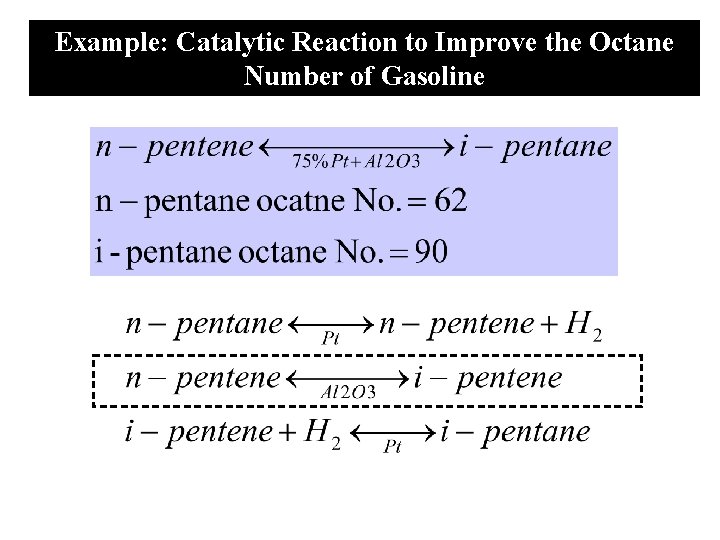
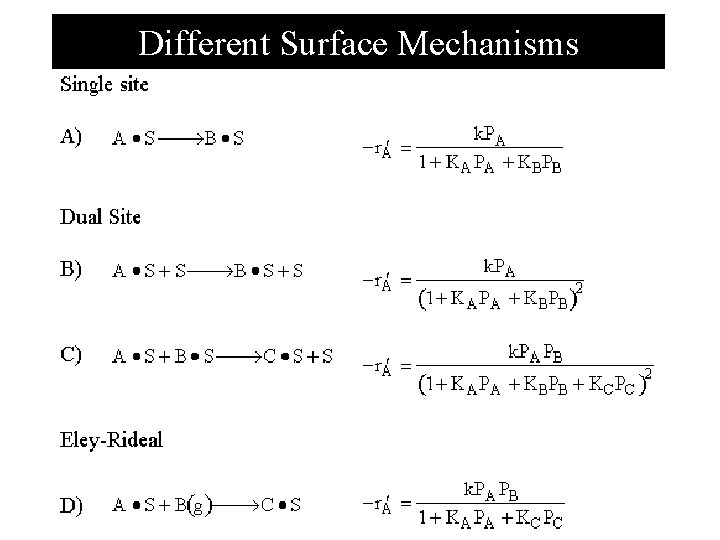
Single Site mechanism N I Al 2 O 3 N=n-pentene I=i-pentene Each step of the reaction mechanism is elementary, the surface reaction rate law is

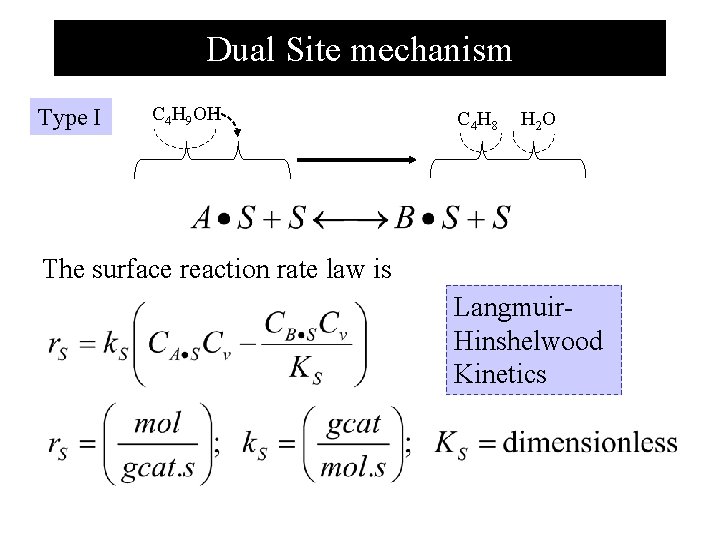
Dual Site mechanism Type I C 4 H 9 OH C 4 H 8 H 2 O The surface reaction rate law is Langmuir. Hinshelwood Kinetics

