Organometallic Reactions and Catalysis Chapter 14 Gain or












- Slides: 34

Organometallic Reactions and Catalysis Chapter 14

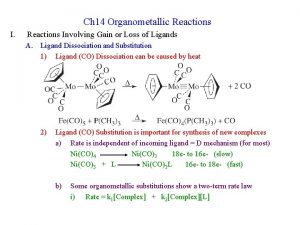
Gain or Loss of Ligands • CO dissociation – In many cases to add another ligand. – Dissociative and associative mechanisms – More complicated reactions. • Dissociation of phosphine (steric effects) – cis-Mo(CO)4 L 2 + CO Mo(CO)5 L + L – Figure 14 -1 and Table 14 -1 (Article) – Rate dependence on cone angle and other factors. • Reaction follows the first-order rate law.

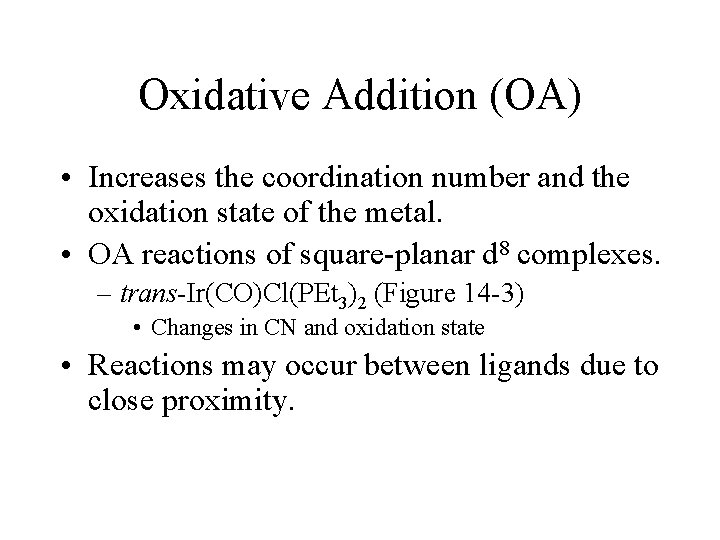
Oxidative Addition (OA) • Increases the coordination number and the oxidation state of the metal. • OA reactions of square-planar d 8 complexes. – trans-Ir(CO)Cl(PEt 3)2 (Figure 14 -3) • Changes in CN and oxidation state • Reactions may occur between ligands due to close proximity.

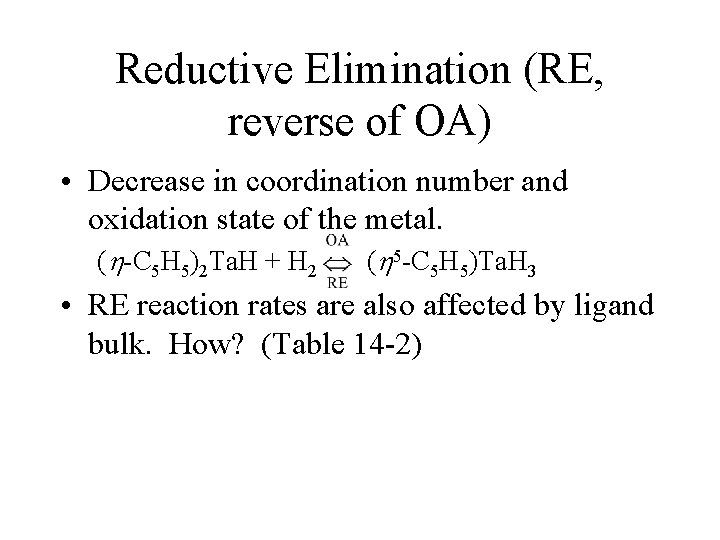
Reductive Elimination (RE, reverse of OA) • Decrease in coordination number and oxidation state of the metal. ( -C 5 H 5)2 Ta. H + H 2 ( 5 -C 5 H 5)Ta. H 3 • RE reaction rates are also affected by ligand bulk. How? (Table 14 -2)

