Chapter 11 Diversification of Magmas Magmatic Differentiation l






























- Slides: 55

Chapter 11: Diversification of Magmas

Magmatic Differentiation l Any process by which a magma is able to diversify and produce a magma or rock of different composition

Magmatic Differentiation l Two essential processes 1. Creates a compositional difference in one or more phases 2. Preserves the chemical difference by segregating (or fractionating) the chemically distinct portions

Partial Melting Separation of a partially melted liquid from the solid residue

Effects of removing liquid at various stages of melting l Eutectic systems First melt always = eutectic composition F Major element composition of eutectic melt is constant until one of the source mineral phases is consumed (trace elements differ) F Once a phase is consumed, the next increment of melt will be different X and T F

l l Separation of a partially melted liquid from the solid residue requires a critical melt % Sufficient melt must be produced for it to F Form a continuous, interconnected film F Have enough interior volume that it is not all of it is adsorbed to the crystal surfaces

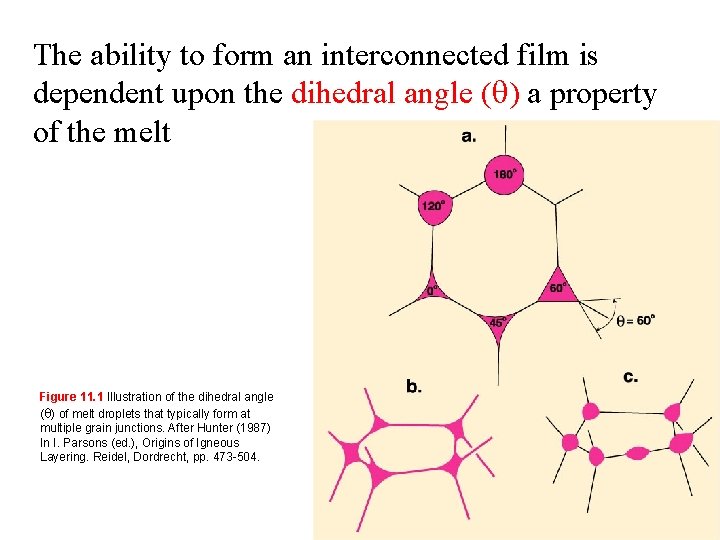
The ability to form an interconnected film is dependent upon the dihedral angle ( ) a property of the melt Figure 11. 1 Illustration of the dihedral angle ( ) of melt droplets that typically form at multiple grain junctions. After Hunter (1987) In I. Parsons (ed. ), Origins of Igneous Layering. Reidel, Dordrecht, pp. 473 -504.

l Gravitational effects (buoyant liquid) Filter pressing, or compaction, of crystal mush Shear - the RCMP drops considerably l RCMP varies with l l T F viscosity FX F

Crystal Fractionation l Dominant mechanism by which most magmas, once formed, differentiate?

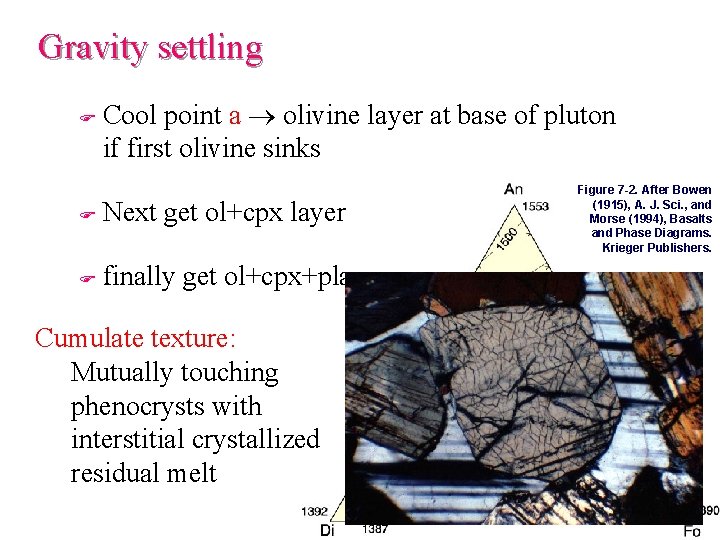
Gravity settling F The differential motion of crystals and liquid under the influence of gravity due to their differences in density

Gravity settling F Cool point a olivine layer at base of pluton if first olivine sinks F Next get ol+cpx layer F finally get ol+cpx+plag Cumulate texture: Mutually touching phenocrysts with interstitial crystallized residual melt Figure 7 -2. After Bowen (1915), A. J. Sci. , and Morse (1994), Basalts and Phase Diagrams. Krieger Publishers.

