Chapter 14 Organometallic Chemistry Reactions and Catalysis Types




- Slides: 32

Chapter 14 Organometallic Chemistry Reactions and Catalysis

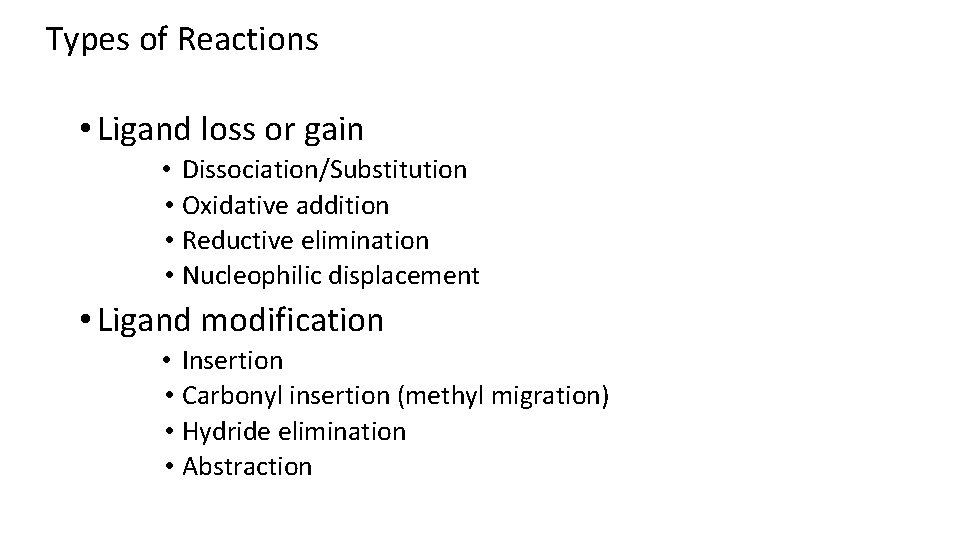
Types of Reactions • Ligand loss or gain • Dissociation/Substitution • Oxidative addition • Reductive elimination • Nucleophilic displacement • Ligand modification • Insertion • Carbonyl insertion (methyl migration) • Hydride elimination • Abstraction

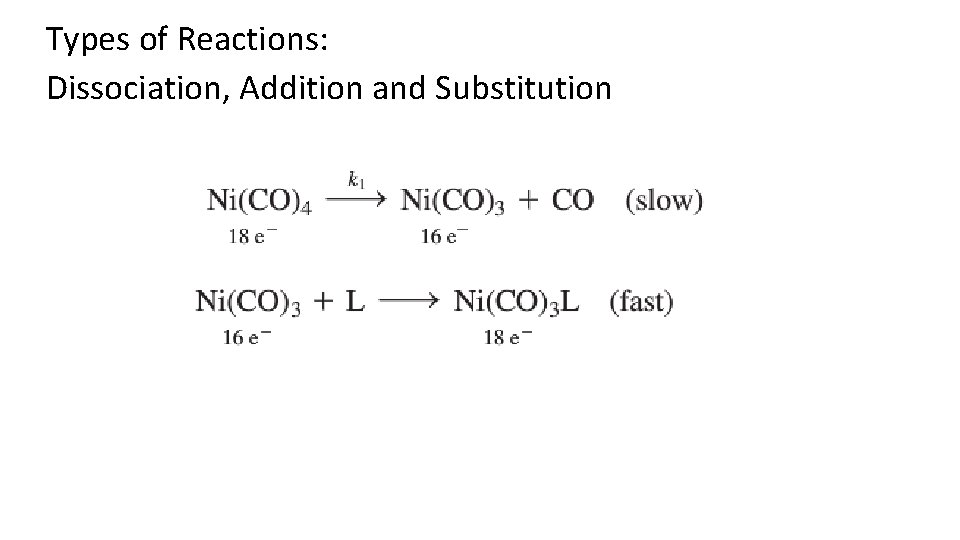
Types of Reactions: Dissociation, Addition and Substitution

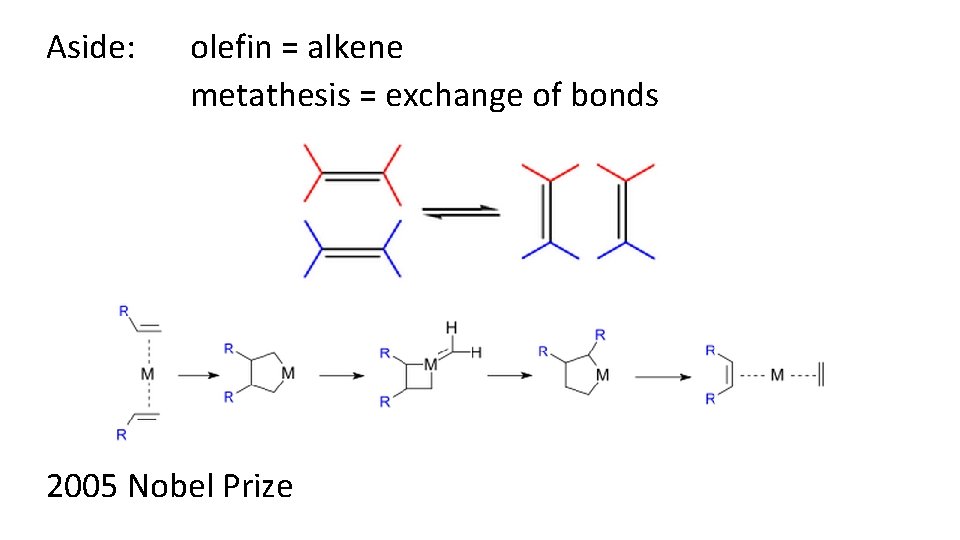
Aside: olefin = alkene metathesis = exchange of bonds 2005 Nobel Prize

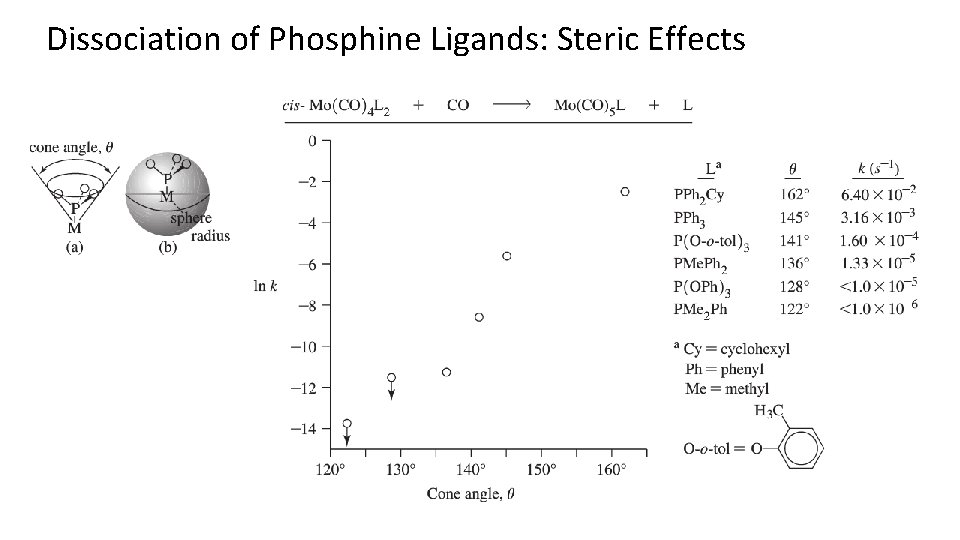
Dissociation of Phosphine Ligands: Steric Effects