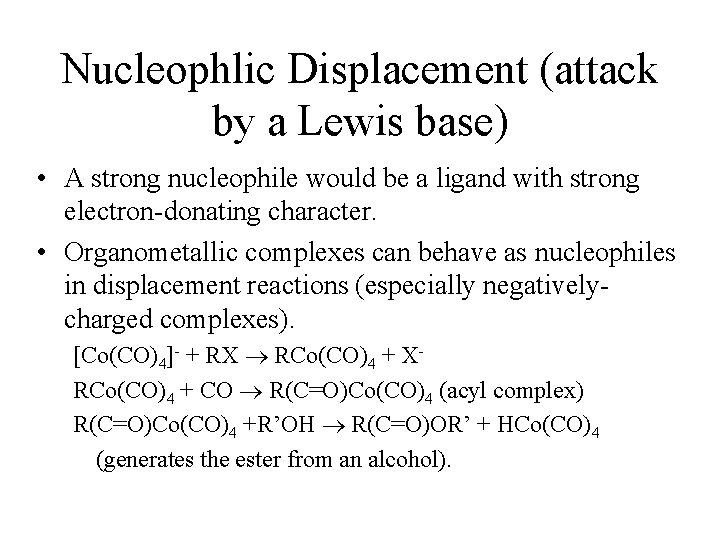
Nucleophlic Displacement (attack by a Lewis base) • A strong nucleophile would be a ligand with strong electron-donating character. • Organometallic complexes can behave as nucleophiles in displacement reactions (especially negativelycharged complexes). [Co(CO)4]- + RX RCo(CO)4 + XRCo(CO)4 + CO R(C=O)Co(CO)4 (acyl complex) R(C=O)Co(CO)4 +R’OH R(C=O)OR’ + HCo(CO)4 (generates the ester from an alcohol).

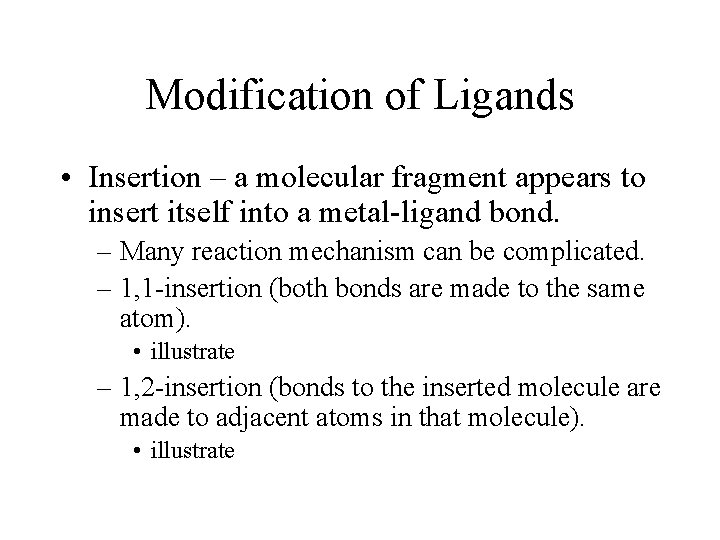
Modification of Ligands • Insertion – a molecular fragment appears to insert itself into a metal-ligand bond. – Many reaction mechanism can be complicated. – 1, 1 -insertion (both bonds are made to the same atom). • illustrate – 1, 2 -insertion (bonds to the inserted molecule are made to adjacent atoms in that molecule). • illustrate

Insertion of Ligands • How is CO inserted in the complex shown previously (1, 1 -insertion)? – Work through this and understand. • 1, 2 -insertions

Hydride Elimination • Transfer of a hydrogen atom from a ligand to a metal. – elimination is the most common type. • position on the alkyl ligand. • Stability – Alkyl complexes that lack hydrogens are more stable. – Coordinatively saturated complexes containing alkyl ligands are also more stable.

Abstraction • Removal of a substituent from a ligand in which the coordination number of the metal does not change (can be removed by an acid).

Organometallic Catalysts (hydroformylation) • Converting terminal alkenes into other organic products. – (oxo process) H and HCO are formally added across a double bond. • Show reaction • Largest-scale industrial process that is homogeneous • Mechanism was suggested by Heck and Breslow in 1961. – Examine each step in the cycle and characterize the reaction according to type. • 18 -, 16 -electron cycling is common.