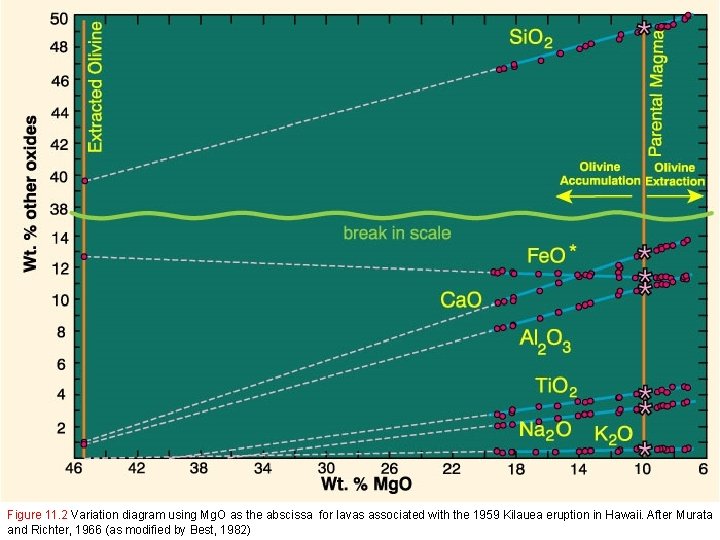
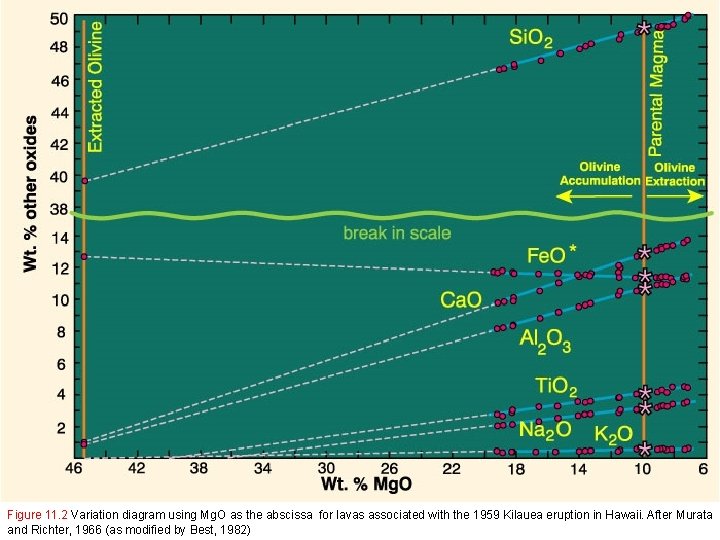
Figure 11. 2 Variation diagram using Mg. O as the abscissa for lavas associated with the 1959 Kilauea eruption in Hawaii. After Murata and Richter, 1966 (as modified by Best, 1982)

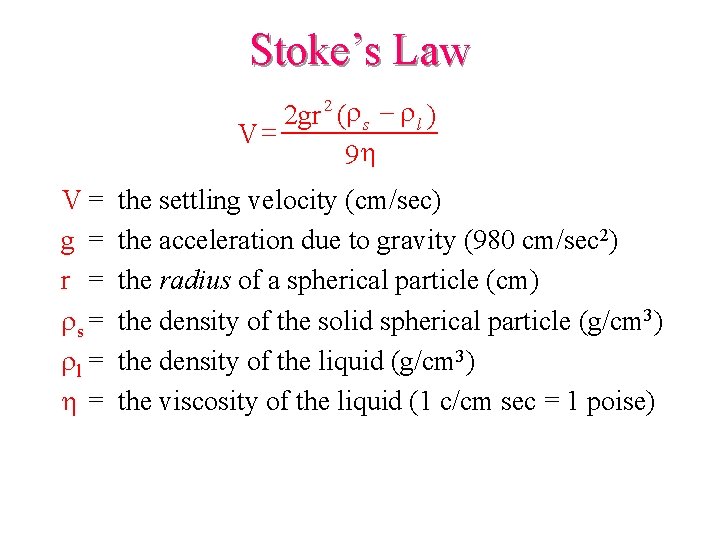
Stoke’s Law 2 gr 2 (r s - r l ) V= 9 h V= g = rs = rl = h= the settling velocity (cm/sec) the acceleration due to gravity (980 cm/sec 2) the radius of a spherical particle (cm) the density of the solid spherical particle (g/cm 3) the density of the liquid (g/cm 3) the viscosity of the liquid (1 c/cm sec = 1 poise)

Olivine in basalt F Olivine (rs = 3. 3 g/cm 3, r = 0. 1 cm) F Basaltic liquid (rl = 2. 65 g/cm 3, h = 1000 poise) F V = 2· 980· 0. 12 (3. 3 -2. 65)/9· 1000 = 0. 0013 cm/sec

Rhyolitic melt h = 107 poise and rl = 2. 3 g/cm 3 F hornblende crystal (rs = 3. 2 g/cm 3, r = 0. 1 cm) -7 cm/sec, or 6 cm/year s V = 2 x 10 F feldspars (rl = 2. 7 g/cm 3) s V = 2 cm/year 4 s = 200 m in the 10 years that a stock might cool s If 0. 5 cm in radius (1 cm diameter) settle at 0. 65 meters/year, or 6. 5 km in 104 year cooling of stock F

Stokes’ Law is overly simplified 1. Crystals are not spherical 2. Only basaltic magmas very near their liquidus temperatures behave as Newtonian fluids