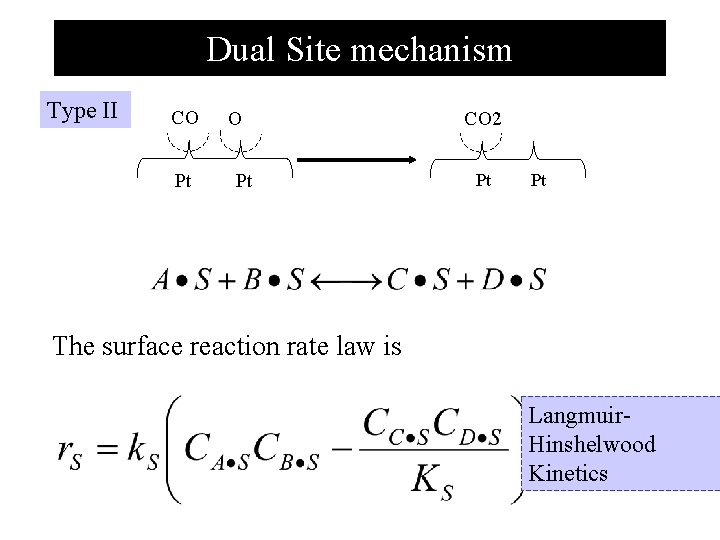
Dual Site mechanism Type II CO Pt CO 2 Pt Pt The surface reaction rate law is Langmuir. Hinshelwood Kinetics

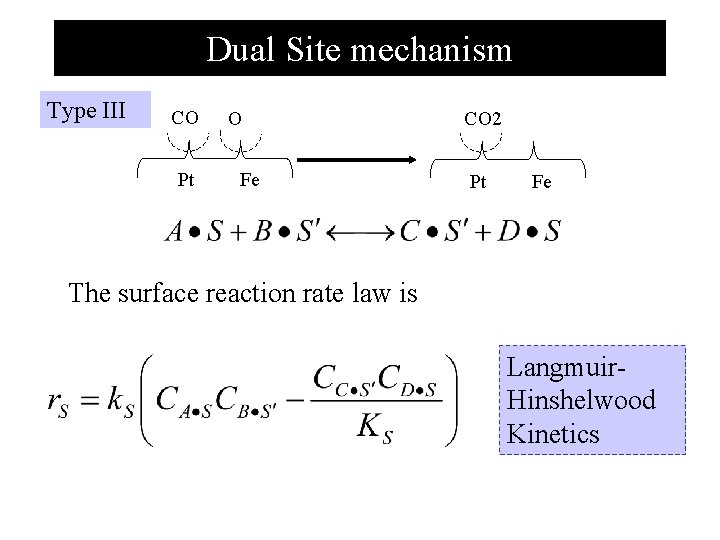
Dual Site mechanism Type III CO Pt O Fe CO 2 Pt Fe The surface reaction rate law is Langmuir. Hinshelwood Kinetics

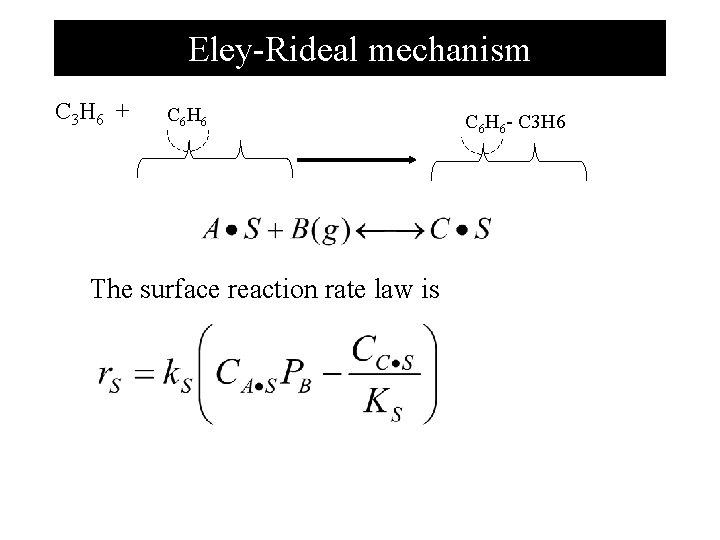
Eley-Rideal mechanism C 3 H 6 + C 6 H 6 The surface reaction rate law is C 6 H 6 - C 3 H 6