Comments on the Hydroformylation Mechanism • CO pressure has to be controlled carefully. Why? • Rate-determining step is the insertion of the olefin (alkene). • Main purpose of the reaction is to produce butanal from propene. CH 3 CH=CH 2 CH 3 CH 2 CHO

Union Carbide Hydroformylation Process • Contain Rh and bulky phosphine groups. How will this affect the mechanism? – (Ph 3 P)3 Rh(CO)H • In many cases, the linear/branched ration needs to high. • The catalyst is also water-soluble.


Hydrogenation of Alkenes • Wilkinson’s catalyst – Show reaction (alkenes and alkynes) • Show mechanism and discuss – Step 9 is slow, the sequence 1 2 3 is favored. – The rate determining step is insertion, 4. • The catalyst hydrogenizes terminal and internal olefins. • Examine Table 14 -3.


Hydrogenation Catalyst • Selective hydrogenation can be observed if the ligand contains multiple double bonds. • Another hydrogenation catalyst, (PPh 3)2 Rh(CO)H, is very selective toward hydrogenation of only terminal olefins. • Asymmetric hydrogenation – If the catalyst, [L 2 RHS 2]+, bears an optically active diphosphane, prochiral unsaturated molecules can be hydrogenated to chiral products (enantiomeric selectivity). • L-Dopa (treatment of Parkinson’s disease).

Alkene Metathesis • Demonstrate – Propene and 1 -butene (what are the 4 new products that may form from methathesis? ) • Ring-opening metathesis (ROM) • Chauvin mechanism is most widely accepted. – Involved a carbene complex – The carbene reacts with an alkene to form a metallocyclobutane intermediate. The intermediate can either revert to reactants or form new products. – Schrock metathesis catalysts are most effective and the most studied (available commercially). • Ring-closing methathesis (page 545)


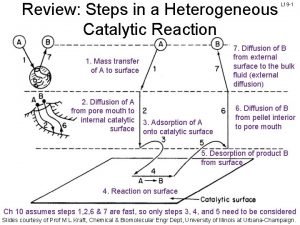
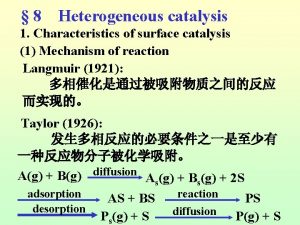
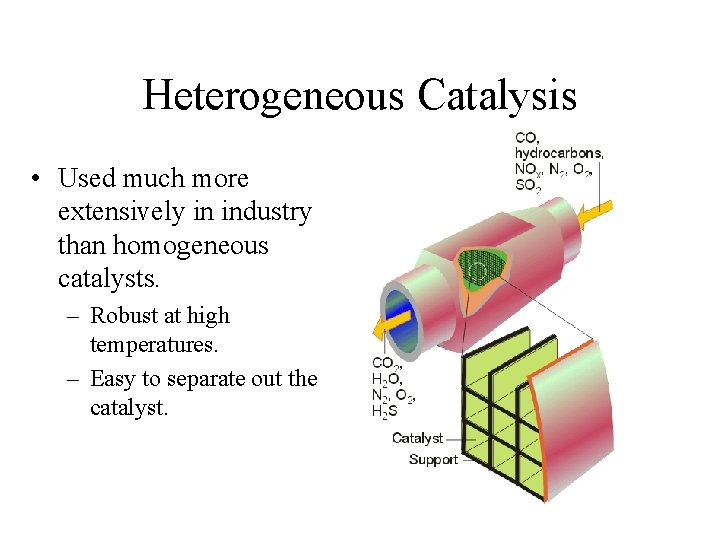
Heterogeneous Catalysis • Used much more extensively in industry than homogeneous catalysts. – Robust at high temperatures. – Easy to separate out the catalyst.