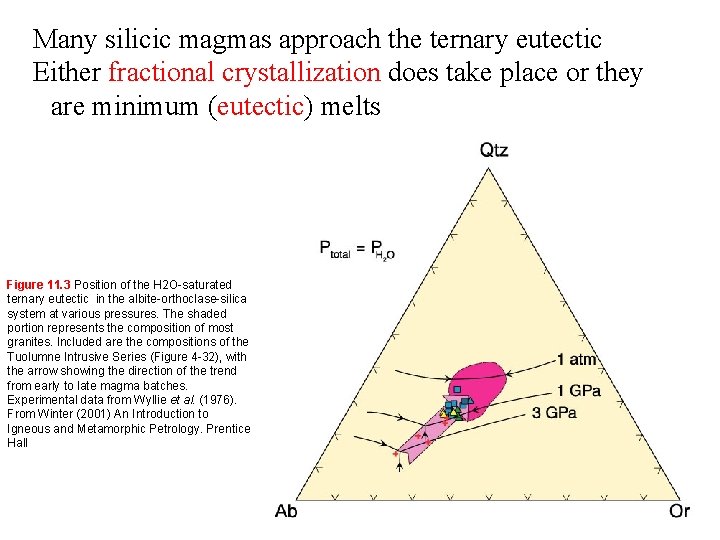
Many silicic magmas approach the ternary eutectic Either fractional crystallization does take place or they are minimum (eutectic) melts Figure 11. 3 Position of the H 2 O-saturated ternary eutectic in the albite-orthoclase-silica system at various pressures. The shaded portion represents the composition of most granites. Included are the compositions of the Tuolumne Intrusive Series (Figure 4 -32), with the arrow showing the direction of the trend from early to late magma batches. Experimental data from Wyllie et al. (1976). From Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall

Polybaric Fractional Crystallization 1. Stability of phases will change (hi-P garnet)

Polybaric Fractional Crystallization 1. Stability of phases changes (hi-P garnet. . . ) 2. Shift of the eutectic point with pressure will cause the quantity of the liquidus phases to vary

High-P (purple tieline) has liq > ol Low-P (blue tie-line) has ol > liquid Hi-P Low-P Ol Pyx Expansion of olivine field at low pressure causes an increase in the quantity of crystallized olivine

Two other mechanisms that facilitate the separation of crystals and liquid 1. Compaction

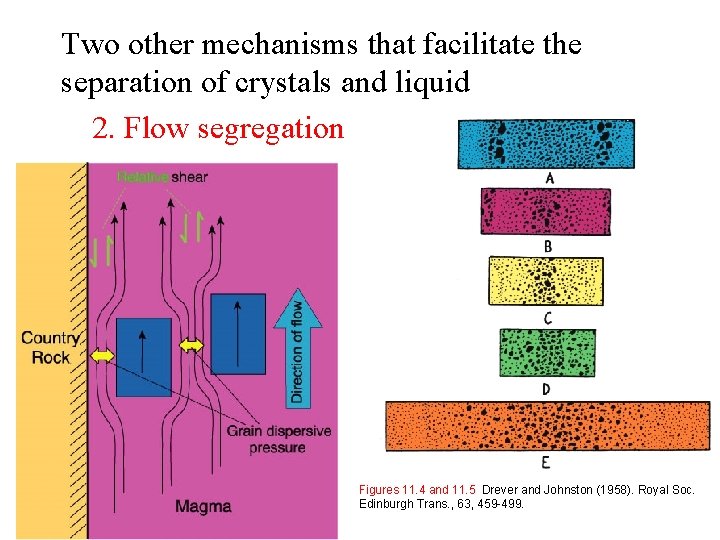
Two other mechanisms that facilitate the separation of crystals and liquid 2. Flow segregation Figures 11. 4 and 11. 5 Drever and Johnston (1958). Royal Soc. Edinburgh Trans. , 63, 459 -499.

Volatile Transport 1. Vapor released by heating of hydrated or carbonated wall rocks

Volatile Transport 2. As a volatile-bearing (but undersaturated) magma rises and pressure is reduced, the magma may eventually become saturated in the vapor, and a free vapor phase will be released Figure 7. 22. From Burnham and Davis (1974). A J Sci. , 274, 902 -940.

3. Late-stage fractional crystallization l l Fractional crystallization enriches late melt in incompatible, LIL, and non-lithophile elements Many concentrate further in the vapor Particularly enriched with resurgent boiling (melt already evolved when vapor phase released) Get a silicate-saturated vapor + a vapor-saturated late derivative silicate liquid

