Homogeneous and heterogeneous catalysis Heterogeneous catalysis This project


















- Slides: 21

Homogeneous and heterogeneous catalysis Heterogeneous catalysis ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

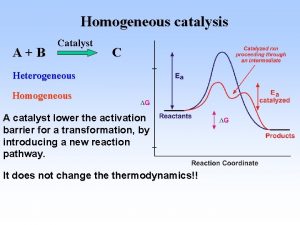
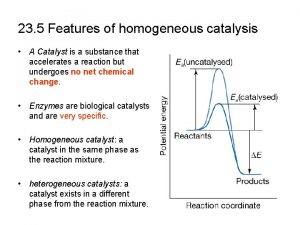
• Reaktanti i katalizator u istoj fazi-homogena • Kat posebna faza – heterogena kataliza • Homog kataliza međuproizvodi imaju stehiometrijski sastav, a u heterogenoj nemaju • Prelazna oblast između hom i hetero nalaze se katalitički procesi u koloidnim sistemima u kojima se supstance koje reaguju nalaze u rast-ru a kat su makromolekuli ili mol agregati. Tu se ubraja ENZIMSKA KATALIZA ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Kataliza u homogenim sistemima • Homogeno kataliticke reakcije odvijaju se u dve faze: • -gasovita • -Tecna • Katalizator je uobliku molekula, jona, atoma ili radikala • Na brzinu rekacija uticu T, p, konc reaktanata, hemijsk apriroda reaktanata i K. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Kataliza u homogenim sistemima • Homogenokatalitičke r-cije mogu da se podele na one koje se odvijaju u gasovitoj i tečnoj fazi. • K koji je u obliki mol, jona, atoma ili radikala je u istoj fazi sa reaktantima. Između čestica K i mol reaktanata dolazi do normalnih hem r-cija. Pa nastaju produkti u kojima je ugrađen K. Za globalnu r-ciju oni predstavljaju međuprodukte koji su nestabilni pa dalje reaguju oslobađajući K i dajući konačne produkte r-cije. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• Na brzinu međureakcija, u kojima učestvuje K, utiču temperatura, pritisak , koncentracija i hemijska priroda reaktanata i K. Tragovi K su često vrlo efikasni, pr: Cu 2+ u konc 10 -9 M veoma ubrzava oksidaciju Na-sulfida sa kiseonikom. • U većini slučajeva je brzina homogenokatalitičkih r-cija s obzirom na konc K, prvoga reda. Isti sistem reaguje merljivom brzinom i u odsustvu katalizatora. Zato je ukupna brzina reakcije, brzina koja se dobija u prisustvu K, jednaka zbiru brzine nekatalitičke i kataliticke u prisustvu K. • k = k 0 + kkat [ckat] • k 0 –specifična brzina reakcije bez K • kkat – katalitički koeficijenat • ckat – koncentracija K u sistemu ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

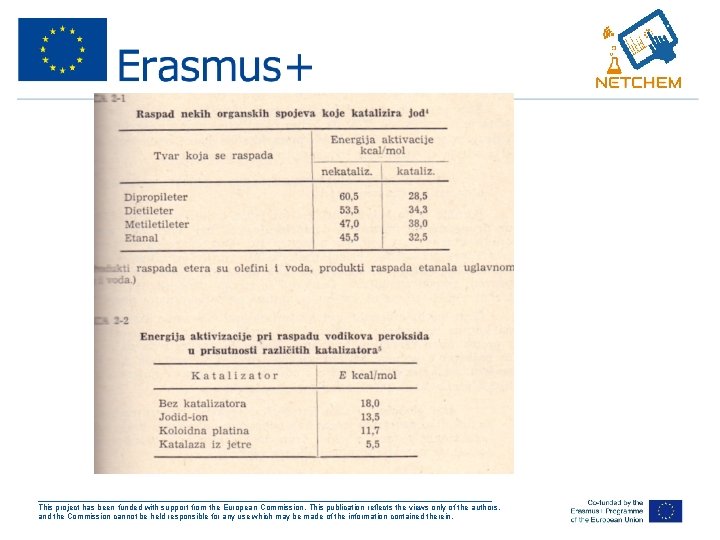
Homogena kataliza u gasovitoj fazi • Ovo je retka pojava, ali kao primer su r-cije tipa: • 1. oksidacija SO 2 u prisustvu NO. Odvija se prema mehanizmu NO + O 2 2 NO 2 + SO 2 NO + SO 3 Znatno brze se odvija r-cija 2 • 2. raspad etanala i raznih estara koje ubrzava J 2. Promena Ea za ove rcije, koja usleđuje dodatkom J 2 u sistem, dat je u tablici 2 -1. • 3. nastajanje hlorovodonika iz elemenata. Ovu r-ciju ubrzavaju pare Na ili K. • 4. raspad metanola na parama Zn ili Cd. Ova r-cija se odvija preko intermedijernog kompleksa CH 3 – O – Zn (Cd). . . H. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• Mnoge gas r-cije su prividno nekatalitičke odvijaju se na zidovima reaktora, pa su ustvari heterogenokatalitičke • Zapaža se da prisustvo vode usporava neku r-ciju u gasovitoj fazi. Tumači se adsorpcijom vode na zidovima reaktora, pri čemu se smanjuje ili sprečava njihovo kat delovanje. • One kat r-cije koje su uistinu homogenokatalitičke se odvijaju po lančanom meh. Pr raspad ozona koji ubrzava hlor: • Cl 2 + O 3 → Cl. O + Cl. O 2 • Cl. O 2 + O 3 → Cl. O 3 + O 2 • Cl. O 3 + O 3 → Cl. O 2 + 2 O 2 Reakc stupnjevi 2 i 3 odigravaju se mnogo puta dok se reakc lanac ne prekine. Do prekida lanca dovodi r-cija: Cl. O 3 + Cl. O 8 → Cl 2 + 3 O 2 ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Homogena kataliza u tečnoj fazi • Ova kataliza je veoma česta. Kao katalizatori deluju: • 1. Kiseline i baze • 2. jedinjenja i joni koji mogu stvarati koordin komplekse • 3. slobodni radikali ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Kiselinsko-bazna kataliza u nevodenoj sredini • Ako je ra-ač takav da se može jonizirati(amfiprotno) kao i voda u njemu postoji ravnoteža tipa: • 2 HB → H 2 B+ + B • Kao disocija vode 2 H 2 O → H 3 O+ + OH- a lionijum i liat joni (H 2 B+ i B-) su analogni hidronijum jonu i hidroksilnom jonu u vodi. • I ovde kao I u vodi konstanta disocijacije rastvaraca odredjuje kiselost koja se postiže u rru. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Joni metala kao Katalizatori u tečnim homogenim sistemima • Joni M u r-rima utiču katalitički najčešće tako što kao međuprodukt r-cije daju koordinativna jnjenja. Primer: okso-sinteza i Fridel-Craftsova rcija. • Koordinaciona jedinjenja nastaju kada se el par sa atoma donora iz reaktanata koordinativno veže na jon M. Primer: kompleks piridina i merkurijona. Piridin je vezan na jon M preko slobodnog el para na N. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uzroci ubrzanja r-cije, koja se odvija preko koordinacionih kompleksa mogu biti: 1. koordinacija omogućava da L i M joni dođu u veoma blizi kontakt gde mogu da lako reaguju. Pr: etanal se lako dobija iz etena u prisustvu Pd. Cl 2. ; eten i voda daju Pd. Cl 2 nestabilno koordinaciono jedinjenje koji se raspada na etanal, HCl i atom Pd • C 2 H 4 + H 2 O + Pd. Cl 2 → CH 3 COH + Pd + 2 HCl • Pd+ 2 Cu. Cl 2 → Pd. Cl 2 + Cu 2 Cl 2 • Cu 2 Cl 2 se regenerise sa O 2 prema j-ni: • Cu 2 Cl 2 + 1/2 O 2 +2 HCl → 2 Cu. Cl 2 + H 2 O • Ova dva stepena r-cije mogu da se odvijaju istovremeno ili sukcesivno. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uzroci ubrzanja r-cije, koja se odvija preko koordinacionih kompleksa mogu biti: 2. Reakcija se ubrzava jer je L, tj reaktant vezan za pozitivan M, polarizovan. Tako da je olakšano uklanjanje e iz L. Kako je polarizacija donora opšta pojava koja prati nastajanje koordinacionih veza, dodatni uticaj se zapaža u velikom br homogeno-katalitičkih r-cija. Primer je katalitička hidroliza etra koju ubrzavaju Lewisove kis kao Al. Cl 3, Zn. Cl 2 C 2 H 5 – O – C 2 H 5 Zn. Cl 2 C 2 H 5: Ö: Zn. Cl 2 H 2 O 2 C 2 H 5 OH + Zn. Cl 2 C 2 H 5 ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

3. Zbog koordinacije stabilizuje se jedan oblik L koji je podesan za željenu r-ciju. Ligandi mogu postojati u više tautomernih oblika od kojih samo jedan stupa u koord vezu. Primer: r-cija bromovanja etilacetoacetata koja se odvija u prisustvu Cu 2+. Na jone bakra veže se etilacetoacetat u enolnoj formi kojoj je na taj način omogućeno da reaguje sa Br. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• 4. koordinacijom se može maskirati jedna aktivna grupa L, tako da on može reagovati samo na slobodnim aktivnim grupama. Tako K deluje selektivno. • Pr: maskiranjem NH 2 grupe α-amino kis pomocu Cu 2+, sprečava se acetiliranje ove grupe ili neka druga r-cija koja bi usledila. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• 5. koordinacija može biti preduslov za prelaz e. Ovakvo delovanje M katjona susreće se kod katalitičke oksidacije organskih jedinjenja. Pri nastajanju kopleksa katjon privlači 1 e iz L i on ostaje u oksidovanom obliku. Katjon M, koji se redukovao, lako se oksiduje molekulskim O 2, i opet prelazi u prvobitni oblik. U ovu grupu spadaju mnoge autooksidacije koje nastaju u prisustvu tragova Cu+, Fe 2+ i dr jona M. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• 6. iz reaktanata, koji pod datim uslovima ne mogu reagovati, i jona M nastaje kompleks u kome jedan L može doživeti unutrašnje pregrupisavanje koji inače nastaje veoma teško ili je nemoguće. U ovu grupu spadaju r-cije transaminacije i transesterifikacije. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Slobodni radikali kao katalizatori u homogenim tečnim i gasovitim sistemima • Slobodni radikali su atomi, joni, molekularni fragmenti ili molekuli, čija je osnovna karakteristika da poseduju barem jedan nespareni e. Zato lako reagujuju sa česticama sa kojima se sudare. Pri toj r-ciji oni zasićuju svoju valencu, tj sparuju svoj nespareni e. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• Iz dva slobodna radikala nastaje stabilan molekul: R + R =R: R Ako slobodni radikal reaguje sa nekim molekulom u kome su svi e bili spareni pre r-cije, nakon r-cije će 1 e ostati nesparen, tj ostaće slobodni radikal. Reakcioni mehanizam će biti lančan. Nastavljaće se dok se ne susretnu 2 čestice sa nesparenim e. Kao pr re-cije ovog tipa je nastajanje bromovodonika iz molekulskog Br i H 2. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Author, Editor and Referee References This remote access laboratory was created thanks to work done primarily at University of Niš. Contributors to this material were: ________ Refereeing of this material was done by: ___________ Editing into NETCHEM Format and onto NETCHEM platform was completed by: __________________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, ___________________________________________________ andhas the been Commission be held responsible for any use which may made of the information contained This project funded cannot with support from the European Commission. Thisbe publication reflects the views only of therein. the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

References and Supplemental Material The NETCHEM platform was established at the University of Nis in 2016 -2019 through the Erasmus Programme. Please contact a NETCHEM representatives at your institution or visit our website for an expanded contact list. The work included had been led by the NETCHEM staff at your institution. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, ___________________________________________________ andhas the been Commission be held responsible for any use which may made of the information contained This project funded cannot with support from the European Commission. Thisbe publication reflects the views only of therein. the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
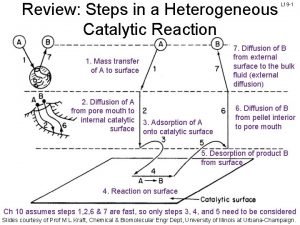
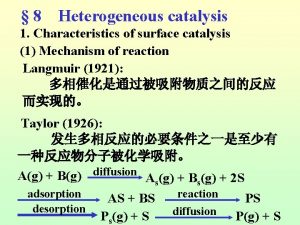
 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Definition of homogeneous differential equation
Definition of homogeneous differential equation Homogeneous mixture and heterogeneous mixture class 9
Homogeneous mixture and heterogeneous mixture class 9 Homogeneous distributed database system
Homogeneous distributed database system Is chunky peanut butter homogeneous or heterogeneous
Is chunky peanut butter homogeneous or heterogeneous Homogeneous vs heterogeneous reactions
Homogeneous vs heterogeneous reactions Homogeneous vs heterogeneous
Homogeneous vs heterogeneous Mixtures and solutions grade 7
Mixtures and solutions grade 7 Heterogeneous vs homogeneous groups
Heterogeneous vs homogeneous groups Tap water pure substance or mixture
Tap water pure substance or mixture Honey homogeneous or heterogeneous
Honey homogeneous or heterogeneous Is tea homogeneous or heterogeneous
Is tea homogeneous or heterogeneous Is a granola bar homogeneous or heterogeneous
Is a granola bar homogeneous or heterogeneous Is jello homogeneous or heterogeneous
Is jello homogeneous or heterogeneous Is raisin bran a heterogeneous mixture
Is raisin bran a heterogeneous mixture A compund
A compund Is flat soda homogeneous or heterogeneous
Is flat soda homogeneous or heterogeneous Is supreme pizza homogeneous or heterogeneous
Is supreme pizza homogeneous or heterogeneous Is cake batter homogeneous or heterogeneous
Is cake batter homogeneous or heterogeneous A pile of rusty iron filings homogeneous or heterogeneous
A pile of rusty iron filings homogeneous or heterogeneous Ctive
Ctive