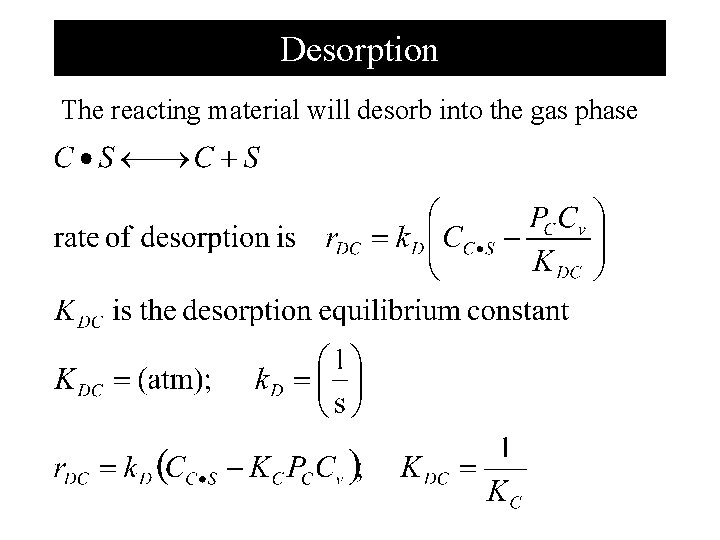
Desorption The reacting material will desorb into the gas phase

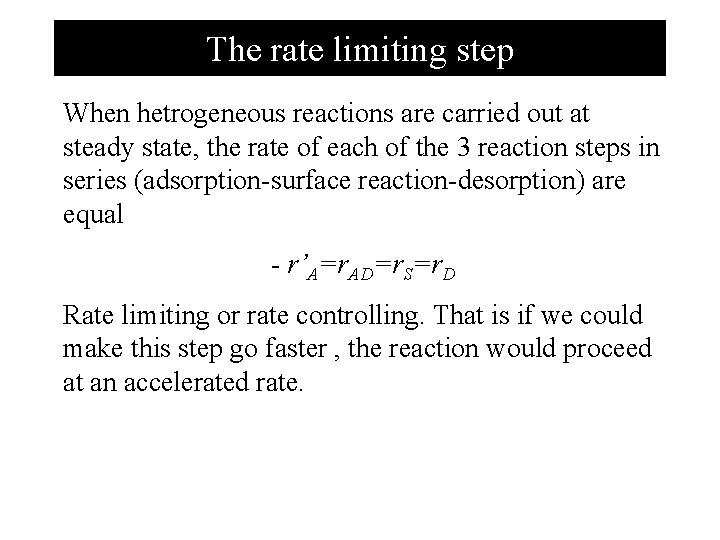
The rate limiting step When hetrogeneous reactions are carried out at steady state, the rate of each of the 3 reaction steps in series (adsorption-surface reaction-desorption) are equal - r’A=r. AD=r. S=r. D Rate limiting or rate controlling. That is if we could make this step go faster , the reaction would proceed at an accelerated rate.

The rate limiting step The approach in determining the catalytic and hetrogeneous mechanisms is usually termed the Langmuir-Hinshelwood approach since it is derived from ideas proposed by Hinshelwood based on Langmuir principles for adsorption

Langmuir-Hinshelwood approach It consist of • 1 st assuming a sequence of steps in the reaction. In writing the sequence, one must choose among such mechanisms as molecular or atomic adsorption, and single or dual site reaction. • 2 nd, rate laws are written for the individual steps. Assuming that all steps are reversible. • 3 rd Finally , a rate – limiting step is postulated , and steps that are not rate limiting are used to eliminate all coverage dependent terms.