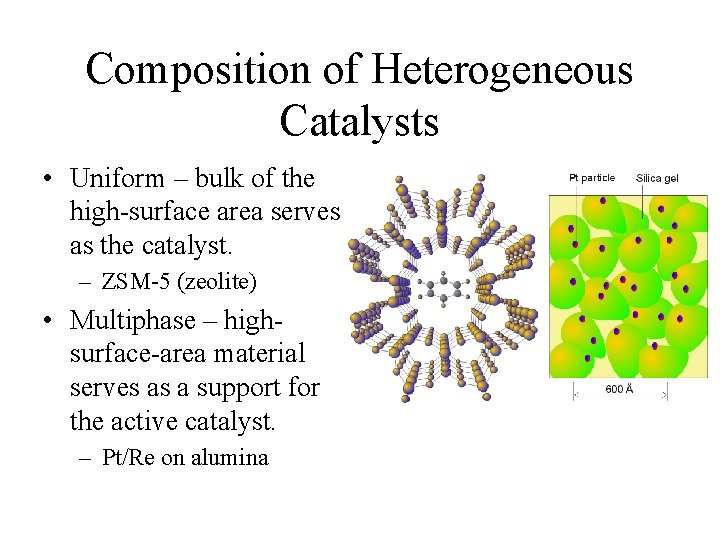
Composition of Heterogeneous Catalysts • Uniform – bulk of the high-surface area serves as the catalyst. – ZSM-5 (zeolite) • Multiphase – highsurface-area material serves as a support for the active catalyst. – Pt/Re on alumina

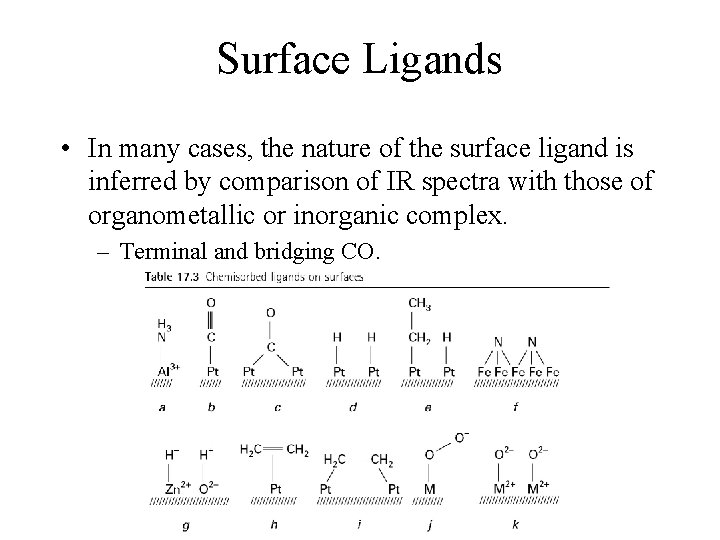
Surface Ligands • In many cases, the nature of the surface ligand is inferred by comparison of IR spectra with those of organometallic or inorganic complex. – Terminal and bridging CO.

Surface-Sensitive Techniqus • Temperature-programmed desorption (mass spectroscopy). • Photoelectron spectroscopy (XPS and Auger) • Low-Energy Electron Diffraction (LEED) • Scanning Tunneling and Atomic Force Microscopies. • Vibrational Techniques (RAIRS and HREELS). • Many othes.

Catalytic Steps • Many parallels can be drawn in comparison to organometallic mechanisms studied previously. • Chemisorption and physisorption – Similar to interactions present in complexes with low oxidation states. – Physisorption and chemisorption.

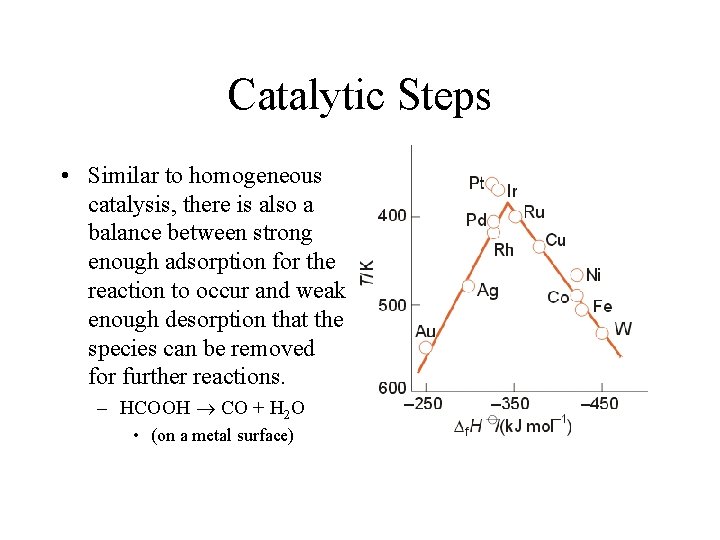
Catalytic Steps • Similar to homogeneous catalysis, there is also a balance between strong enough adsorption for the reaction to occur and weak enough desorption that the species can be removed for further reactions. – HCOOH CO + H 2 O • (on a metal surface)

