Crystallization Concepts Development Manufacturing Strategies 1 Agenda Crystallization


















- Slides: 31

Crystallization: Concepts, Development & Manufacturing Strategies 1

Agenda Crystallization in the Pharmaceutical Industry n. Crystallization kinetics n. Crystallization development n. Particle size engineering n. Analytical tools – Lasentec n. Practical examples n 2

Crystallization in the Pharma Industry n n Most of the Active Product Ingredients (API’s) and the intermediate products form stable crystalline compounds at room temperature. Crystallization is an efficient process to isolate these compounds with high productivity and high purity. 3

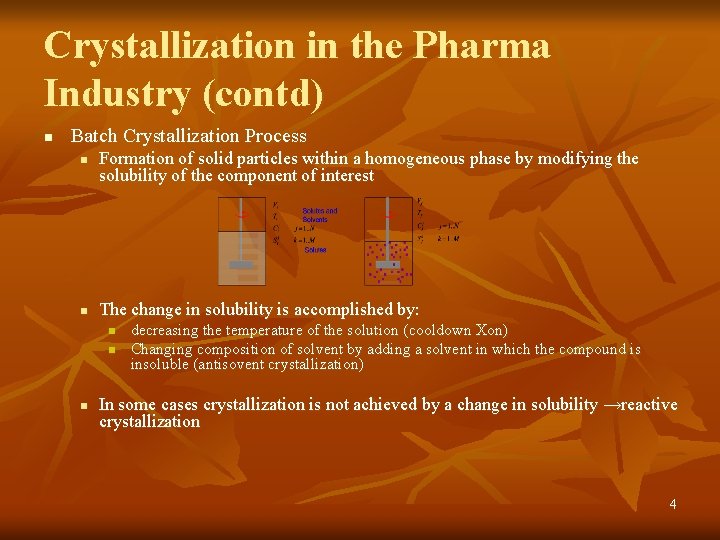
Crystallization in the Pharma Industry (contd) n Batch Crystallization Process n n Formation of solid particles within a homogeneous phase by modifying the solubility of the component of interest The change in solubility is accomplished by: n n n decreasing the temperature of the solution (cooldown Xon) Changing composition of solvent by adding a solvent in which the compound is insoluble (antisovent crystallization) In some cases crystallization is not achieved by a change in solubility →reactive crystallization 4

Crystallization Objectives n Isolate substrate n n PSD, Crystal habit Solvent Selection, PSD Solubility, T Purify Substrate n n Filtration Drying Yield Impurity Rejection Solvent removal Washing Properties Relative Solubility PSD Cake porosity Downstream Manufacturability n n Flow Properties of product Filtration (specific cake resistance) Drying rate/LOD Physical Attributes PSD, habit, agglomeration PSD, habit, solvate PSD, Polymorph 5

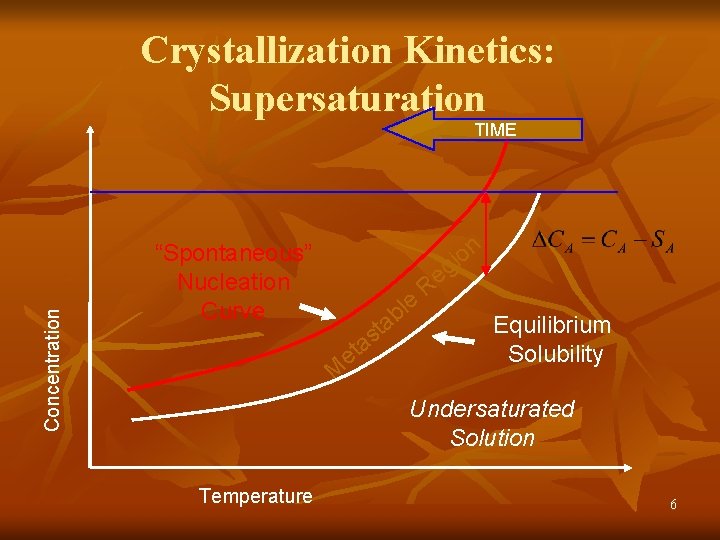
Crystallization Kinetics: Supersaturation Concentration TIME n “Spontaneous” Nucleation Curve o i g et t s a M le b a Re Equilibrium Solubility Undersaturated Solution Temperature 6

