Aim How to identify Organic Reactions Organic Reactions















- Slides: 31

Aim: How to identify Organic Reactions

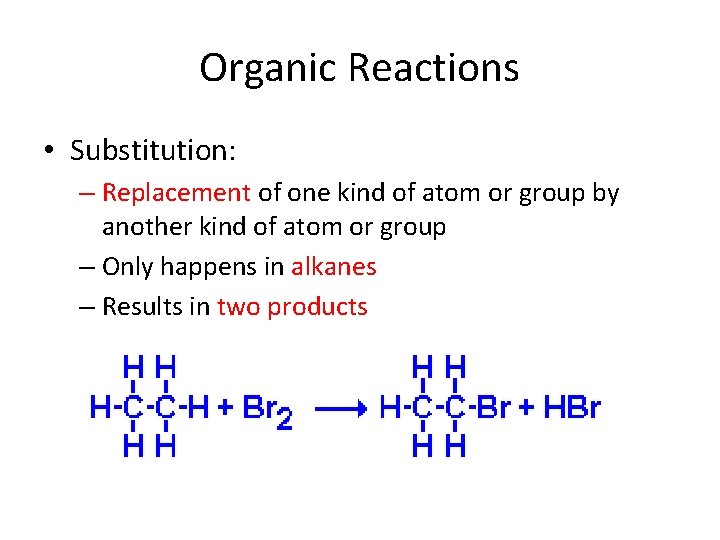
Organic Reactions • Substitution: – Replacement of one kind of atom or group by another kind of atom or group – Only happens in alkanes – Results in two products

Question 1 The reaction A) addition B) hydrogenation C) substitution D) polymerization is an example of

Question 2 Which hydrocarbon will undergo a substitution reaction with chlorine? A) methane B) ethyne C) propene D) butene

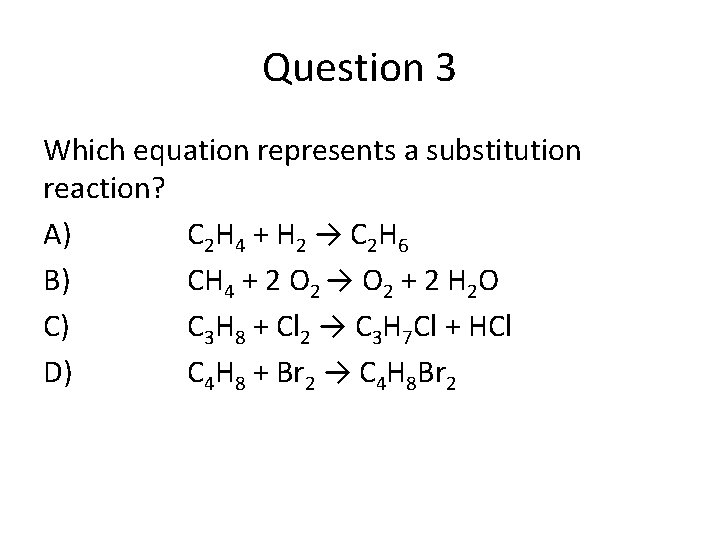
Question 3 Which equation represents a substitution reaction? A) C 2 H 4 + H 2 → C 2 H 6 B) CH 4 + 2 O 2 → O 2 + 2 H 2 O C) C 3 H 8 + Cl 2 → C 3 H 7 Cl + HCl D) C 4 H 8 + Br 2 → C 4 H 8 Br 2

Question 4 When methane reacts with a halogen, the type of reaction is A) addition B) saturation C) substitution D) hydrogenation