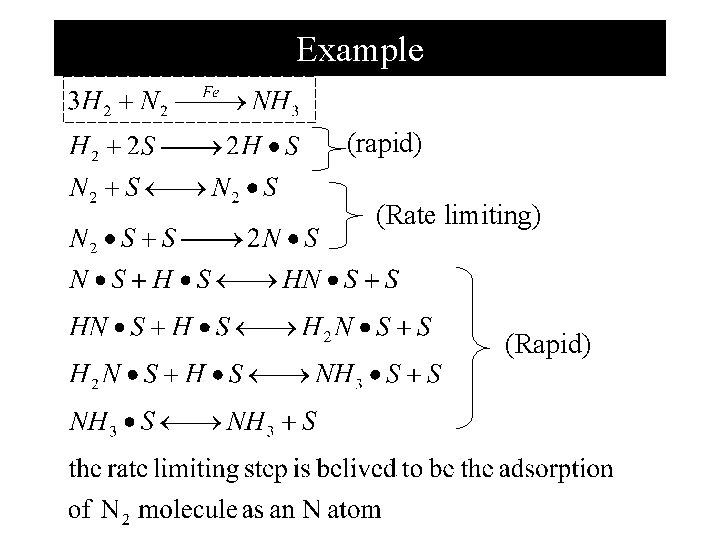
Example (rapid) (Rate limiting) (Rapid)