Crystallization Kinetics: Nucleation Two common types of nucleation mechanisms n Primary nucleation: n n n Homegeneous: occurs at the onset of crystallization, when the concentration of the solvent exceeds the metastable region. Heterogeneous: occurs when solid particles of foreign substances cause an increase in the rate of nucleation. Secondary nucleation: is caused by contacts between a crystal and another surface, and occurs within the metastable region (difficult to scale up) 7

Crystallization Kinetics: Growth n n Typically follows an initial stage of either homogeneous or heterogeneous nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. Addition of solute to faces of crystal For controlled growth operate crystallization under low supersaturation levels Growth & nucleation are competing processes! 8

Crystallization development n n Requirement to isolate as many solid forms as possible in order to select the form with best attribute for further development (screening of polymorphs) Develop best crystallization procedure with means available at hand to enable scale-up for New Drug Toxicology and other campaigns. 9

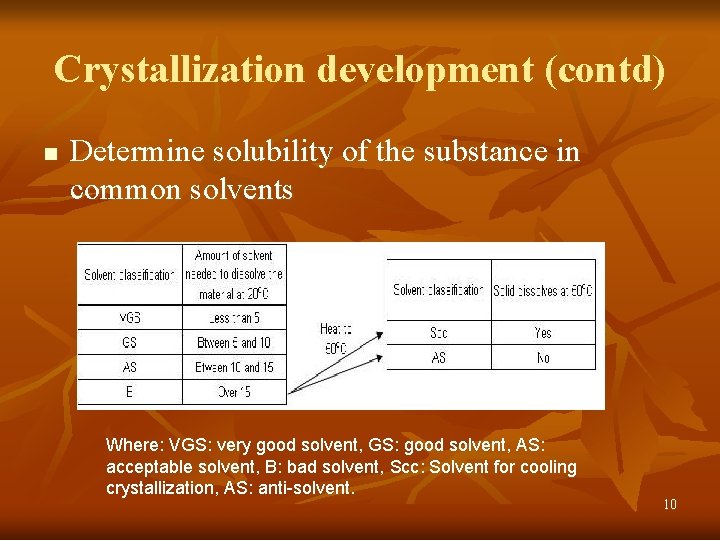
Crystallization development (contd) n Determine solubility of the substance in common solvents Where: VGS: very good solvent, GS: good solvent, AS: acceptable solvent, B: bad solvent, Scc: Solvent for cooling crystallization, AS: anti-solvent. 10

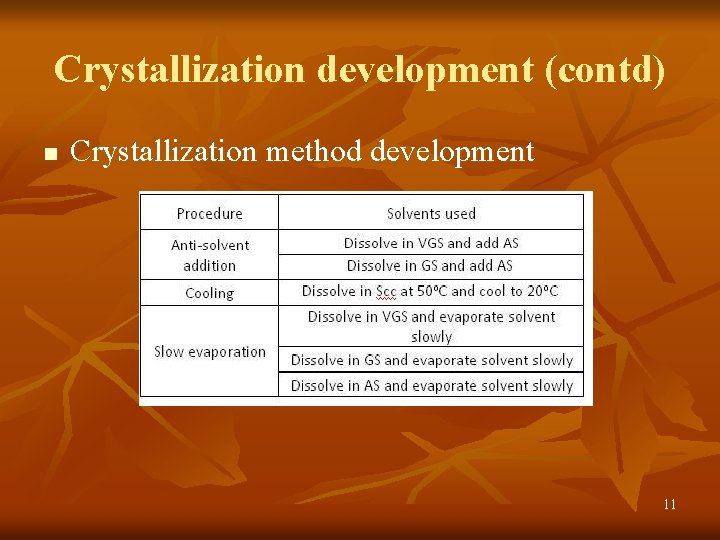
Crystallization development (contd) n Crystallization method development 11