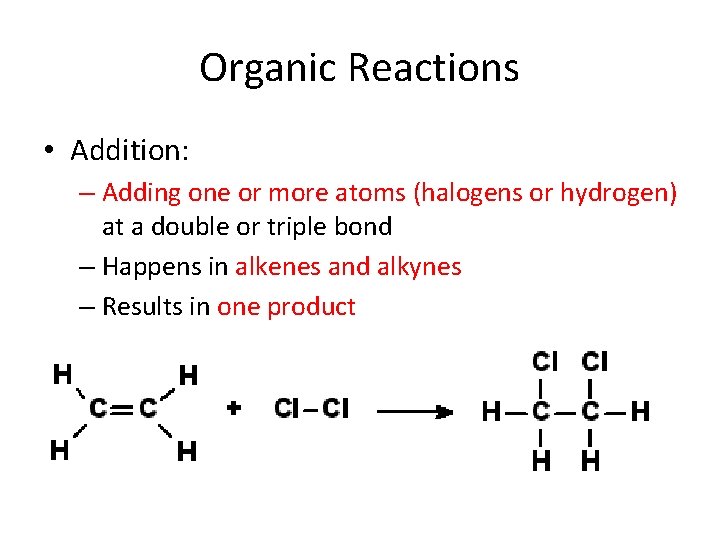
Organic Reactions • Addition: – Adding one or more atoms (halogens or hydrogen) at a double or triple bond – Happens in alkenes and alkynes – Results in one product

Question 5 • Which formula correctly represents the product of an addition reaction between ethene and chlorine? A) CH 2 Cl 2 B) CH 3 Cl C) C 2 H 4 Cl 2 D) C 2 H 3 Cl

Question 6 • Which equation represents an addition reaction? A) CH 4 + 2 O 2 → CO 2 + 2 H 2 O B) C 2 H 6 + Br 2 → C 2 H 5 Br + HBr C) C 3 H 6 + Cl 2 → C 3 H 6 Cl 2 D) C 4 H 10 + Cl 2 → C 4 H 9 Cl + HCl

Question 7 • A) B) C) D) Which hydrocarbon will most likely undergo an addition reaction with Br 2?

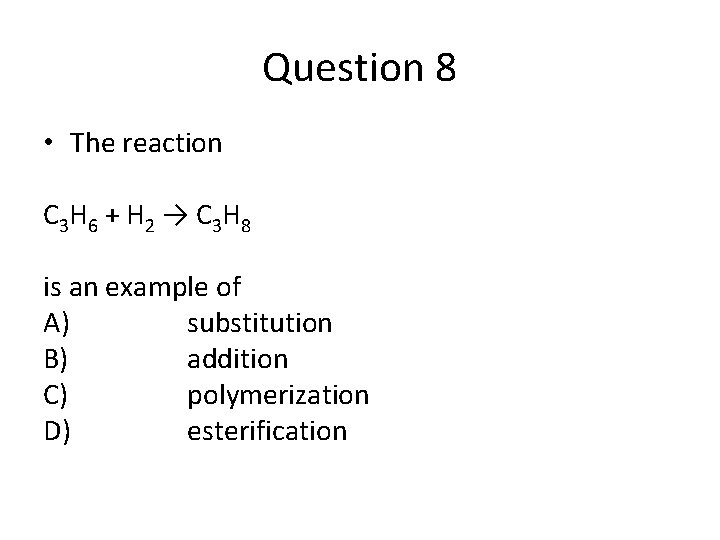
Question 8 • The reaction C 3 H 6 + H 2 → C 3 H 8 is an example of A) substitution B) addition C) polymerization D) esterification

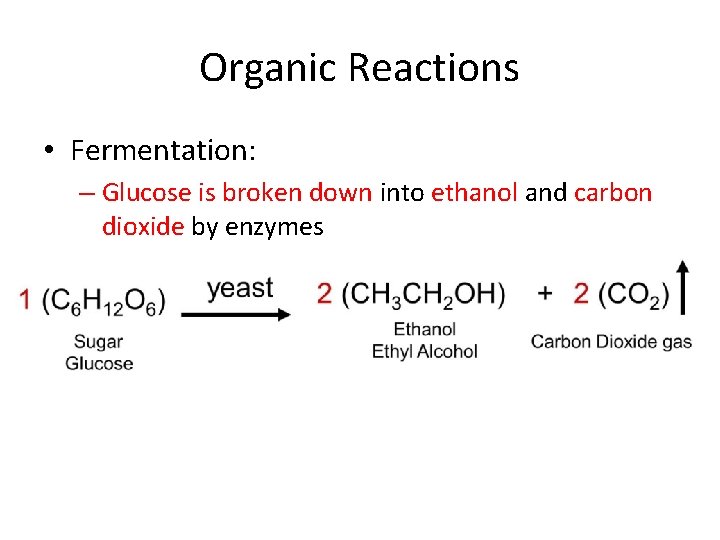
Organic Reactions • Fermentation: – Glucose is broken down into ethanol and carbon dioxide by enzymes