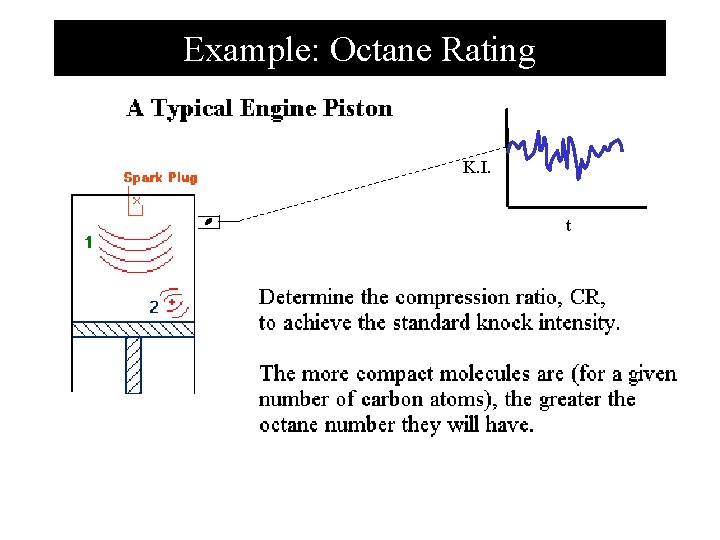
Example: Octane Rating K. I. t

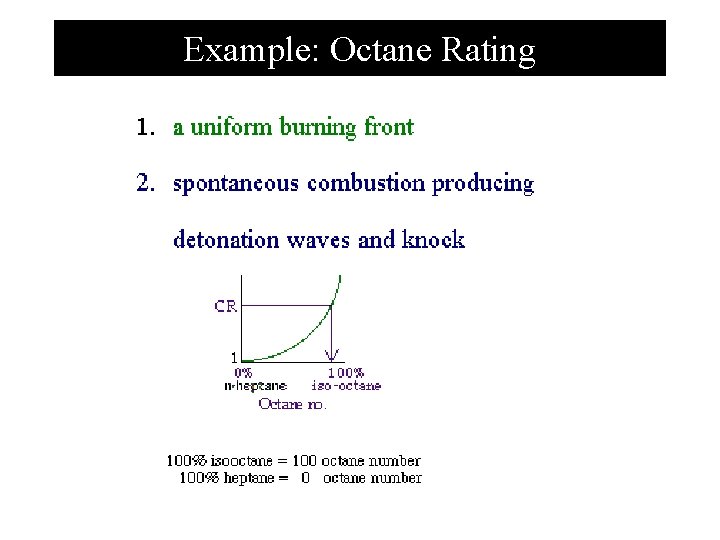
Example: Octane Rating

Example: Catalytic Reaction to Improve the Octane Number of Gasoline

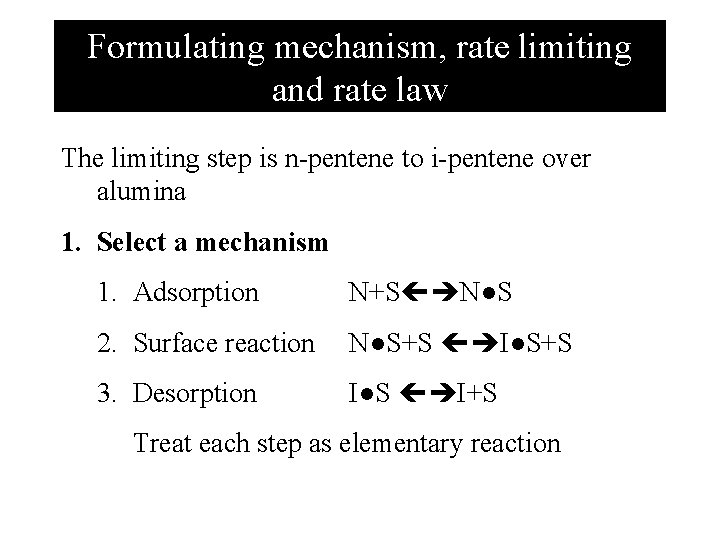
Formulating mechanism, rate limiting and rate law The limiting step is n-pentene to i-pentene over alumina 1. Select a mechanism 1. Adsorption N+S N●S 2. Surface reaction N●S+S I●S+S 3. Desorption I●S I+S Treat each step as elementary reaction

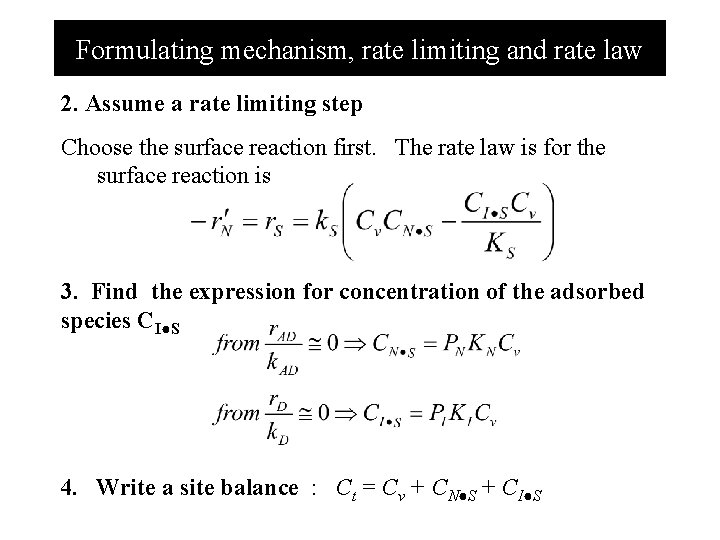
Formulating mechanism, rate limiting and rate law 2. Assume a rate limiting step Choose the surface reaction first. The rate law is for the surface reaction is 3. Find the expression for concentration of the adsorbed species CI●S 4. Write a site balance : Ct = Cv + CN●S + CI●S

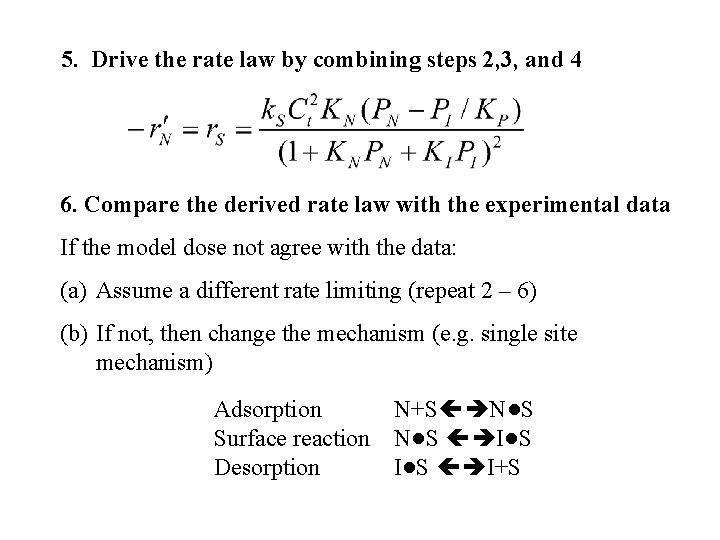
5. Drive the rate law by combining steps 2, 3, and 4 6. Compare the derived rate law with the experimental data If the model dose not agree with the data: (a) Assume a different rate limiting (repeat 2 – 6) (b) If not, then change the mechanism (e. g. single site mechanism) Adsorption N+S N●S Surface reaction N●S I●S Desorption I●S I+S