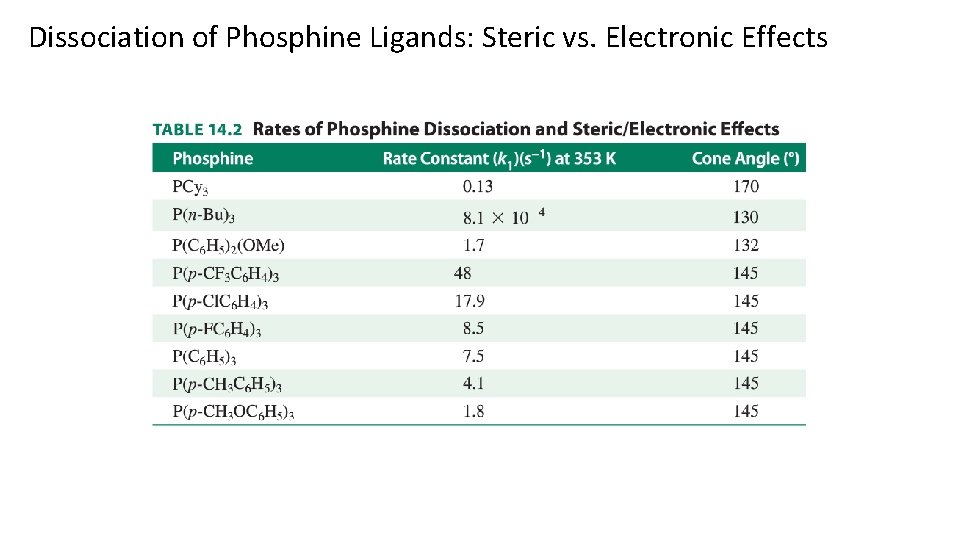
Dissociation of Phosphine Ligands: Steric vs. Electronic Effects

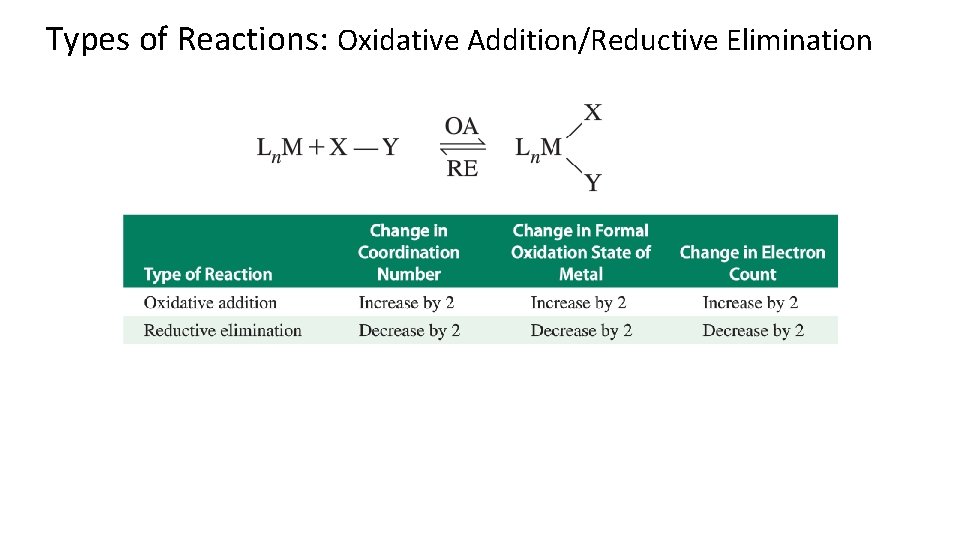
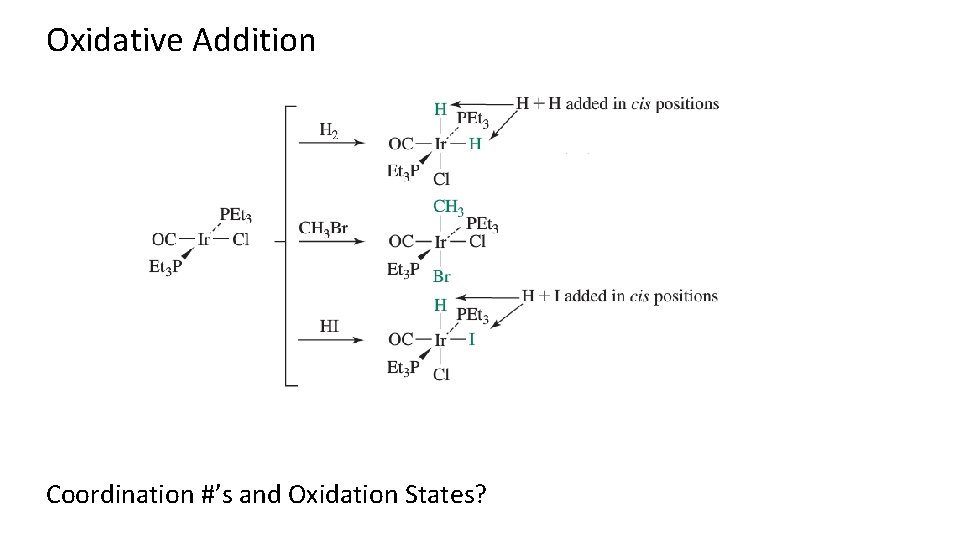
Types of Reactions: Oxidative Addition/Reductive Elimination

Oxidative Addition Coordination #’s and Oxidation States?

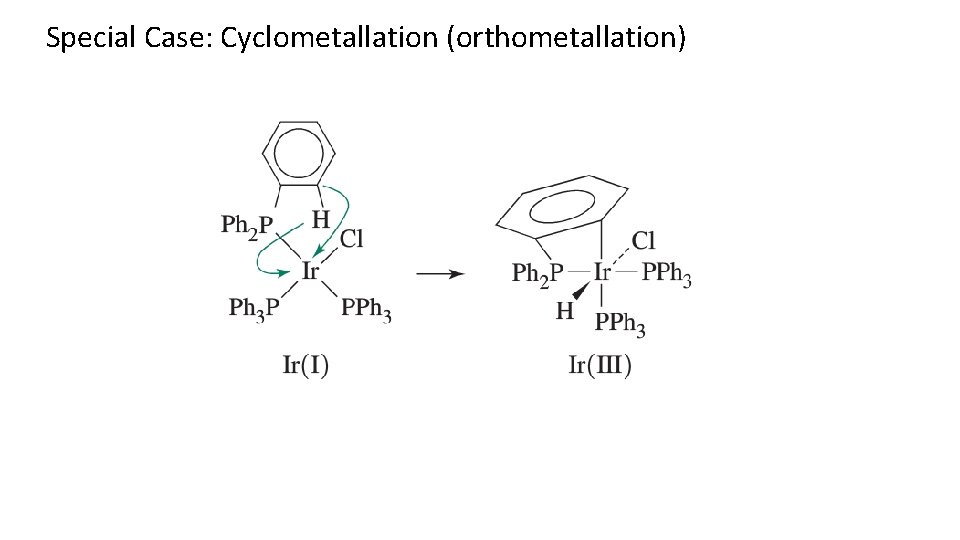
Special Case: Cyclometallation (orthometallation)

Reductive Elimination: Key usefulness– forms a new bond H-H, C-X (X = halide, amide, alkoxide, othes) Rates depend on electronic and steric effects. Electronic effects: more weakly bonded ligands are eliminated faster.

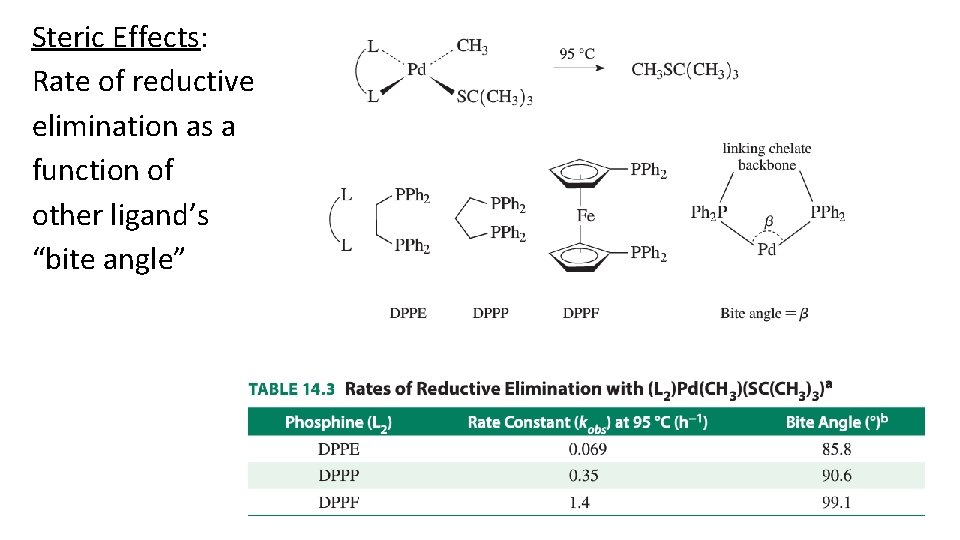
Steric Effects: Rate of reductive elimination as a function of other ligand’s “bite angle”

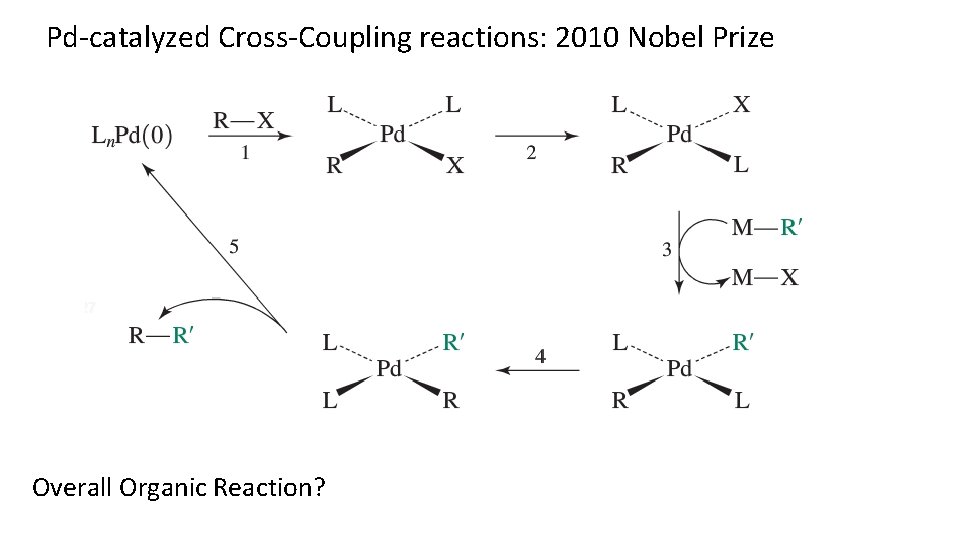
Pd-catalyzed Cross-Coupling reactions: 2010 Nobel Prize Overall Organic Reaction?

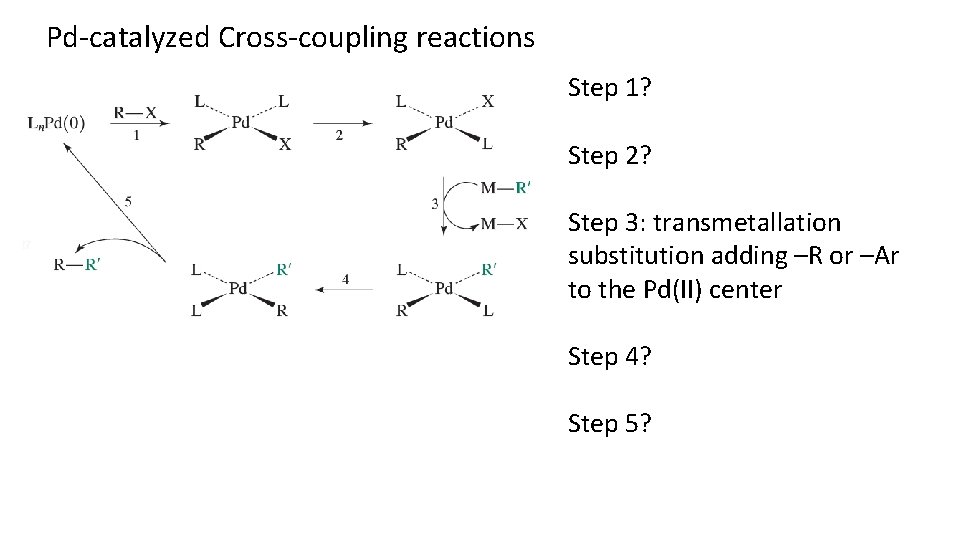
Pd-catalyzed Cross-coupling reactions Step 1? Step 2? Step 3: transmetallation substitution adding –R or –Ar to the Pd(II) center Step 4? Step 5?