Question 9 • Which substances are products of a fermentation reaction? A) water and carbon dioxide B) soap and glycerol C) alcohol and carbon dioxide D) ester and water

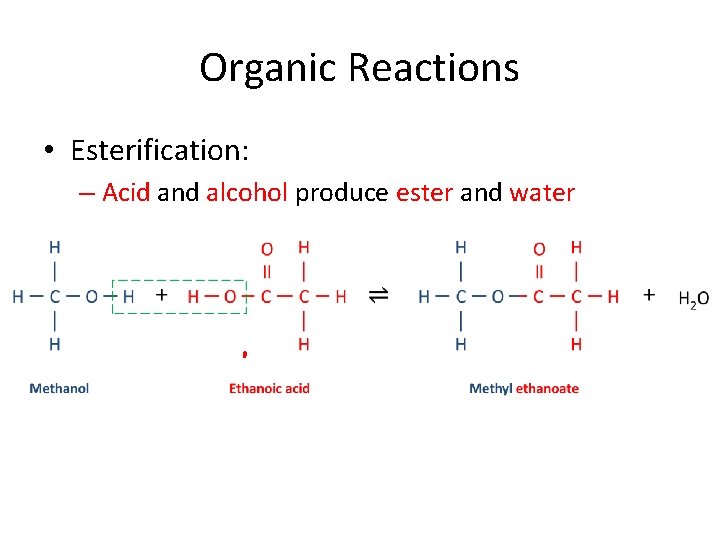
Organic Reactions • Esterification: – Acid and alcohol produce ester and water

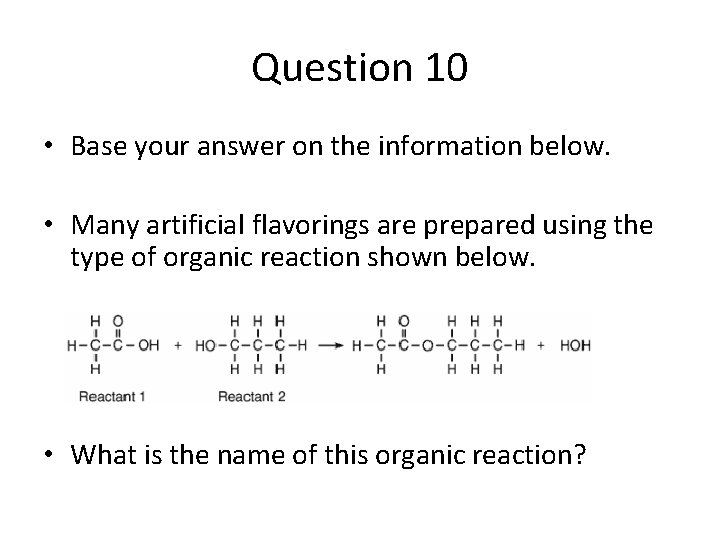
Question 10 • Base your answer on the information below. • Many artificial flavorings are prepared using the type of organic reaction shown below. • What is the name of this organic reaction?

Question 11 • Base your answer on the information below. • Ethyl butanoate is an organic compound that contributes to the odor of pineapple. Ethyl butanoate is one of the products formed by the reaction of butanoic acid with ethanol. • Identify the type of organic reaction that produces the compound that contributes to the odor of pineapple.