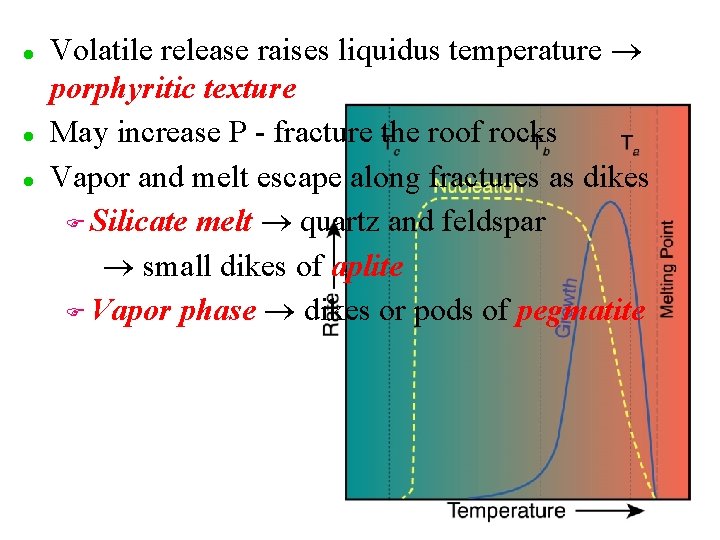
l l l Volatile release raises liquidus temperature porphyritic texture May increase P - fracture the roof rocks Vapor and melt escape along fractures as dikes F Silicate melt quartz and feldspar small dikes of aplite F Vapor phase dikes or pods of pegmatite

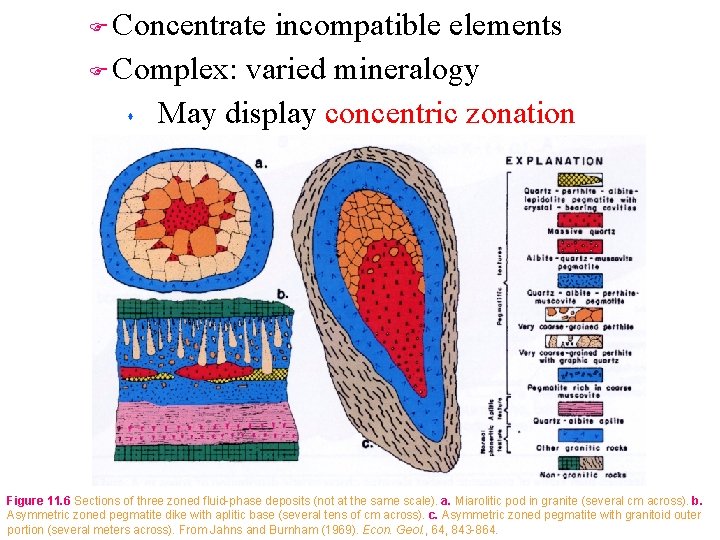
Concentrate incompatible elements F Complex: varied mineralogy s May display concentric zonation F Figure 11. 6 Sections of three zoned fluid-phase deposits (not at the same scale). a. Miarolitic pod in granite (several cm across). b. Asymmetric zoned pegmatite dike with aplitic base (several tens of cm across). c. Asymmetric zoned pegmatite with granitoid outer portion (several meters across). From Jahns and Burnham (1969). Econ. Geol. , 64, 843 -864.

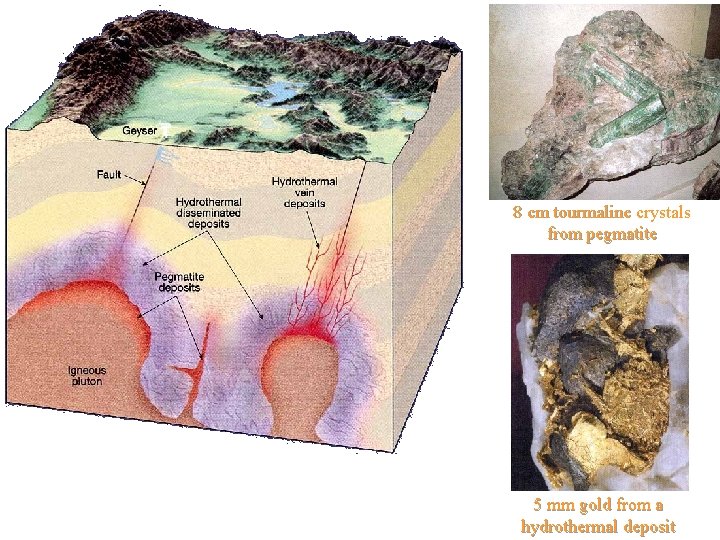
8 cm tourmaline crystals from pegmatite 5 mm gold from a hydrothermal deposit

Pegmatites



Aplite dikes

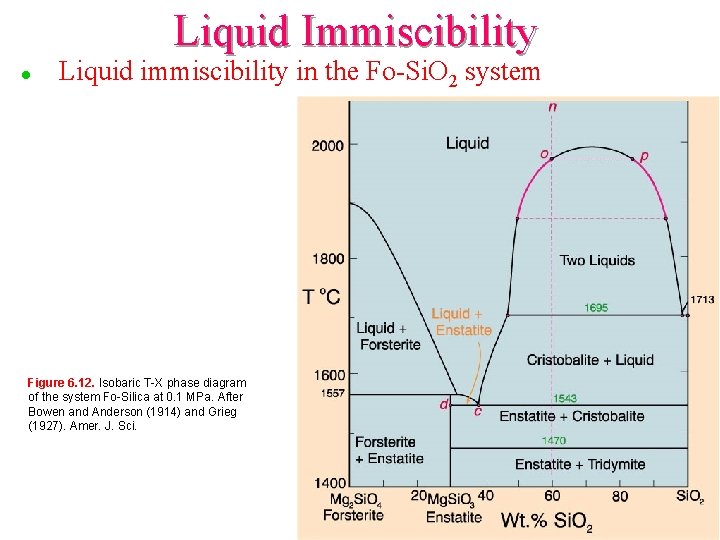
Liquid Immiscibility l Liquid immiscibility in the Fo-Si. O 2 system Figure 6. 12. Isobaric T-X phase diagram of the system Fo-Silica at 0. 1 MPa. After Bowen and Anderson (1914) and Grieg (1927). Amer. J. Sci.

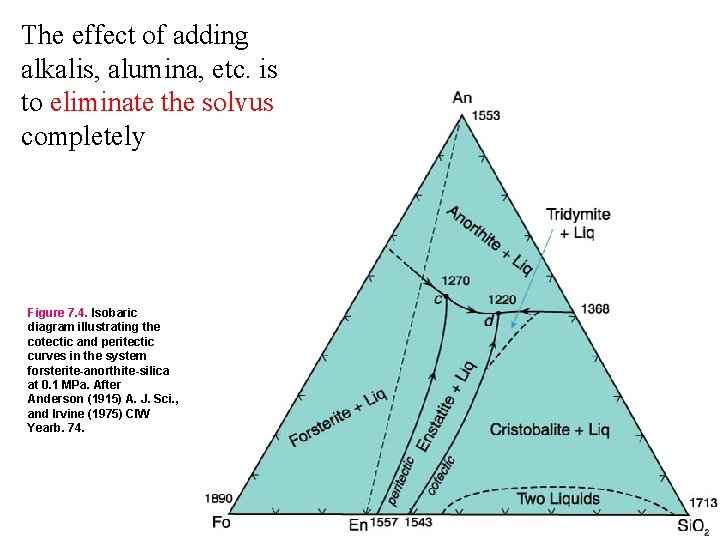
The effect of adding alkalis, alumina, etc. is to eliminate the solvus completely Figure 7. 4. Isobaric diagram illustrating the cotectic and peritectic curves in the system forsterite-anorthite-silica at 0. 1 MPa. After Anderson (1915) A. J. Sci. , and Irvine (1975) CIW Yearb. 74.

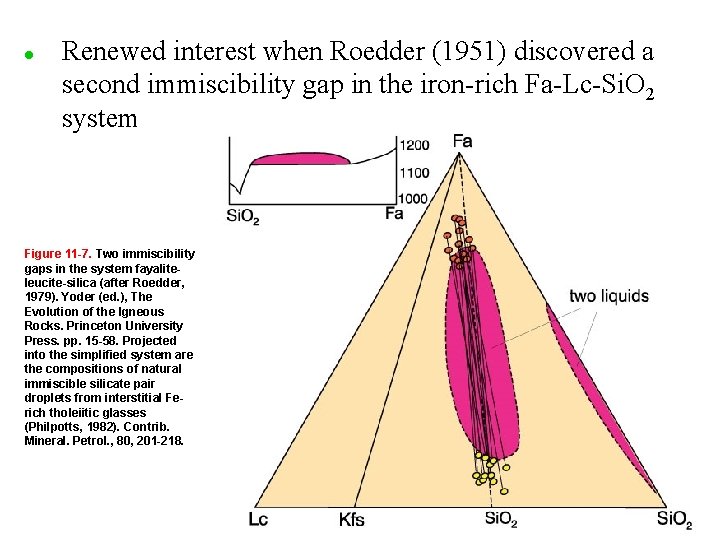
l Renewed interest when Roedder (1951) discovered a second immiscibility gap in the iron-rich Fa-Lc-Si. O 2 system Figure 11 -7. Two immiscibility gaps in the system fayaliteleucite-silica (after Roedder, 1979). Yoder (ed. ), The Evolution of the Igneous Rocks. Princeton University Press. pp. 15 -58. Projected into the simplified system are the compositions of natural immiscible silicate pair droplets from interstitial Ferich tholeiitic glasses (Philpotts, 1982). Contrib. Mineral. Petrol. , 80, 201 -218.

Some Examples l l l Late silica-rich immiscible droplets in Fe-rich tholeiitic basalts (as in Roedder) Sulfide-silicate immiscibility (massive sulfide deposits) Carbonatite-nephelinite systems (Chapter 19)

Tests for immiscible origin of associated rock pairs 1. The magmas must be immiscible when heated experimentally, or they must plot on the boundaries of a known immiscibility gap, as in Fig. 11. 7

Tests for immiscible origin of associated rock pairs 2. Immiscible liquids are in equilibrium with each other, and thus they must be in equilibrium with the same minerals

Compositional Convection and In Situ Differentiation Processes l l In-situ: crystals don’t sink/move Typically involves F Diffusion F Convective separation of liquid and crystals

The Soret Effect and Thermogravitational Diffusion l l Thermal diffusion, or the Soret effect Heavy elements/molecules migrate toward the colder end and lighter ones to the hotter end of the gradient

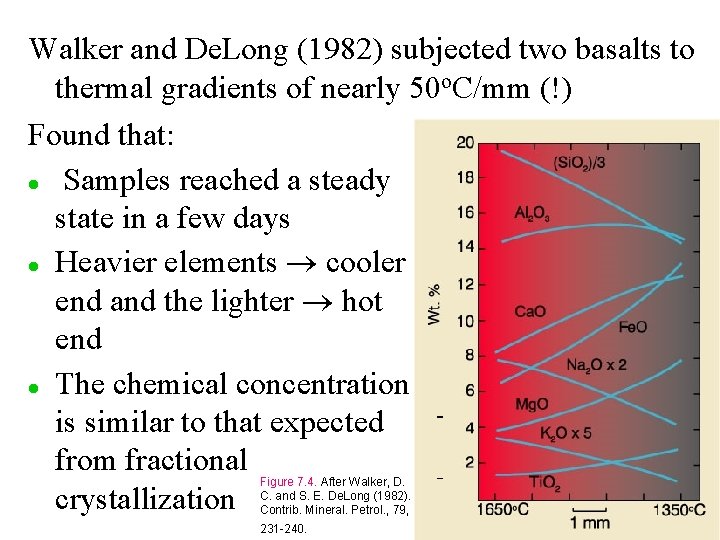
Walker and De. Long (1982) subjected two basalts to thermal gradients of nearly 50 o. C/mm (!) Found that: l Samples reached a steady state in a few days l Heavier elements cooler end and the lighter hot end l The chemical concentration is similar to that expected from fractional crystallization Figure 7. 4. After Walker, D. C. and S. E. De. Long (1982). Contrib. Mineral. Petrol. , 79, 231 -240.

Thermogravitational diffusion Stable and persistent stagnant boundary layers have been shown to occur near the top and sides of magma chambers

Hildreth (1979) 0. 7 Ma Bishop Tuff at Long Valley, California l Vertical compositional variation in the stratified tuff l Thermal gradient in chamber

Model Figure 11 -11. Schematic section through a rhyolitic magma chamber undergoing convection-aided in-situ differentiation. After Hildreth (1979). Geol. Soc. Amer. Special Paper, 180, 43 -75.

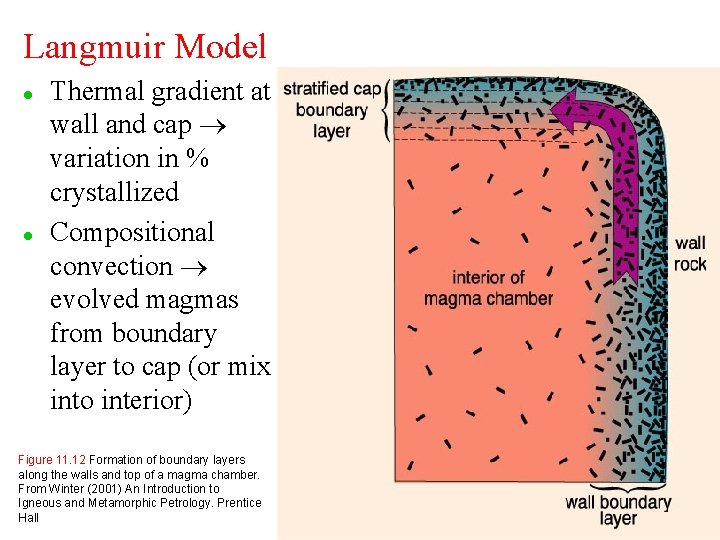
Langmuir Model l l Thermal gradient at wall and cap variation in % crystallized Compositional convection evolved magmas from boundary layer to cap (or mix into interior) Figure 11. 12 Formation of boundary layers along the walls and top of a magma chamber. From Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall

Magma Mixing l l End member mixing for a suite of rocks Variation on Harker-type diagrams should lie on a straight line between the two most extreme compositions

Figure 11. 2 Variation diagram using Mg. O as the abscissa for lavas associated with the 1959 Kilauea eruption in Hawaii. After Murata and Richter, 1966 (as modified by Best, 1982)

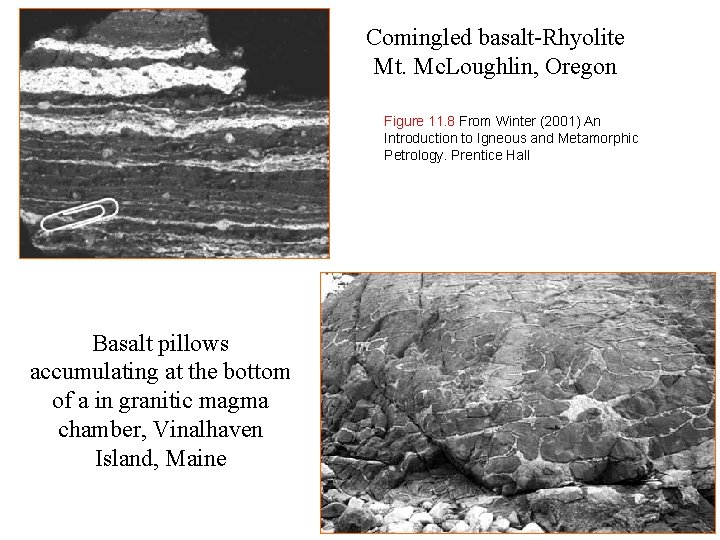
Comingled basalt-Rhyolite Mt. Mc. Loughlin, Oregon Figure 11. 8 From Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall Basalt pillows accumulating at the bottom of a in granitic magma chamber, Vinalhaven Island, Maine

Assimilation l l Incorporation of wall rocks (diffusion, xenoliths) Assimilation by melting is limited by the heat available in the magma

l Zone melting F F Crystallizing igneous material at the base equivalent to the amount melted at the top Transfer heat by convection

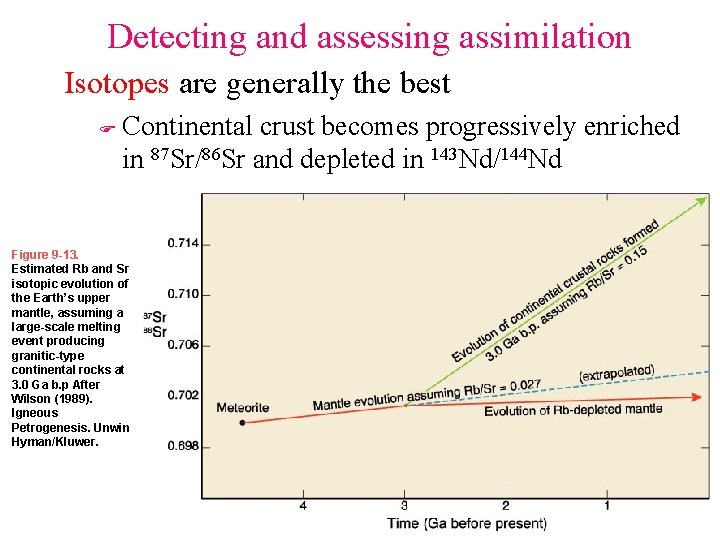
Detecting and assessing assimilation Isotopes are generally the best F Continental crust becomes progressively enriched in 87 Sr/86 Sr and depleted in 143 Nd/144 Nd Figure 9 -13. Estimated Rb and Sr isotopic evolution of the Earth’s upper mantle, assuming a large-scale melting event producing granitic-type continental rocks at 3. 0 Ga b. p After Wilson (1989). Igneous Petrogenesis. Unwin Hyman/Kluwer.

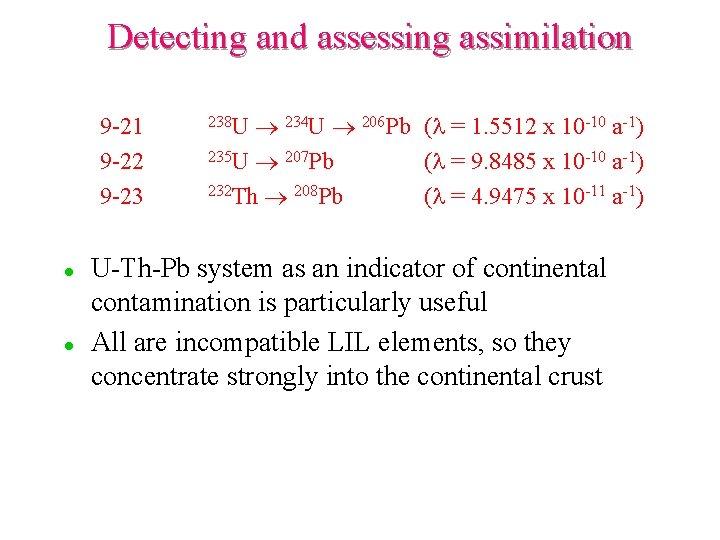
Detecting and assessing assimilation 9 -21 9 -22 9 -23 l l 234 U 206 Pb (l = 1. 5512 x 10 -10 a-1) 235 U 207 Pb (l = 9. 8485 x 10 -10 a-1) 232 Th 208 Pb (l = 4. 9475 x 10 -11 a-1) 238 U U-Th-Pb system as an indicator of continental contamination is particularly useful All are incompatible LIL elements, so they concentrate strongly into the continental crust

Mixed Processes l May be more than coincidence: two processes may operate in conjunction (cooperation? ) F AFC: FX supplies the necessary heat for assimilation F Fractional crystallization + recharge of more primitive magma

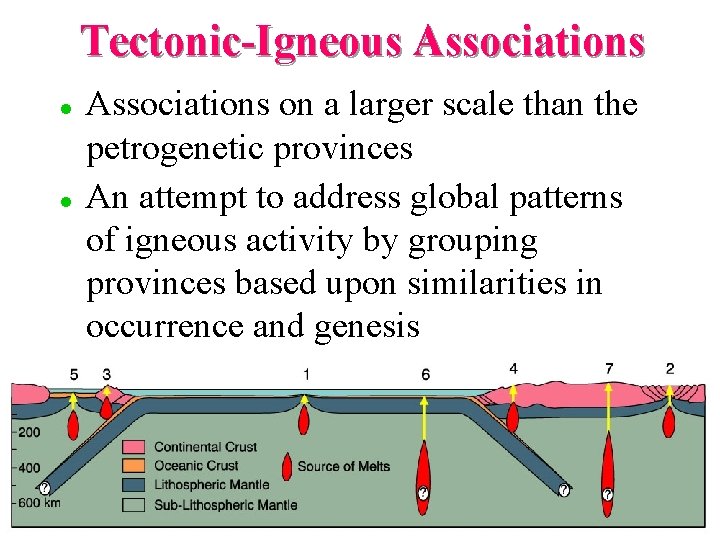
Tectonic-Igneous Associations l l Associations on a larger scale than the petrogenetic provinces An attempt to address global patterns of igneous activity by grouping provinces based upon similarities in occurrence and genesis

Tectonic-Igneous Associations Mid-Ocean Ridge Volcanism F Ocean Intra-plate (Island) volcanism F Continental Plateau Basalts F Subduction-related volcanism and plutonism s Island Arcs s Continental Arcs F Granites (not a true T-I Association) F Mostly alkaline igneous processes of stable craton interiors F Anorthosite Massifs F
 The process of crystallization enriches a magma in
The process of crystallization enriches a magma in Magmatic intrusion
Magmatic intrusion Magmás kőzetek csoportosítása
Magmás kőzetek csoportosítása Magmas dosage form
Magmas dosage form What is diversification 6
What is diversification 6 Unrelated diversification companies
Unrelated diversification companies What is related constrained diversification
What is related constrained diversification What is related constrained diversification
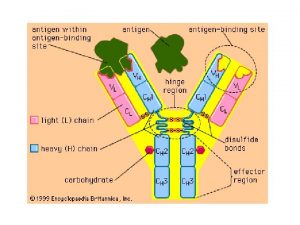
What is related constrained diversification Antibody diversification
Antibody diversification Diversification de confortement
Diversification de confortement Diversification types
Diversification types Forward and backward integration
Forward and backward integration Backward and forward integration
Backward and forward integration Unrelated diversification companies
Unrelated diversification companies Combination related unrelated diversification strategies
Combination related unrelated diversification strategies Related and unrelated diversification
Related and unrelated diversification Intensive integrative and diversification growth strategies
Intensive integrative and diversification growth strategies Unrelated diversification companies
Unrelated diversification companies Lack of diversification
Lack of diversification Intensive integrative and diversification growth strategies
Intensive integrative and diversification growth strategies Diversification of samsung
Diversification of samsung Diversification des espaces et des acteurs de la production
Diversification des espaces et des acteurs de la production Portfolio diversification eliminates
Portfolio diversification eliminates Diversification de confortement
Diversification de confortement 5 dimensions of diversification
5 dimensions of diversification Decentralisation, diversification, connectivity, simplicity
Decentralisation, diversification, connectivity, simplicity Benefits of diversification
Benefits of diversification Optimal portfolio formula
Optimal portfolio formula Les axes de developpement strategique
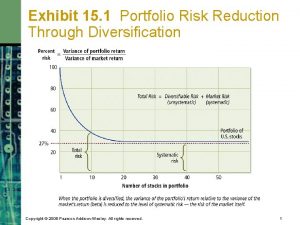
Les axes de developpement strategique Risk reduction through diversification
Risk reduction through diversification Samsung unrelated diversification strategy
Samsung unrelated diversification strategy Random diversification
Random diversification Types of corporate level strategies
Types of corporate level strategies Retrenching to a narrower diversification base
Retrenching to a narrower diversification base What is superfluous diversification
What is superfluous diversification Motives of merger
Motives of merger Horizontal diversification
Horizontal diversification Economic diversification drive strategy botswana
Economic diversification drive strategy botswana Efficient frontier benefits
Efficient frontier benefits Prinsip diversification
Prinsip diversification Conglomerate diversification
Conglomerate diversification Markowitz diversification
Markowitz diversification Gmvp meaning
Gmvp meaning Vertical integration
Vertical integration Volkswagen diversification strategy
Volkswagen diversification strategy Diversification geography definition
Diversification geography definition Porter's three essential tests
Porter's three essential tests Diversification becomes a prime strategic option
Diversification becomes a prime strategic option Amazon forward integration
Amazon forward integration Retrenching to a narrower diversification base
Retrenching to a narrower diversification base The nine-cell attractiveness-strength matrix
The nine-cell attractiveness-strength matrix Chapter 2 differentiation
Chapter 2 differentiation Language objective differentiation for proficiency levels
Language objective differentiation for proficiency levels Difference between udl and differentiation
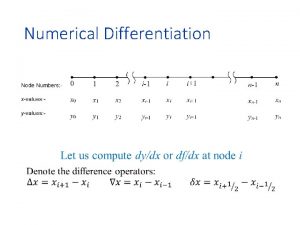
Difference between udl and differentiation High accuracy differentiation formulas
High accuracy differentiation formulas Differentiation formulas of trigonometric functions
Differentiation formulas of trigonometric functions