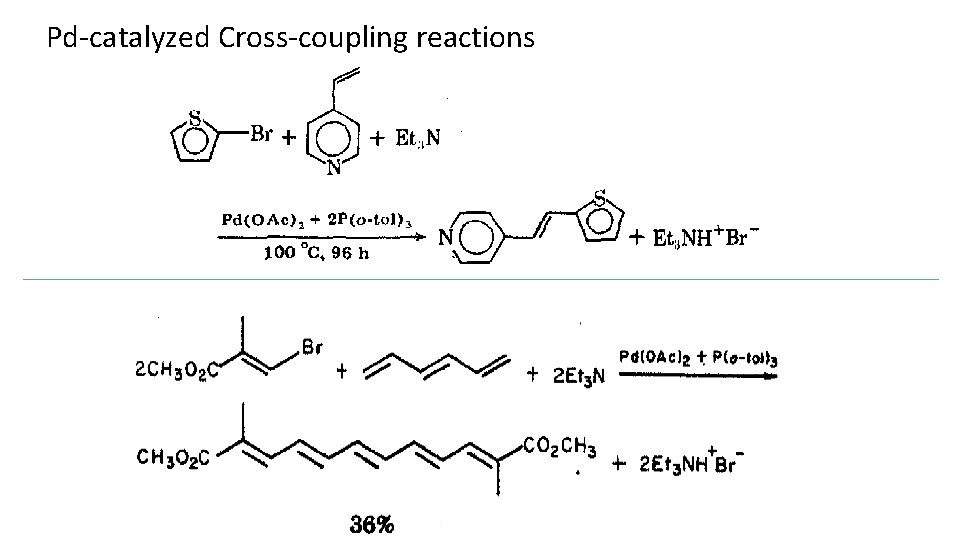
Pd-catalyzed Cross-coupling reactions

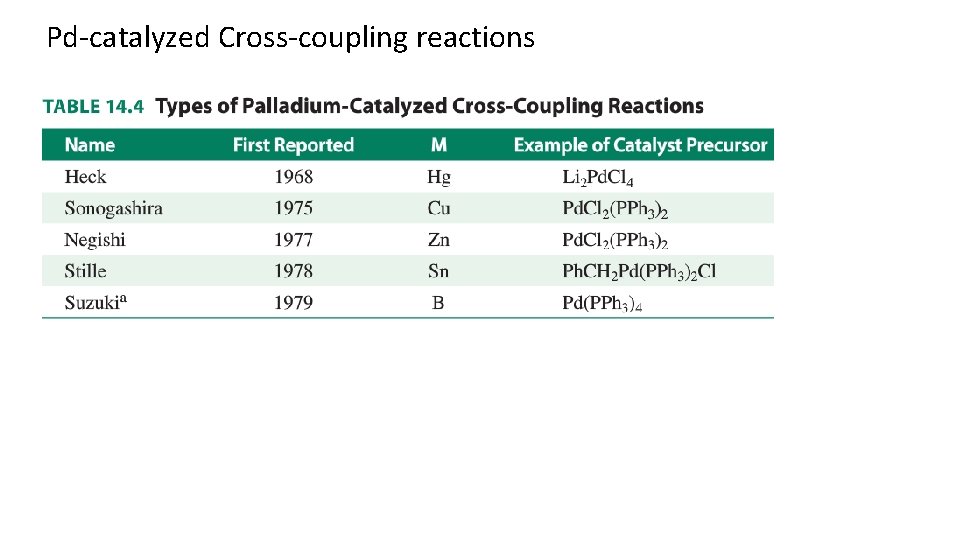
Pd-catalyzed Cross-coupling reactions

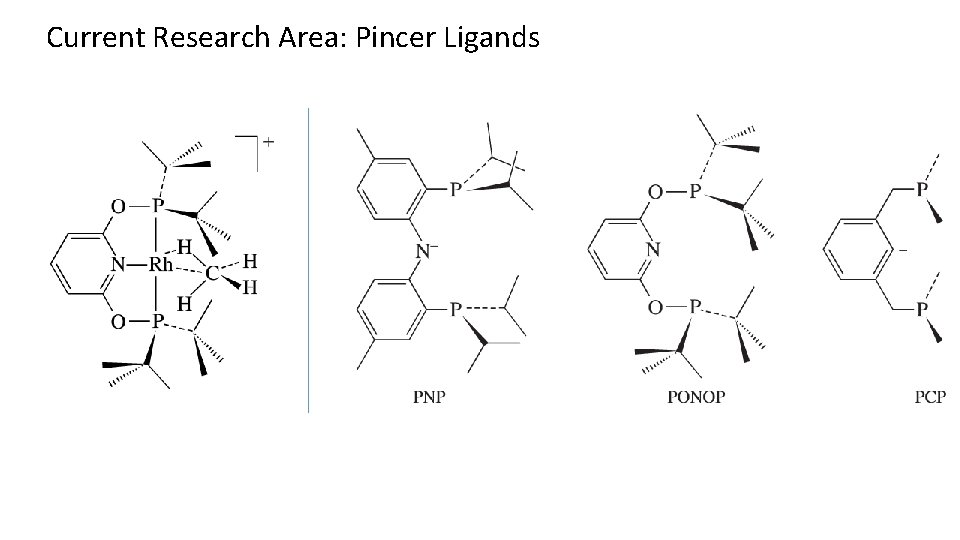
Current Research Area: Pincer Ligands

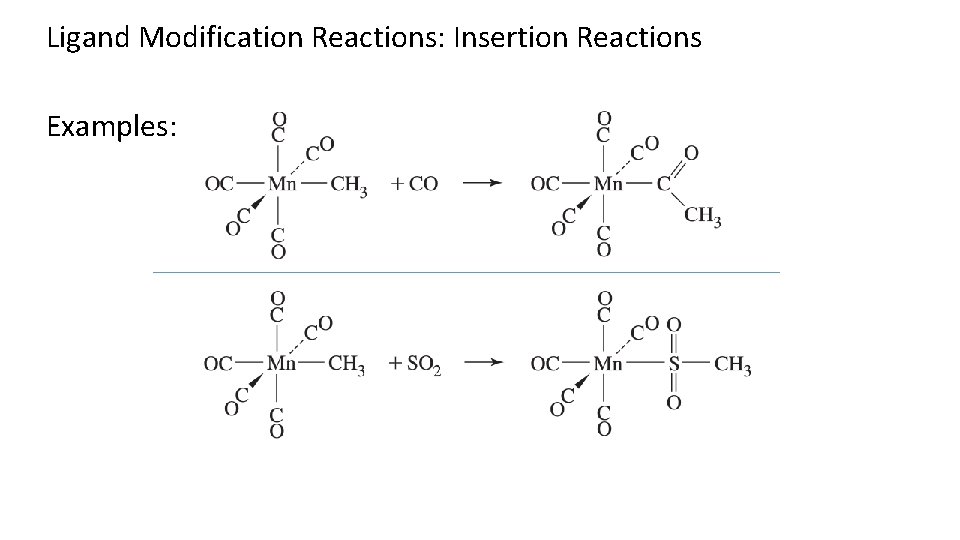
Ligand Modification Reactions: Insertion Reactions Examples:

