Adsorption What is Adsorption Adsorption is the transfer




- Slides: 7

Adsorption

What is Adsorption? Adsorption is the transfer of a material from one liquid or gaseous state to a surface. The substance that is transferred to the surafce is the adsorbate. The material on which the adsorbate deposits is the adsorbent. Example: Silica gel, Activated carbon, Alumina, Zeolites and molecular sieves, Polymers.

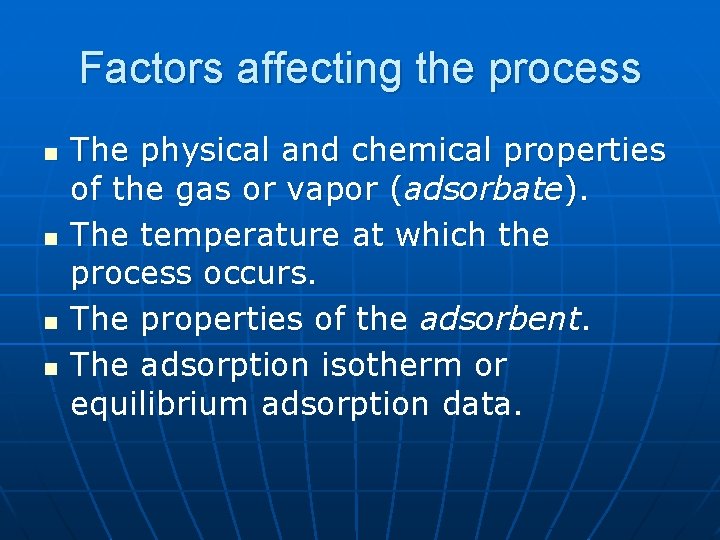
Factors affecting the process n n The physical and chemical properties of the gas or vapor (adsorbate). The temperature at which the process occurs. The properties of the adsorbent. The adsorption isotherm or equilibrium adsorption data.

Physical and chemical adsorption Physical adsorption occurs when the bonding forces are dispersion and coulombic type. The amount of heat released during this process is equal to the heat of condensation. 1. Chemical adsorption occurs when there is sharing of electrons between adsorbent and adsorbate. 1. The amount of heat released during this process is equal to the heat of reaction.

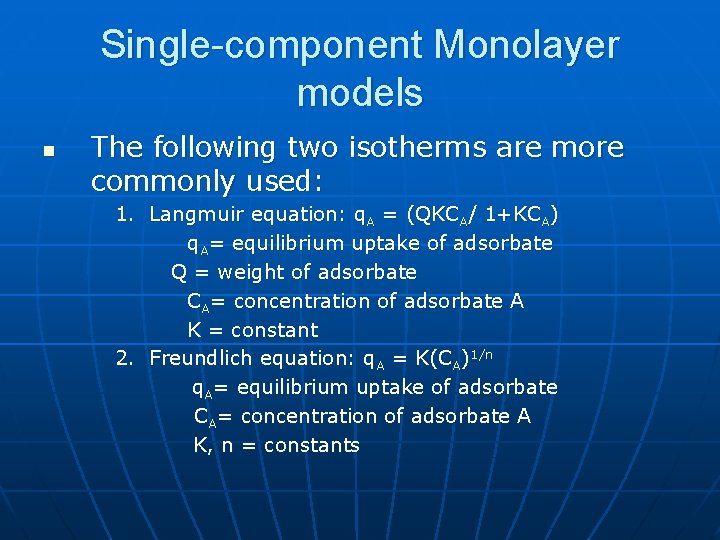
Single-component Monolayer models n The following two isotherms are more commonly used: 1. Langmuir equation: q. A = (QKCA/ 1+KCA) q. A= equilibrium uptake of adsorbate Q = weight of adsorbate CA= concentration of adsorbate A K = constant 2. Freundlich equation: q. A = K(CA)1/n q. A= equilibrium uptake of adsorbate CA= concentration of adsorbate A K, n = constants

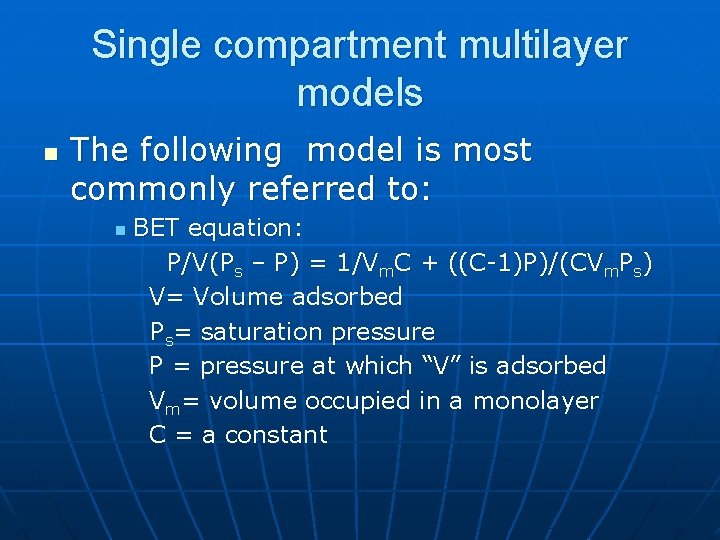
Single compartment multilayer models n The following model is most commonly referred to: n BET equation: P/V(Ps – P) = 1/Vm. C + ((C-1)P)/(CVm. Ps) V= Volume adsorbed Ps= saturation pressure P = pressure at which “V” is adsorbed Vm= volume occupied in a monolayer C = a constant

Multicomponent models Such a model is employede when the adsornber is to remove two or more pollutants. Markham and Benton model: The equation for an n component system qi = Qi. Ki. Pi/(1+∑K /(1+ j. Pj) (for j=1 to n) Pi= partial pressure of component i Qi = amount adsorbed for component I Ki= a constant. Total quantity adsorbed q. T = ∑qi= ∑Qj. Kj. Pj/(1+ ∑Kj. Pj) (for i and j from 1 to n)