Crystallization- Engineering • Particle size distribution: n Particle size reduction n n Greater surface area Faster dissolution Better bioavailability Better compactibility n Particle size increase n n n Faster filtration/drying Better handling Better flowability • Crystal shape: - Influence the flowability of the resulting powder. 12

Crystallization/Particle Engineering n n Particle Size Enhancement: n Cubic Crystallization n Strategic Seeding n Thermal methods Target Property Improvements n Flowability n Filtration n Bulk density n Drying rate Linear cooling Thermal cycle 13

Crystallization/Particle Engineering n Particle Size Reduction n Wet Milling Ultrasounds n High-shear Polymorph Transformation n Dry milling Wet milling Target Property Improvements n Dissolution rate n Exposure, bioavailability n n PSD Compactability/ Compressibility Dry milling 14

Particle size reduction Why not just mill all the API’s? n n n n Usually undesired in manufacturing Safety issues related to dust explosion potential Issues of physical stability of crystals--potential loss of crystallinity due to stresses applied to crystals Wide particle size distribution, more fines Possibility of reduced yield Noise Another unit operation Productivity, equipment/facility issues 15

Particle size increase • Salt crystallization at low supersaturation by cubic addition of sulfuric acid into the solution with seeds • Cubic addition: addition at a variable rate, slow at first and gradually faster towards the end as the surface area for growth increases • Increased filtration rate and wash efficiency From linear crystallization From cubic crystallization 16

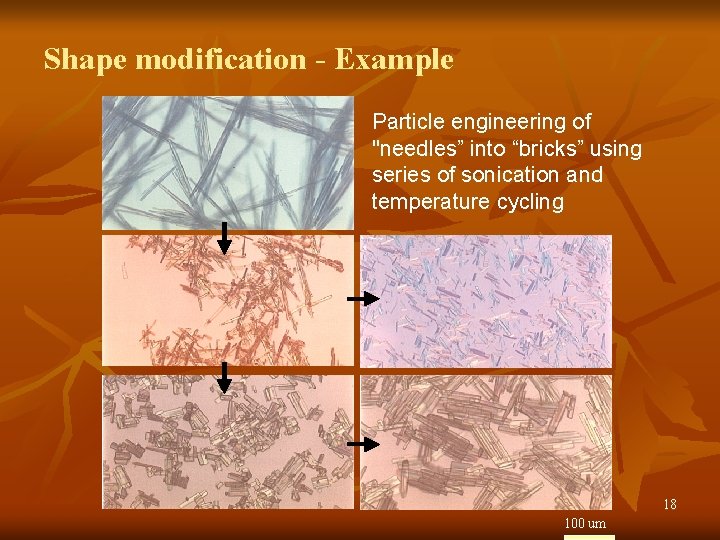
Particle shape modification n To improve flowability, bulk density, and handling To increase filtration rate “Needles to bricks” or “plates” to “cubes” 17

Shape modification - Example Particle engineering of "needles” into “bricks” using series of sonication and temperature cycling 18 100 um

Spherical agglomeration n Uniform agglomerates sized 20 -100 mm consisting of smaller primary crystals Excellent flowability and handling Compactibility needs to be tested 19

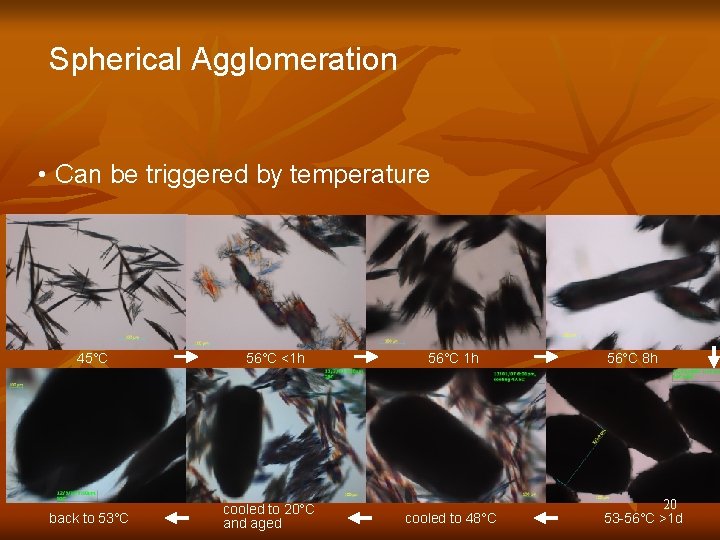
Spherical Agglomeration • Can be triggered by temperature 45°C back to 53°C 56°C <1 h cooled to 20°C and aged 56°C 1 h cooled to 48°C 56°C 8 h 20 53 -56°C >1 d

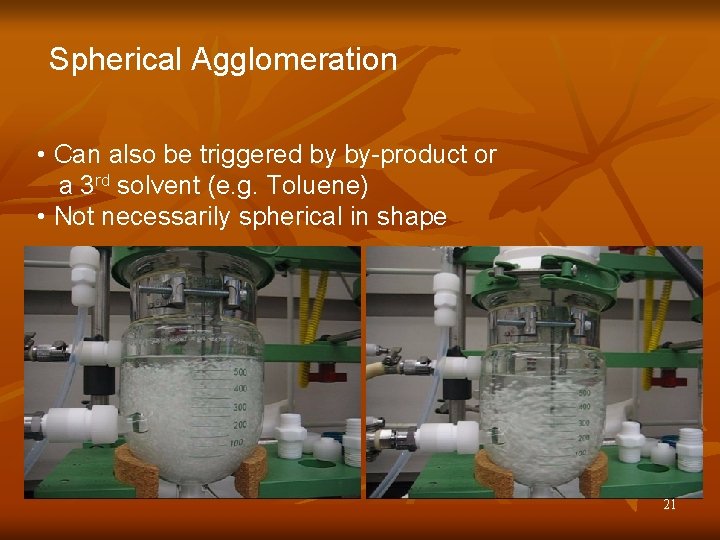
Spherical Agglomeration • Can also be triggered by by-product or a 3 rd solvent (e. g. Toluene) • Not necessarily spherical in shape 21

Crystallization Analytical Tools —In. Process PSD by Lasentec FBRM • Particle size distribution • Crystallization kinetics 22

Lasentec FBRM applications n Practical case 1: low flowability n n n 2007 campaign: 30% batches did not meet flow specifications 2008 campaign: modifications done into crystallization protocol→ 17% batches did not meet flow spec 2009 campaign: modifications done into crystallization protocol→All the batches met the flow specifications 2010: Same crystallization protocol as per 2008→ 25% batches did not meet flow specifications 2011: investigation on-going 23

Practical case 1: low flowability (contd) n n Compound A Crystallisation is p. H and temperature controlled. Crystallisation sequence starts when p. H is lowered below p. H 6 Controlled HCl charge rates for p. H <6. 0 are used to control saturation, nucleation and crystal growth Crystal growth is achieved by a combination of controlled HCl charge rates, specific p. H ranges & seeding coupled with short hold periods at constant temperature for optimal, controlled crystal growth. 24

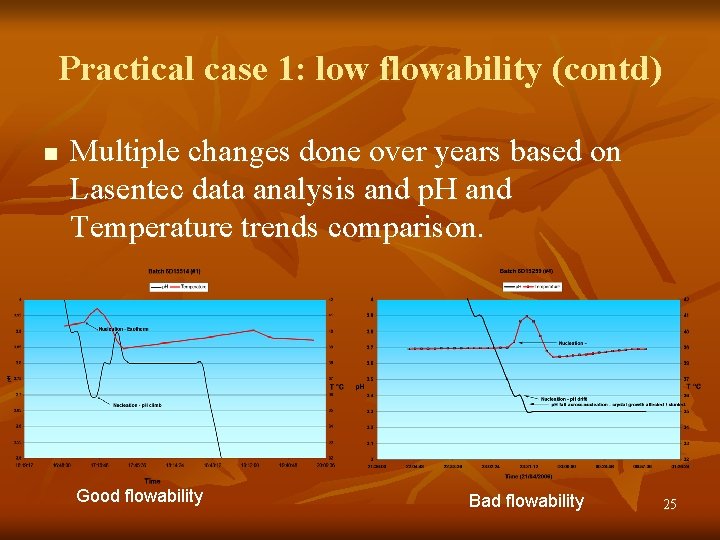
Practical case 1: low flowability (contd) n Multiple changes done over years based on Lasentec data analysis and p. H and Temperature trends comparison. Good flowability Bad flowability 25

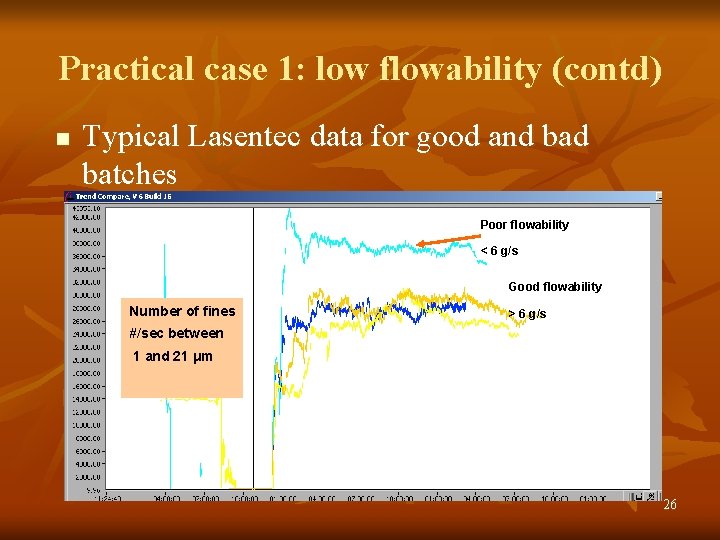
Practical case 1: low flowability (contd) n Typical Lasentec data for good and batches Poor flowability < 6 g/s Good flowability Number of fines > 6 g/s #/sec between 1 and 21 μm 26

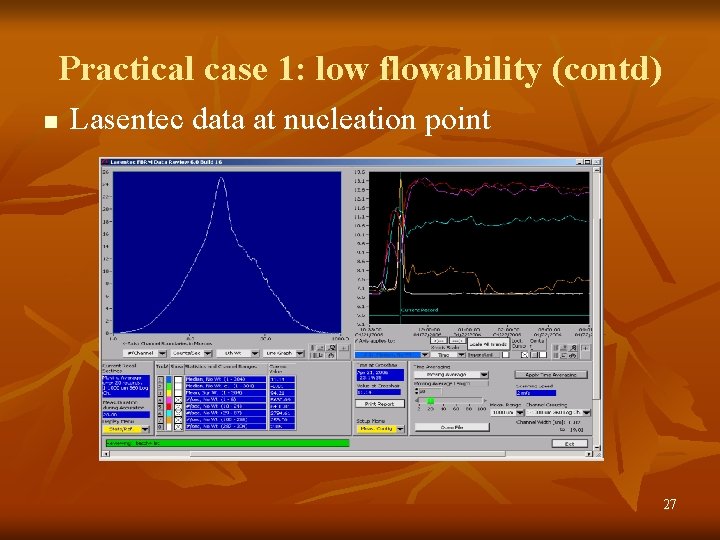
Practical case 1: low flowability (contd) n Lasentec data at nucleation point 27