If the single site mechanism is correct then the rate law is Note : if more than one mechanism agrees with the experimental data then we need to compare there residuals

Different Surface Mechanisms

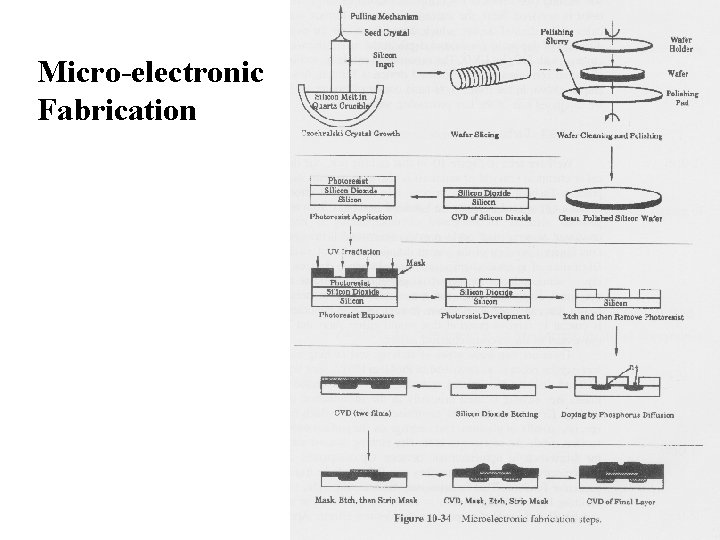
Micro-electronic Fabrication

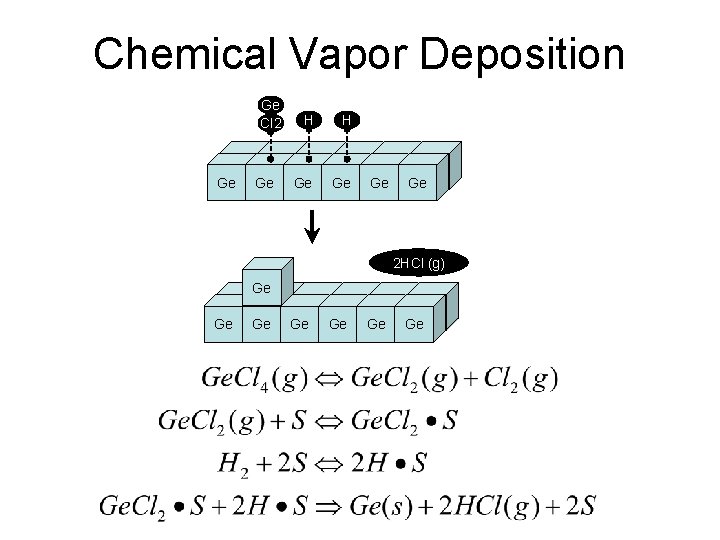
Chemical Vapor Deposition Ge Cl 2 Ge Ge H H Ge Ge 2 HCl (g) Ge Ge





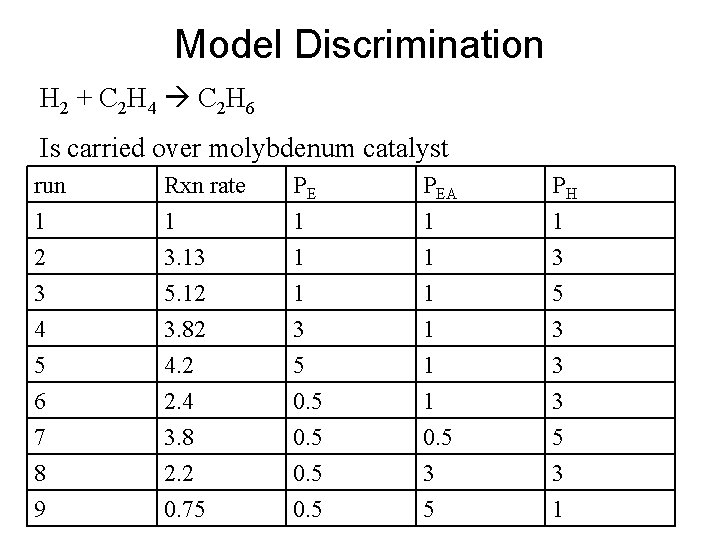
Model Discrimination H 2 + C 2 H 4 C 2 H 6 Is carried over molybdenum catalyst run 1 2 3 Rxn rate 1 3. 13 5. 12 PE 1 1 1 PEA 1 1 1 PH 1 3 5 4 5 6 7 8 9 3. 82 4. 2 2. 4 3. 8 2. 2 0. 75 3 5 0. 5 1 1 1 0. 5 3 3 3 5 3 1

 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Langmuir-hinshelwood mechanism heterogeneous catalysis
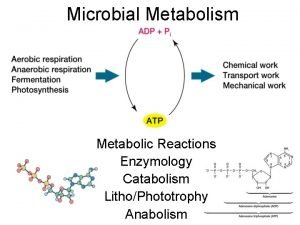
Langmuir-hinshelwood mechanism heterogeneous catalysis Energy catalysis and biosynthesis
Energy catalysis and biosynthesis Catabolism
Catabolism Catalysis by approximation
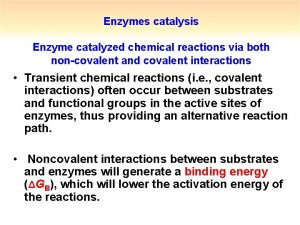
Catalysis by approximation What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Km in enzyme kinetics
Km in enzyme kinetics Erzeng xue
Erzeng xue Catalytic combustion
Catalytic combustion Honh2 dissociation equation
Honh2 dissociation equation Is windex homogeneous or heterogeneous
Is windex homogeneous or heterogeneous Homogeneous distributed database is which of the following
Homogeneous distributed database is which of the following Heterogeneous lipids
Heterogeneous lipids What are lipids
What are lipids Heterogeneous hypoechoic lesion in liver
Heterogeneous hypoechoic lesion in liver Is chunky peanut butter homogeneous or heterogeneous
Is chunky peanut butter homogeneous or heterogeneous What is homogeneous mixture
What is homogeneous mixture Heterogeneous markets
Heterogeneous markets Homogeneous vs heterogeneous reactions
Homogeneous vs heterogeneous reactions Mixtures and compound
Mixtures and compound Homogeneous vs heterogeneous
Homogeneous vs heterogeneous Homogeneous vinegar
Homogeneous vinegar Heterogeneous mixture definition
Heterogeneous mixture definition Heterogeneous audience means
Heterogeneous audience means Lipids are heterogeneous group of compounds
Lipids are heterogeneous group of compounds Homogeneous mixture
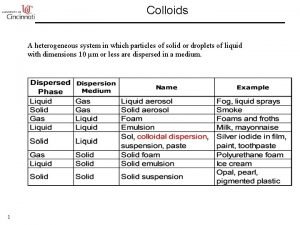
Homogeneous mixture Colloidal system is heterogeneous
Colloidal system is heterogeneous Heterogeneous vs homogeneous groups
Heterogeneous vs homogeneous groups Tap water homogeneous or heterogeneous
Tap water homogeneous or heterogeneous Is tap water a heterogeneous mixture
Is tap water a heterogeneous mixture Heterogeneous nucleation
Heterogeneous nucleation A heterogenous mixture of intermediate sized particles
A heterogenous mixture of intermediate sized particles Heterogenous team
Heterogenous team Suspension vs solution
Suspension vs solution Homogeneous nucleation
Homogeneous nucleation Is limestone a pure substance or mixture
Is limestone a pure substance or mixture Cloudy mixture with particles that move erratically
Cloudy mixture with particles that move erratically Substances and mixtures
Substances and mixtures Is jello homogeneous or heterogeneous
Is jello homogeneous or heterogeneous Homogeneous equilibrium
Homogeneous equilibrium What is a heterogeneous mixture that never settles
What is a heterogeneous mixture that never settles Elements, compounds and mixtures worksheet
Elements, compounds and mixtures worksheet Kool aid homogeneous or heterogeneous
Kool aid homogeneous or heterogeneous