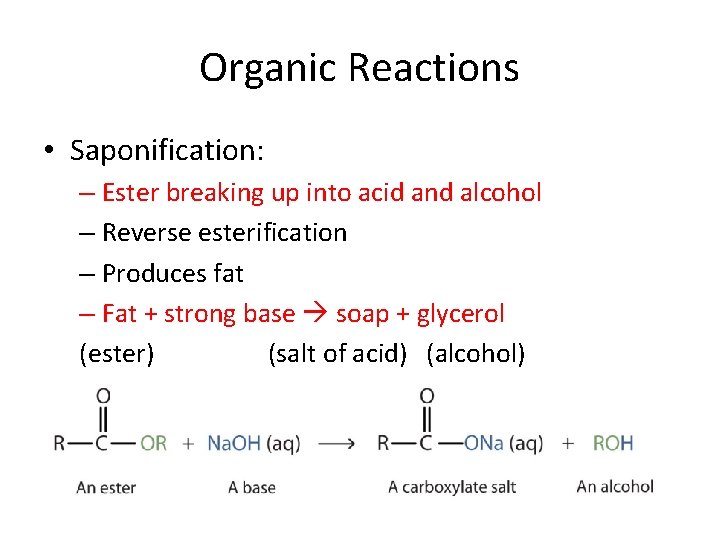
Organic Reactions • Saponification: – Ester breaking up into acid and alcohol – Reverse esterification – Produces fat – Fat + strong base soap + glycerol (ester) (salt of acid) (alcohol)

Question 12 In which kind of reaction is soap one of the products? A) oxidation B) saponification C) neutralization D) fermentation •

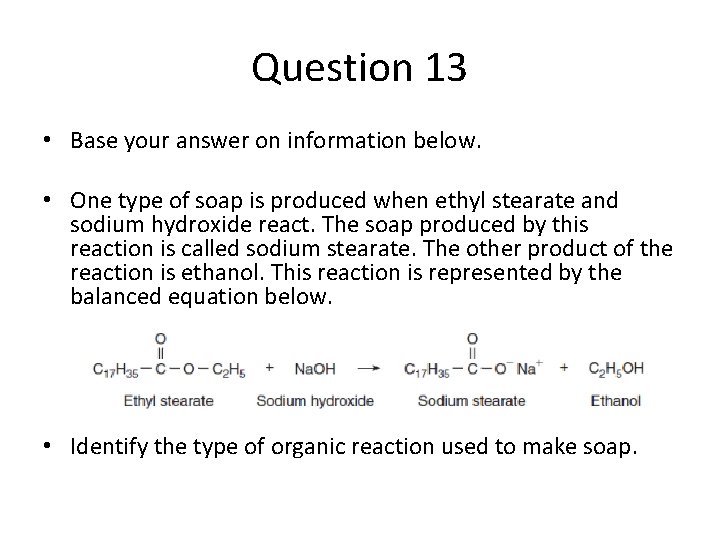
Question 13 • Base your answer on information below. • One type of soap is produced when ethyl stearate and sodium hydroxide react. The soap produced by this reaction is called sodium stearate. The other product of the reaction is ethanol. This reaction is represented by the balanced equation below. • Identify the type of organic reaction used to make soap.

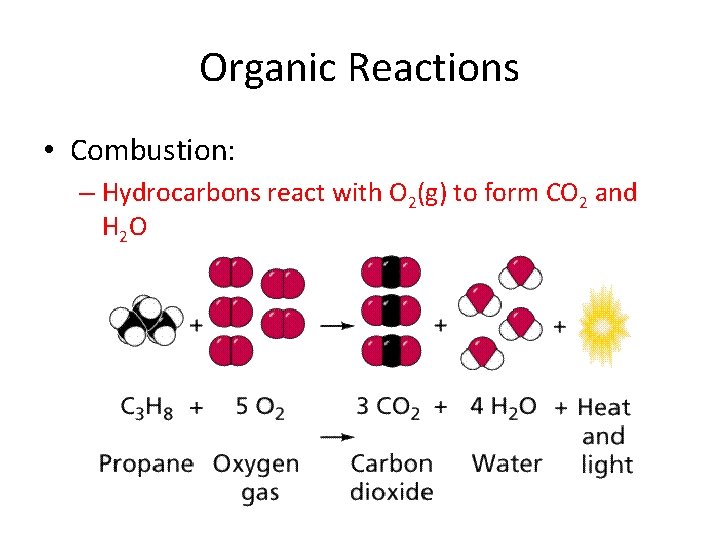
Organic Reactions • Combustion: – Hydrocarbons react with O 2(g) to form CO 2 and H 2 O