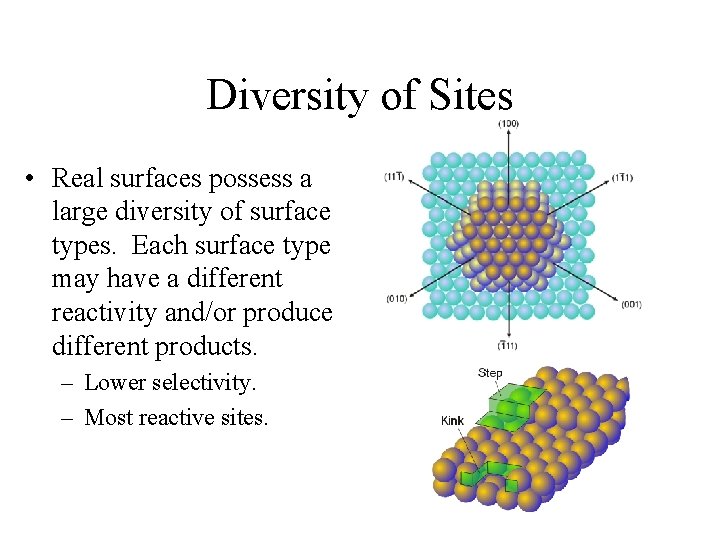
Diversity of Sites • Real surfaces possess a large diversity of surface types. Each surface type may have a different reactivity and/or produce different products. – Lower selectivity. – Most reactive sites.

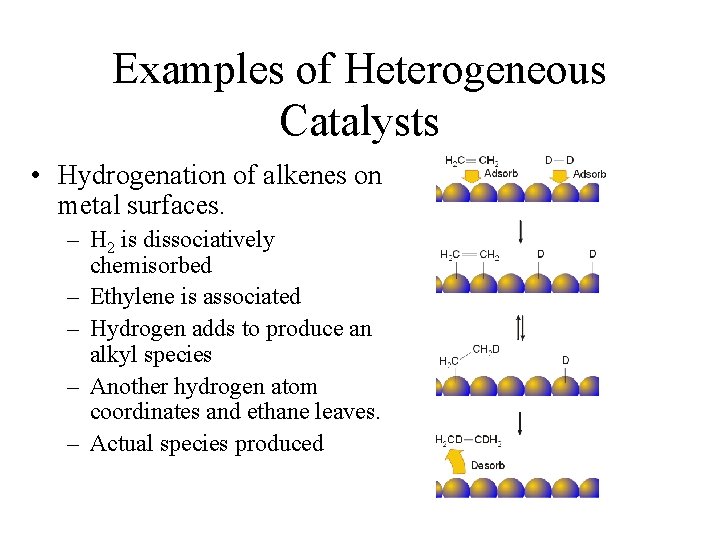
Examples of Heterogeneous Catalysts • Hydrogenation of alkenes on metal surfaces. – H 2 is dissociatively chemisorbed – Ethylene is associated – Hydrogen adds to produce an alkyl species – Another hydrogen atom coordinates and ethane leaves. – Actual species produced

Ziegler-Natta Polymerization • Ti. Cl 4 + Al(C 2 H 5)3 – A titanium alkyl complex is produce. – Ethylene or propylene associates and inserts into the titanium-carbon bond. – The 1, 2 -insertion continues. – Mechanism has proved difficult to understand. In Miessler and Tarr

Fundamental Studies of Hydrocarbons on Platinum Surfaces • The techniques used. – Reflection-absorption infrared spectroscopy. – Auger electron spectroscopy – Temperature-programmed desorption/reaction spectroscopy. – Others as needed.