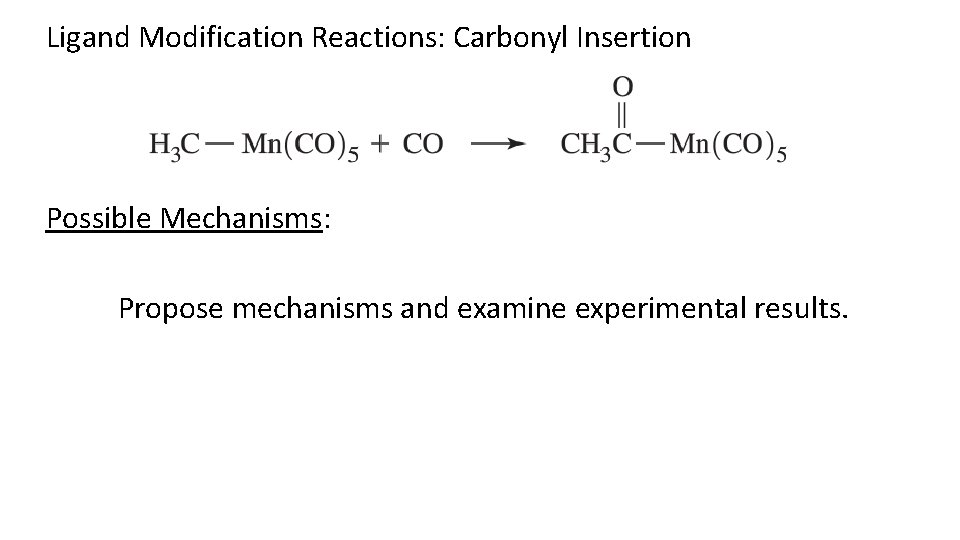
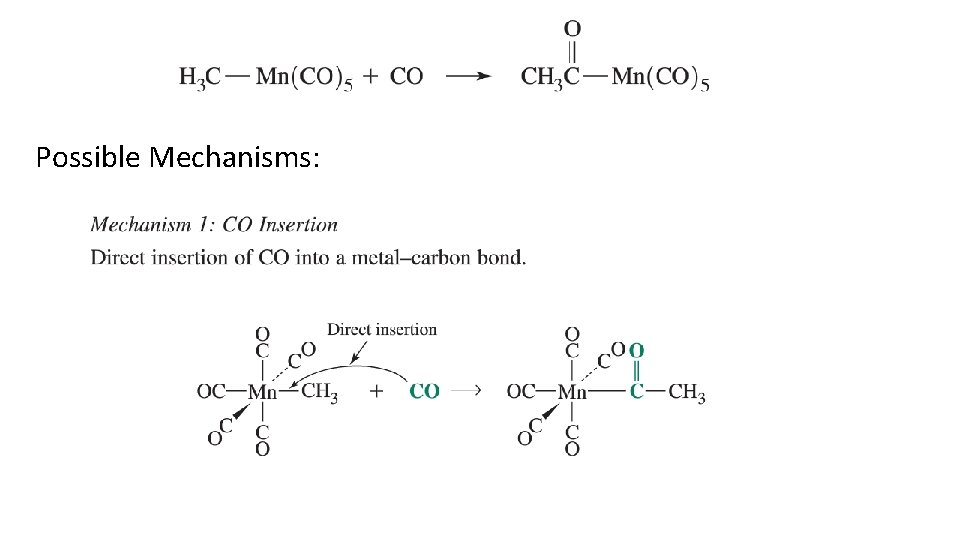
Ligand Modification Reactions: Carbonyl Insertion Possible Mechanisms: Propose mechanisms and examine experimental results.

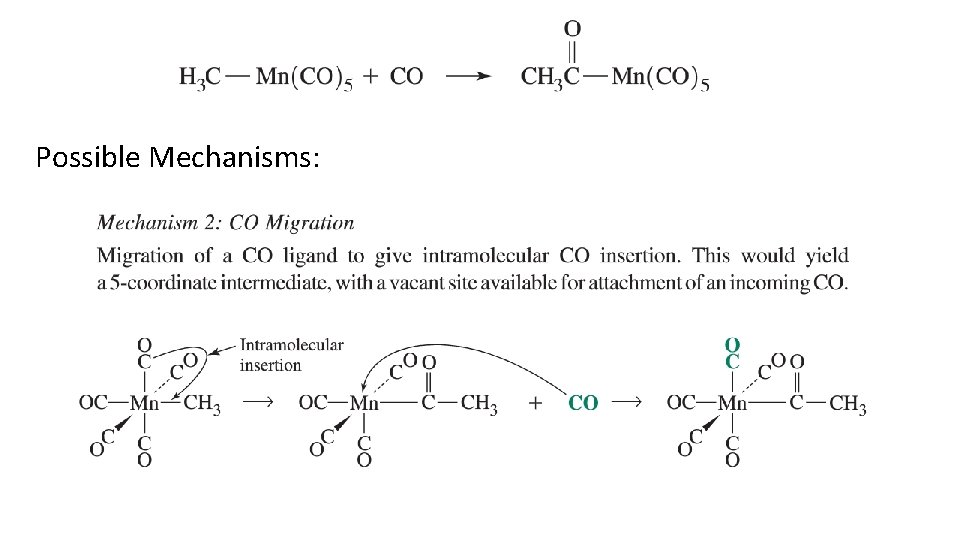
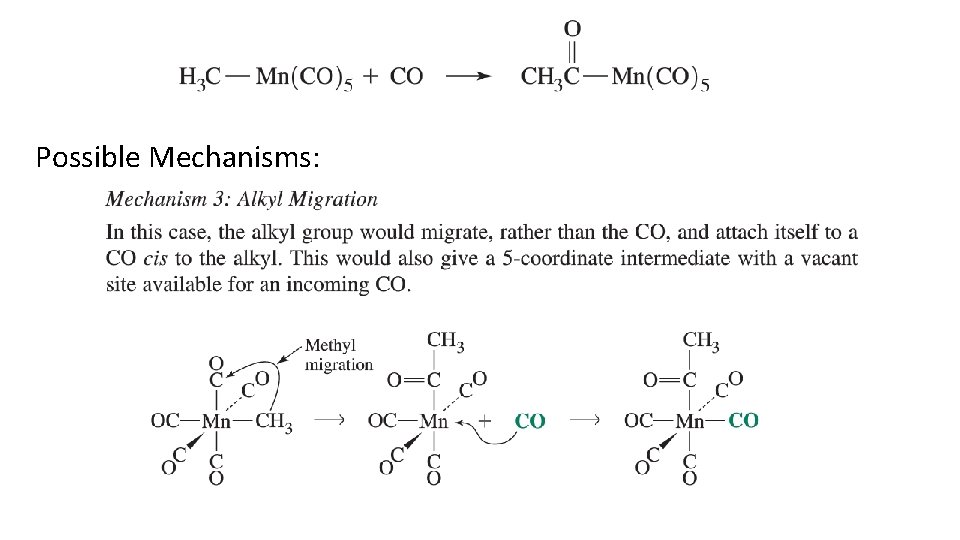
Possible Mechanisms:

Possible Mechanisms:

Possible Mechanisms:

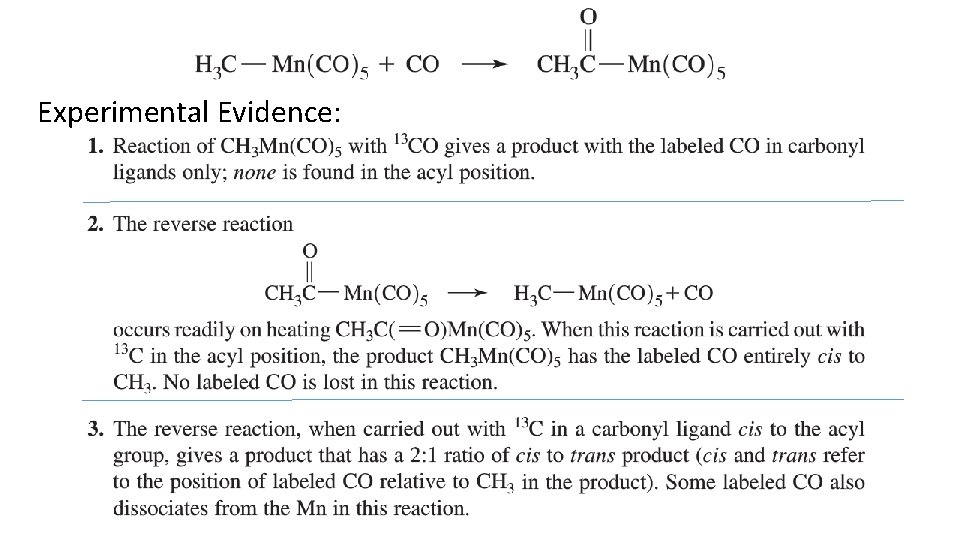
Experimental Evidence:

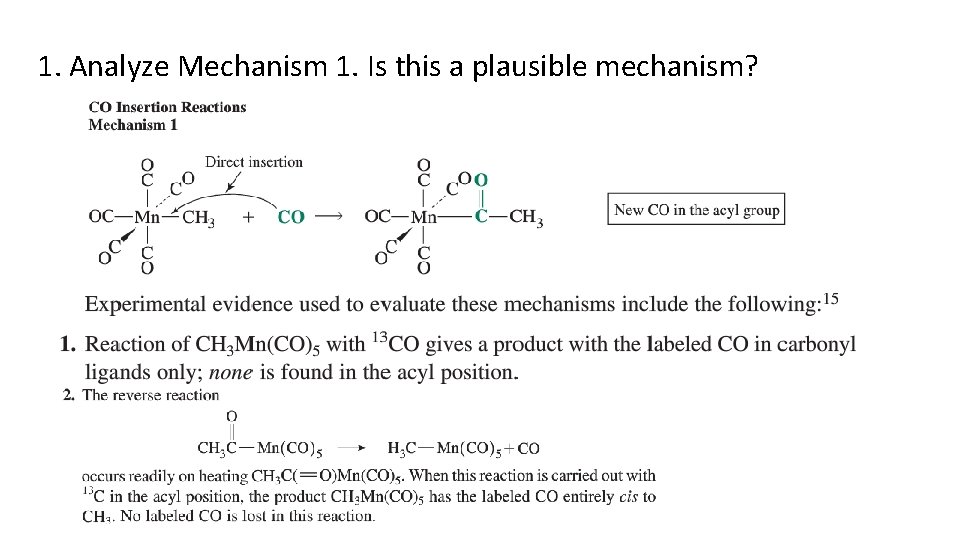
Which mechanism is best supported? 1. Analyze Mechanism 1. Is this a plausible mechanism? 2. Compare Mechanisms 2 and 3. Which is better supported by the evidence.

1. Analyze Mechanism 1. Is this a plausible mechanism?

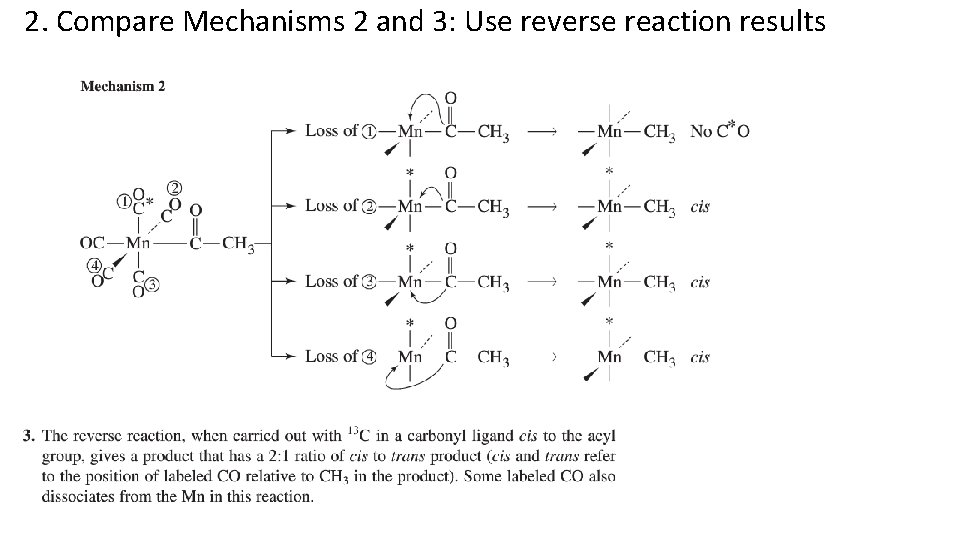
2. Compare Mechanisms 2 and 3: Use reverse reaction results