Question 14 • Which reaction best represents the complete combustion of ethene? A) C 2 H 4 + HCl → C 2 H 5 Cl B) C 2 H 4 + Cl 2 → C 2 H 4 Cl 2 C) C 2 H 4 + 3 O 2 → 2 CO 2 + 2 H 2 O D) C 2 H 4 + H 2 O → C 2 H 5 OH

Question 15 • Given the incomplete equation for the combustion of ethane: 2 C 2 H 6+ 7 O 2 4 CO 2 + 6 ___ • What is the formula of the missing product? A) CH 3 OH B) HCOOH C) H 2 O D) H 2 O 2

Organic Reactions • Polymerization: – Joining smaller molecules (monomers) together to form one big molecule (polymer) – Used in the formation of plastics Amino acid + amino acid protein Monomer monomer polymer

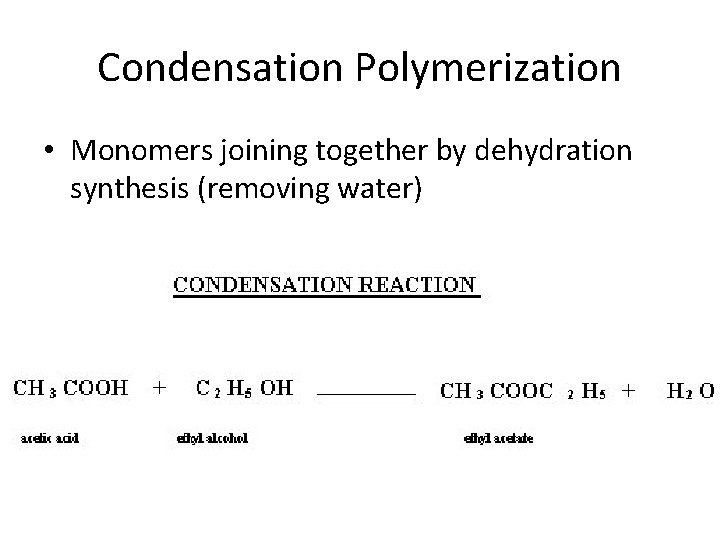
Condensation Polymerization • Monomers joining together by dehydration synthesis (removing water)

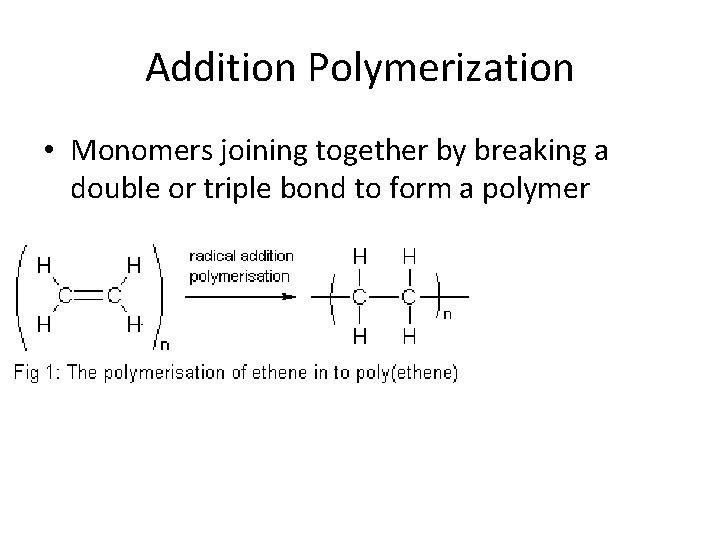
Addition Polymerization • Monomers joining together by breaking a double or triple bond to form a polymer

Question 16 • The formation of large molecules from small molecules is an example of A) polymerization B) decomposition C) saponification D) substitution