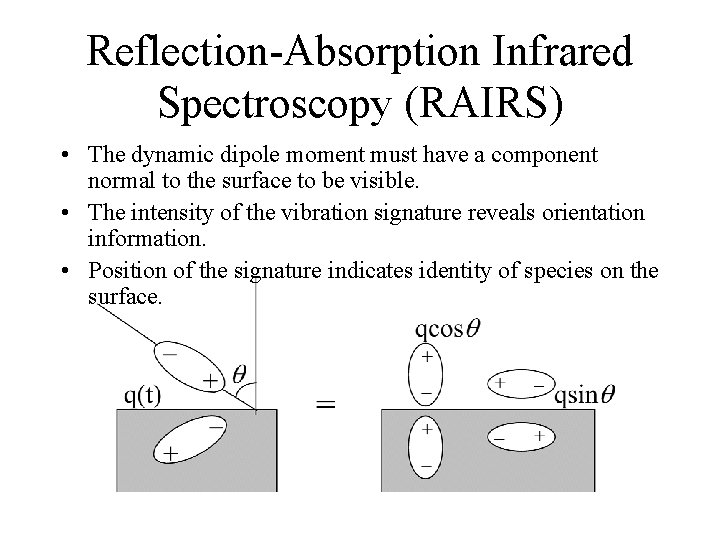
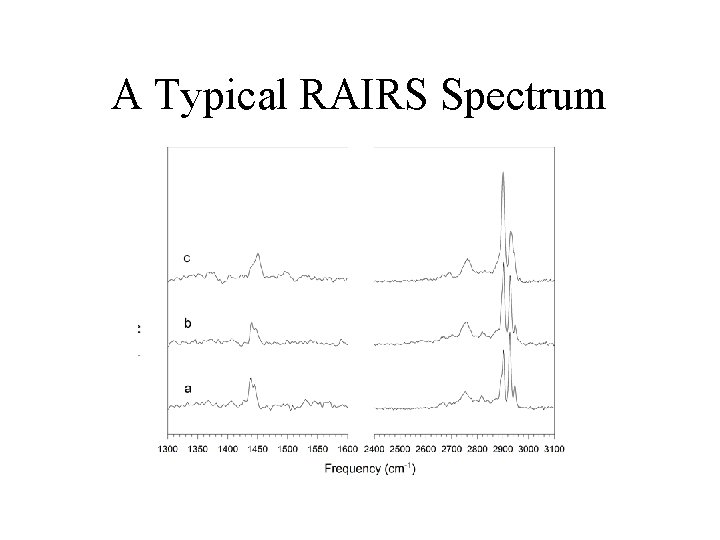
Reflection-Absorption Infrared Spectroscopy (RAIRS) • The dynamic dipole moment must have a component normal to the surface to be visible. • The intensity of the vibration signature reveals orientation information. • Position of the signature indicates identity of species on the surface.