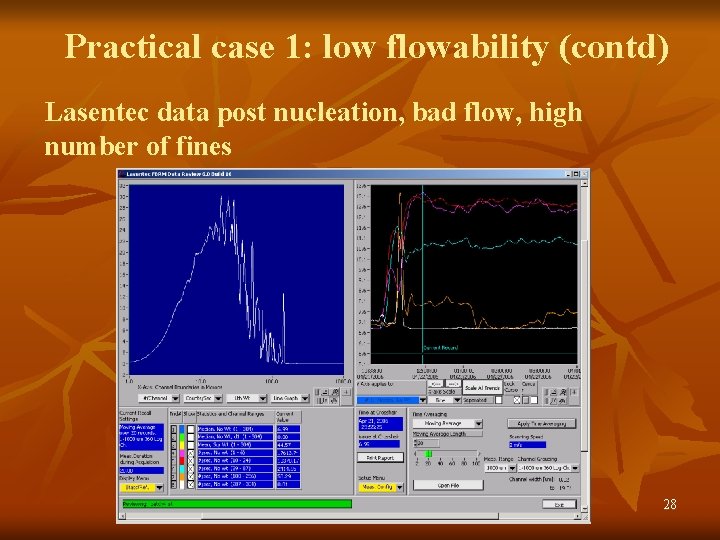
Practical case 1: low flowability (contd) Lasentec data post nucleation, bad flow, high number of fines 28

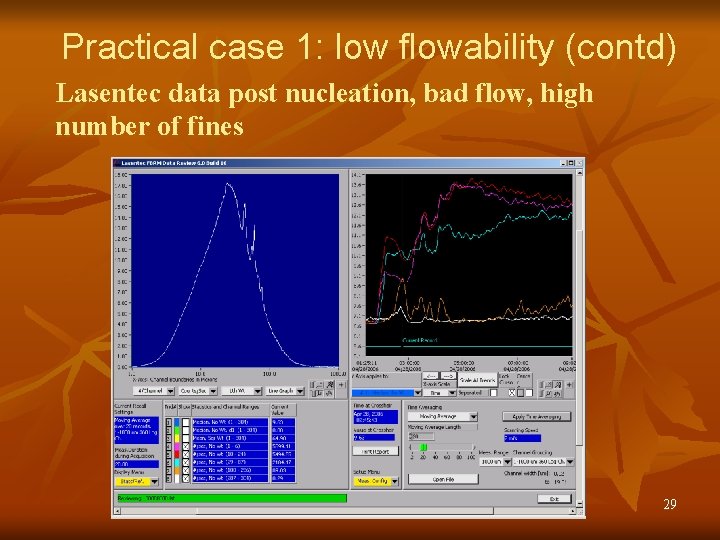
Practical case 1: low flowability (contd) Lasentec data post nucleation, bad flow, high number of fines 29

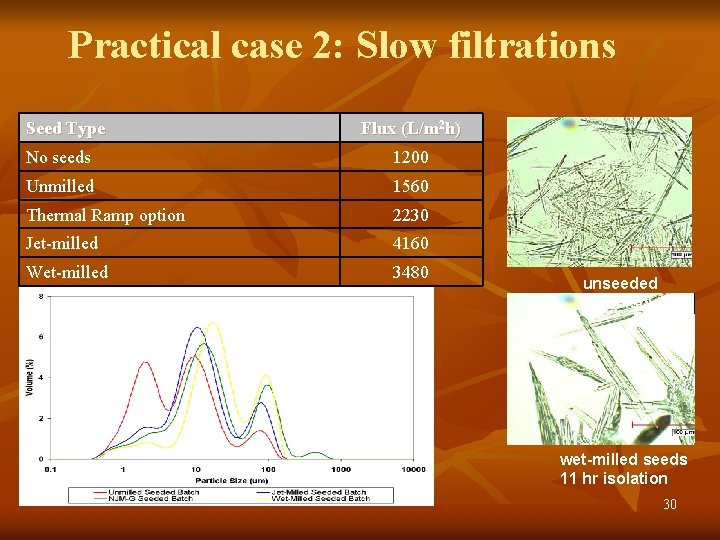
Practical case 2: Slow filtrations Seed Type Flux (L/m 2 h) No seeds 1200 Unmilled 1560 Thermal Ramp option 2230 Jet-milled 4160 Wet-milled 3480 unseeded 32 hr isolation wet-milled seeds 11 hr isolation 30

n Q&A 31