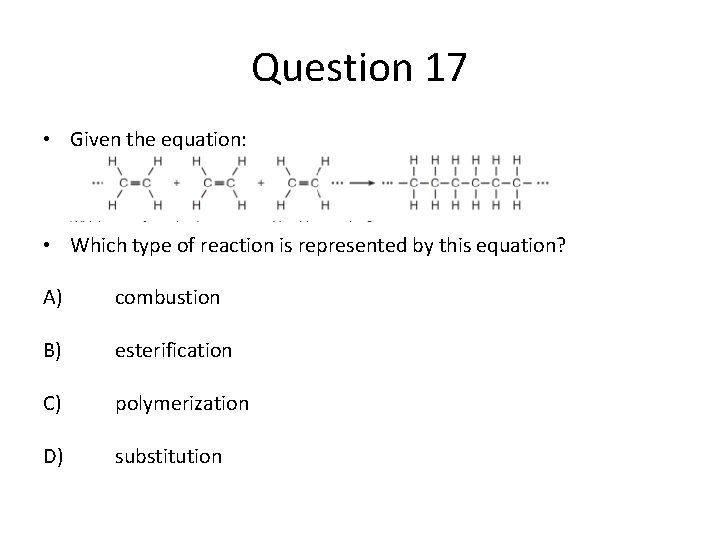
Question 17 • Given the equation: • Which type of reaction is represented by this equation? A) combustion B) esterification C) polymerization D) substitution

Questions The reaction CH 2 + H 2 -> CH 3 is an example of 1. substitution 2. addition 3. esterification 4. fermentation

Questions The products of the fermentation of sugar are ethanol and 1. water 2. oxygen 3. carbon dioxide 4. sulfur dioxide

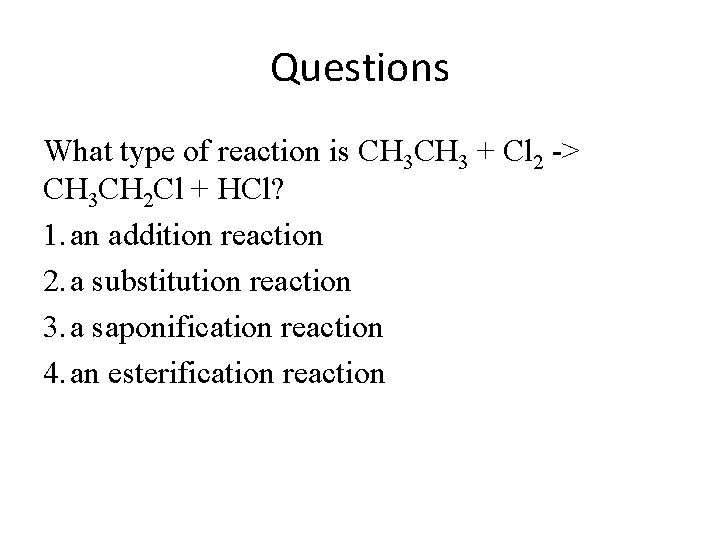
Questions What type of reaction is CH 3 + Cl 2 -> CH 3 CH 2 Cl + HCl? 1. an addition reaction 2. a substitution reaction 3. a saponification reaction 4. an esterification reaction

Questions What substance is made up of monomers joined together in long chains? 1. ketone 2. protein 3. ester 4. acid
 Pyranoses
Pyranoses Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers How to write redox half reactions
How to write redox half reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Identify types of reactions
Identify types of reactions Aim of argumentative essay
Aim of argumentative essay Aim time management
Aim time management Aimlab freezing
Aimlab freezing Aim spark software
Aim spark software Aim isen boyfriend
Aim isen boyfriend Aim chapter 7
Aim chapter 7 Function of meiosis
Function of meiosis Red cat electrochemistry
Red cat electrochemistry Aim in biology
Aim in biology Aim words list
Aim words list Types of aim
Types of aim Emeril
Emeril Reamed slang
Reamed slang Past perfect simple structure
Past perfect simple structure Percussion welding
Percussion welding Spartan aim
Spartan aim Aim who
Aim who Components of rch 2
Components of rch 2 Aim mutual funds
Aim mutual funds Vfr flight plan
Vfr flight plan Aim
Aim Aim dollz icons
Aim dollz icons Direct aim tooling
Direct aim tooling Aim who
Aim who Pans aim doc 10066
Pans aim doc 10066