A Typical RAIRS Spectrum

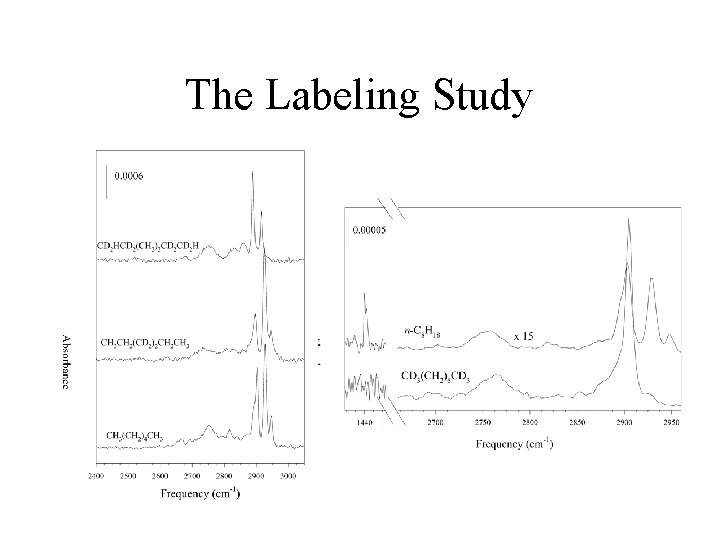
The Labeling Study

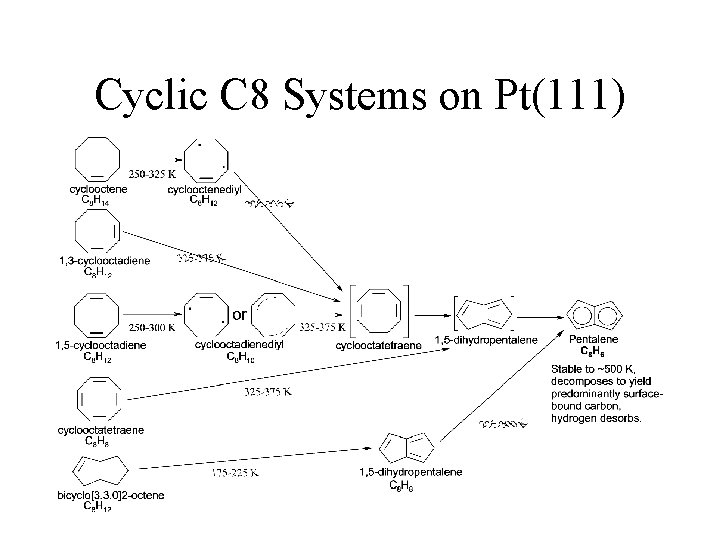
Cyclic C 8 Systems on Pt(111)

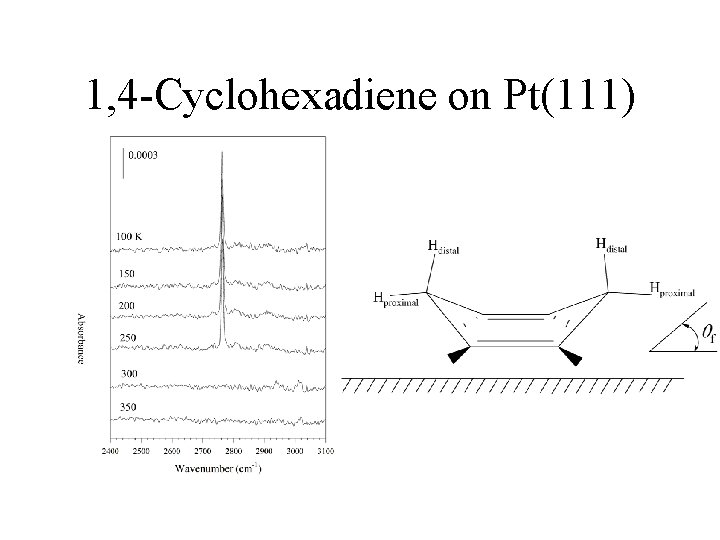
1, 4 -Cyclohexadiene on Pt(111)
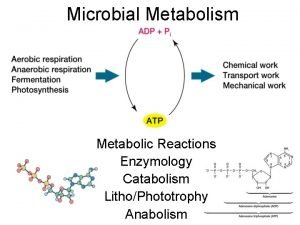
 Energy catalysis and biosynthesis
Energy catalysis and biosynthesis Bonding in organometallic compounds
Bonding in organometallic compounds Sidwick rule
Sidwick rule Organometallic
Organometallic Organometallic
Organometallic Organometallic complex
Organometallic complex Decao
Decao Apoenzyme
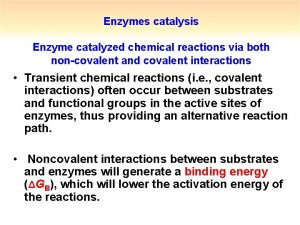
Apoenzyme What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
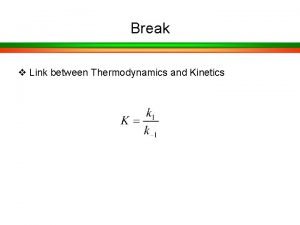
Specific acid base catalysis Km in enzyme kinetics
Km in enzyme kinetics Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Boreskov institute of catalysis
Boreskov institute of catalysis Sodium hydroxide iupac id sodium oxidanide
Sodium hydroxide iupac id sodium oxidanide Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Redox reaction example
Redox reaction example Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 19 redox reactions study guide answers
Chapter 19 redox reactions study guide answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 20 oxidation-reduction reactions answer key
Chapter 20 oxidation-reduction reactions answer key Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions.