Adsorption Lecture 18 Heterogeneous Nucleation Consider for example







- Slides: 10

Adsorption Lecture 18

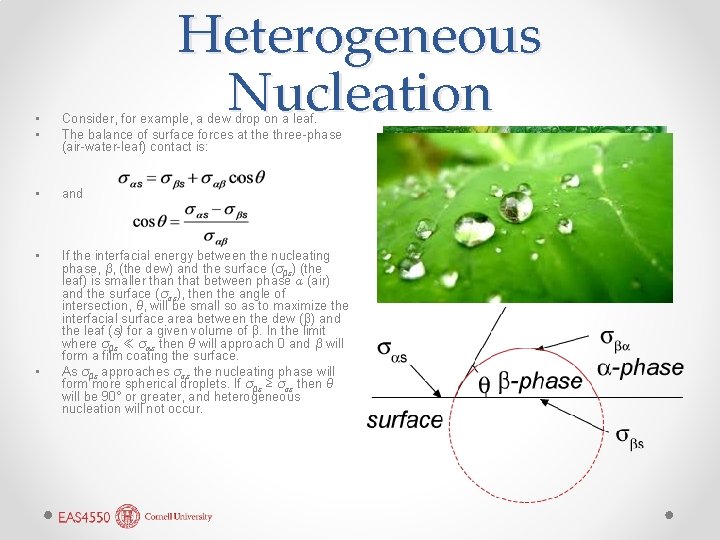
Heterogeneous Nucleation • • Consider, for example, a dew drop on a leaf. The balance of surface forces at the three-phase (air-water-leaf) contact is: • and • If the interfacial energy between the nucleating phase, β, (the dew) and the surface (σβs) (the leaf) is smaller than that between phase α (air) and the surface (σαs), then the angle of intersection, θ, will be small so as to maximize the interfacial surface area between the dew (β) and the leaf (s) for a given volume of β. In the limit where σβs ≪ σαs then θ will approach 0 and β will form a film coating the surface. As σβs approaches σαs the nucleating phase will form more spherical droplets. If σβs ≥ σαs then θ will be 90° or greater, and heterogeneous nucleation will not occur. •

• • Heterogeneous Nucleation In metamorphic reactions, nucleation will necessarily always be heterogeneous. Provided the necessary components of the nucleating phase are available and delivered rapidly enough by fluid transport and diffusion, interfacial energy will dictate where new phases will nucleate, nucleation being favored on phases where the interfacial energy is lowest. (Where transport of components limit growth, however, this may not be the case, as phases will nucleate where the components necessary for growth are available. )

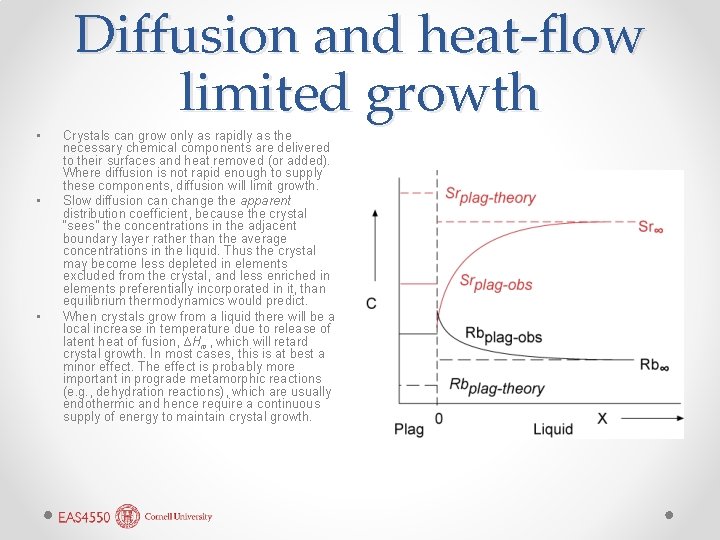
• • • Diffusion and heat-flow limited growth Crystals can grow only as rapidly as the necessary chemical components are delivered to their surfaces and heat removed (or added). Where diffusion is not rapid enough to supply these components, diffusion will limit growth. Slow diffusion can change the apparent distribution coefficient, because the crystal “sees” the concentrations in the adjacent boundary layer rather than the average concentrations in the liquid. Thus the crystal may become less depleted in elements excluded from the crystal, and less enriched in elements preferentially incorporated in it, than equilibrium thermodynamics would predict. When crystals grow from a liquid there will be a local increase in temperature due to release of latent heat of fusion, ∆Hm, which will retard crystal growth. In most cases, this is at best a minor effect. The effect is probably more important in prograde metamorphic reactions (e. g. , dehydration reactions), which are usually endothermic and hence require a continuous supply of energy to maintain crystal growth.

Zoning • Diffusion limits the ability of the interior of a crystal to maintain equilibrium with the magma from which it is precipitating. • This leads to zoning in crystals, apparent under the microscope. • Zoning can record magma history. Zoned pyx and plag in lava.

Adsorption A surprisingly important geochemical phenomenon

Adsorption • Physical o attachment of atom or molecule to a surface through van der Waals or intermolecular forces. o Weak: ∆Had ≈ 4 -12 k. J/mol • Chemical o formation of new chemical bond between adsorbed species and atoms on surface o Stronger: ∆Had >40 k. J/mol • Importance of Adsorption o The first step in precipitation is generally the adsorption of an ion or molecule to the crystal surface. • Also in weathering reactions the first step is adsorption of a H+ (or OH-) ion to the surface. o Adsorption/desorption is critically important in supplying soil nutrients to plants. o The seawater concentrations of many trace elements is controlled by adsorption on pre-existing surfaces, not precipitation.

Adsorption and Surface Free Energy • Adsorption changes the nature of the surface and therefore the surface free energy. • We can express the surface free energy change as: • where ni, s is the number of moles of component i adsorbed to the surface, s, and A is the area of the surface. • We define Γi as the Gibbs Absorption Density: moles of i absorbed on the surface per unit area.

Equilibrium Adsorption • Consider adsorption of species M on surface S: M + S = M S • Let the fraction of surface sites occupied by M be θM. • Fraction of free sites is then 1 - θM. • Assuming elementary reactions, rate of adsorption is: • Rate of desorption is • At equilibrium • and

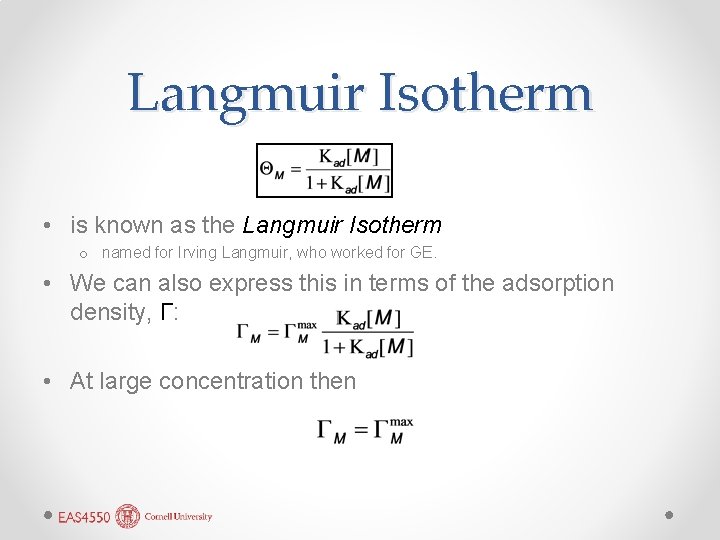
Langmuir Isotherm • is known as the Langmuir Isotherm o named for Irving Langmuir, who worked for GE. • We can also express this in terms of the adsorption density, Γ: • At large concentration then