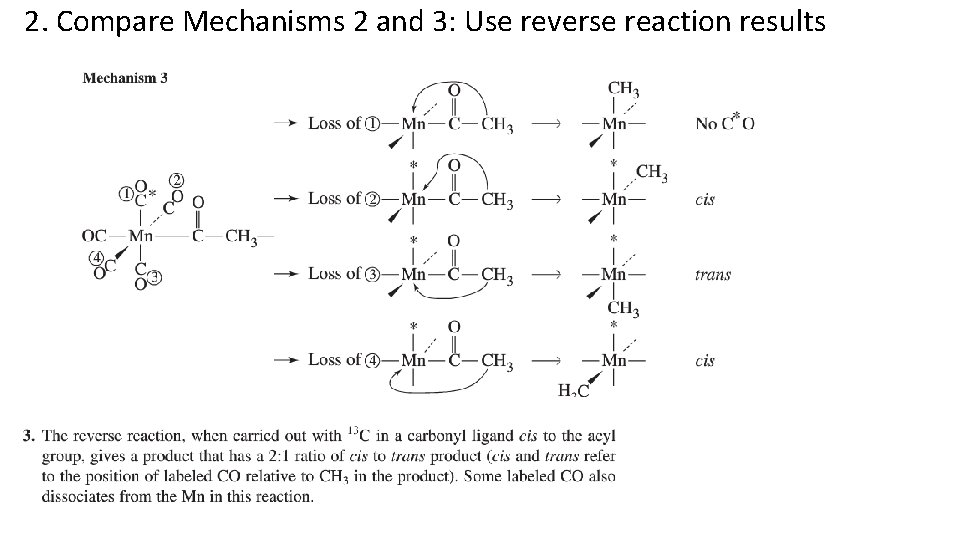
2. Compare Mechanisms 2 and 3: Use reverse reaction results

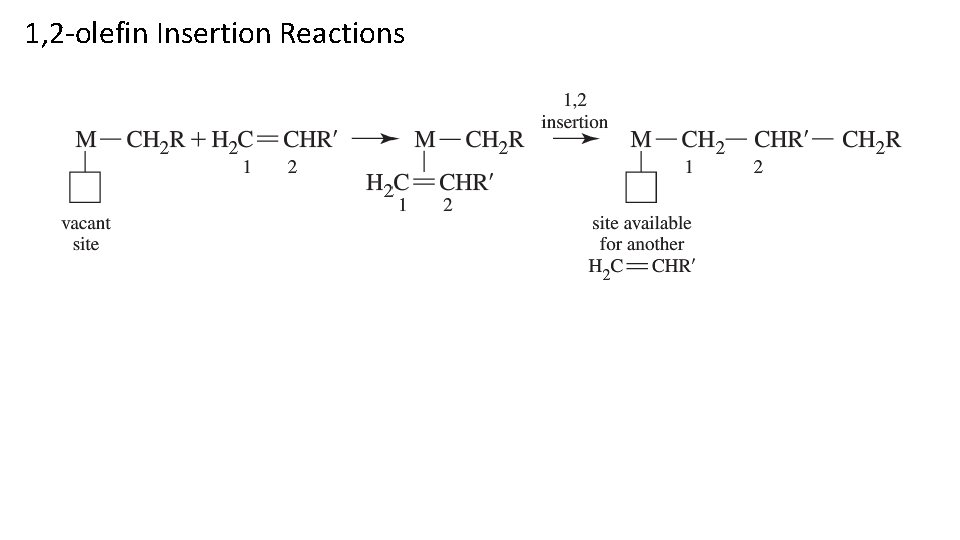
1, 2 -olefin Insertion Reactions

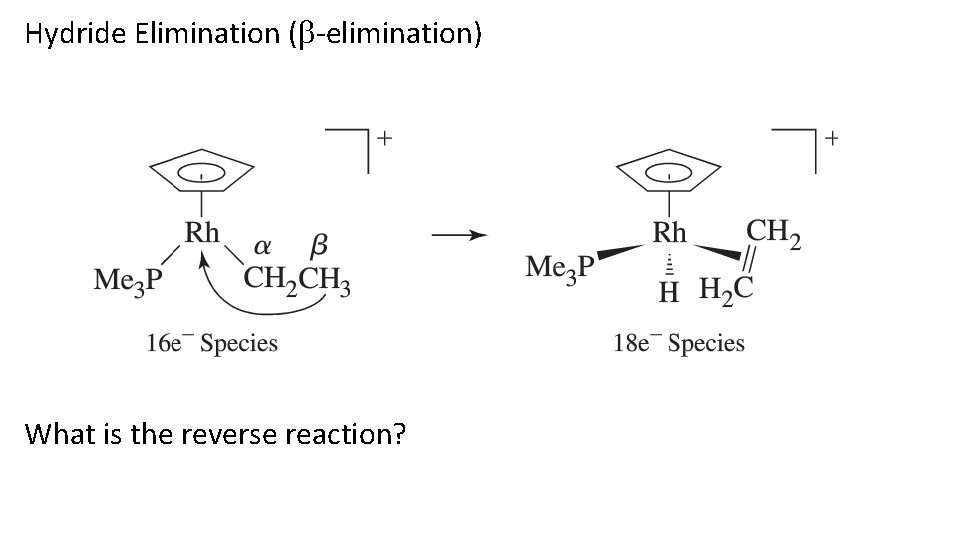
Hydride Elimination ( -elimination) What is the reverse reaction?

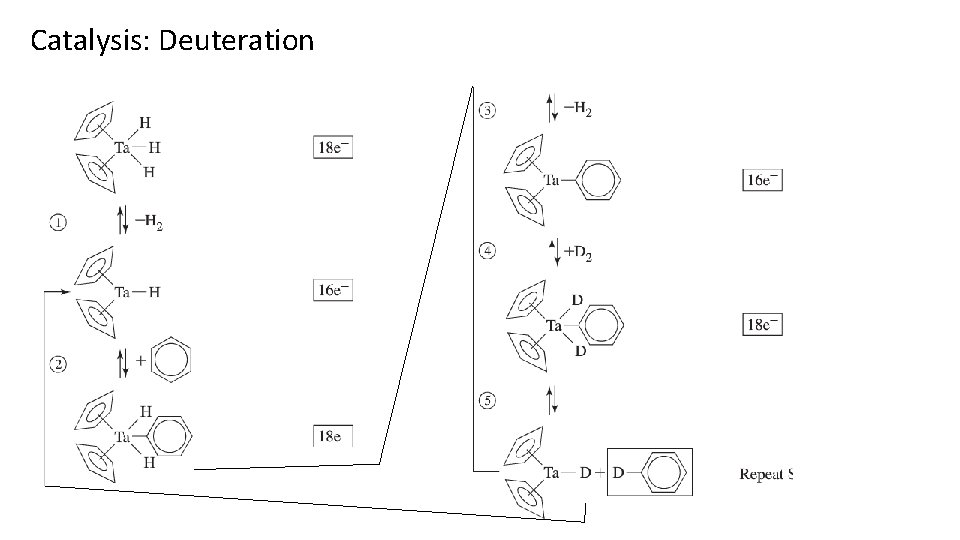
Catalysis: Deuteration

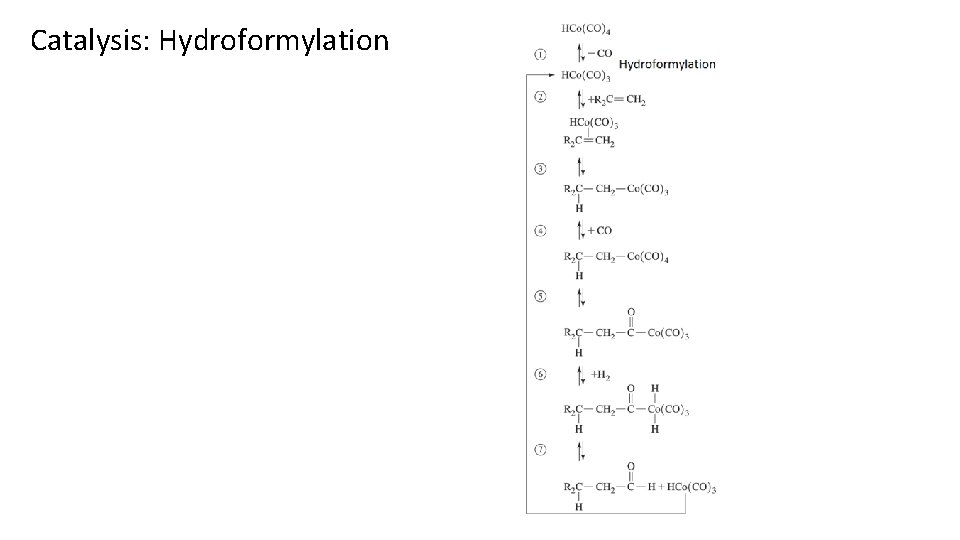
Catalysis: Hydroformylation

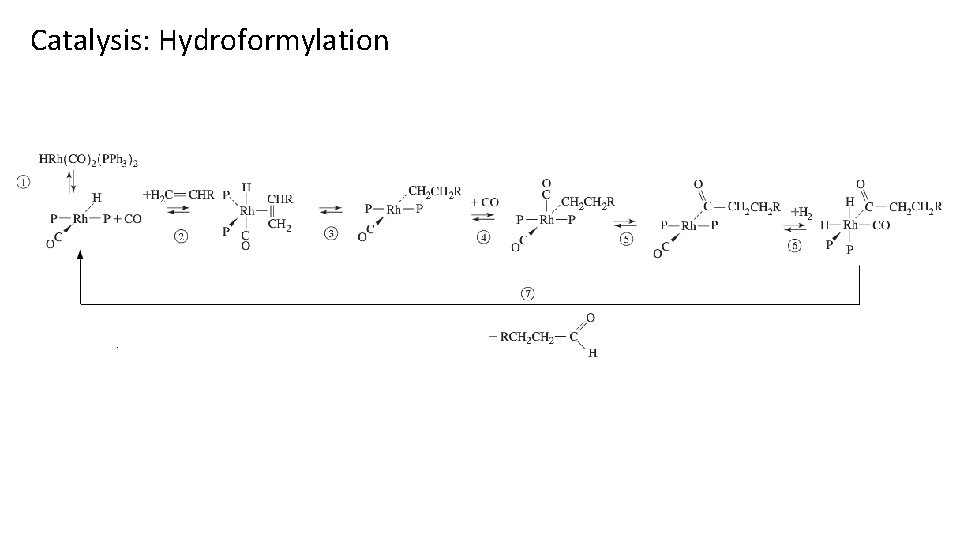
Catalysis: Hydroformylation

Turnover Frequency http: //pubs. acs. org/doi/pdf/10. 1021/om 100552 h Turnover Number: http: //pubs. acs. org/doi/abs/10. 1021/om 060605 p
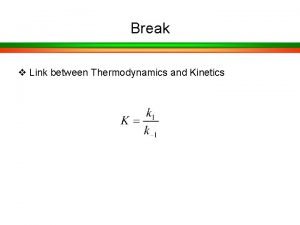
 Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Bonding in organometallic compounds
Bonding in organometallic compounds Chapter 8 review chemical equations and reactions section 2
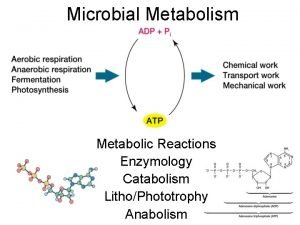
Chapter 8 review chemical equations and reactions section 2 Energy catalysis and biosynthesis
Energy catalysis and biosynthesis Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Symbols in chemical equations
Symbols in chemical equations Sidwick rule
Sidwick rule Chem part 3
Chem part 3 Organometallic
Organometallic 오비탈
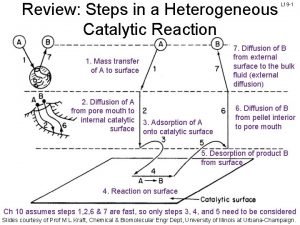
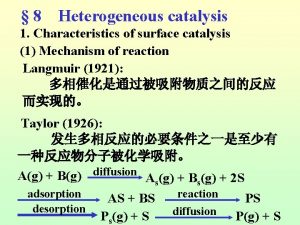
오비탈 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Catabolism
Catabolism Catalysis by approximation
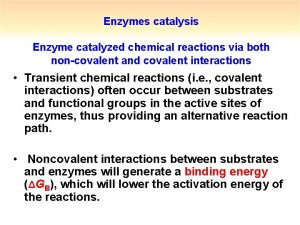
Catalysis by approximation What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Mixed inhibitor km and vmax
Mixed inhibitor km and vmax Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Erzeng
Erzeng Boreskov institute of catalysis
Boreskov institute of catalysis Sodium hydroxide iupac id sodium oxidanide
Sodium hydroxide iupac id sodium oxidanide Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers 20 examples of redox reaction
20 examples of redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Type of reactions chemistry
Type of reactions chemistry Chemistry reactions
Chemistry reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chapter 10 chemical reactions
Chapter 10 chemical reactions