Ch 14 Organometallic Reactions Involving Gain or Loss

- Slides: 13

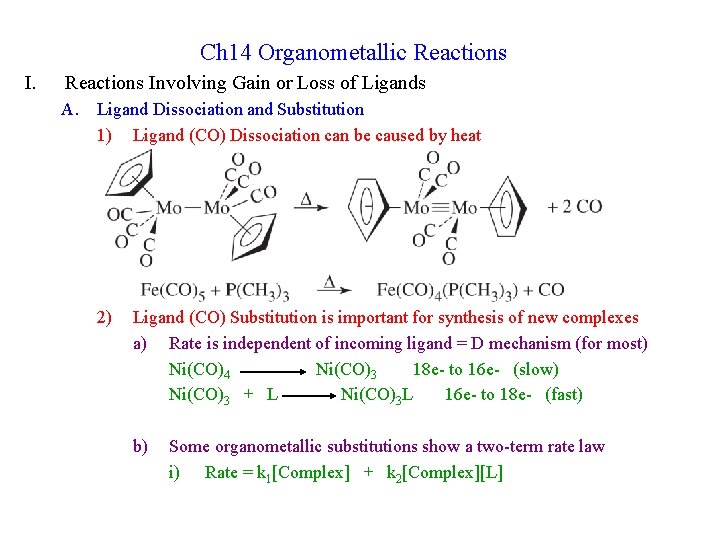
Ch 14 Organometallic Reactions Involving Gain or Loss of Ligands A. Ligand Dissociation and Substitution 1) Ligand (CO) Dissociation can be caused by heat 2) Ligand (CO) Substitution is important for synthesis of new complexes a) Rate is independent of incoming ligand = D mechanism (for most) Ni(CO)4 Ni(CO)3 18 e- to 16 e- (slow) Ni(CO)3 + L Ni(CO)3 L 16 e- to 18 e- (fast) b) Some organometallic substitutions show a two-term rate law i) Rate = k 1[Complex] + k 2[Complex][L]

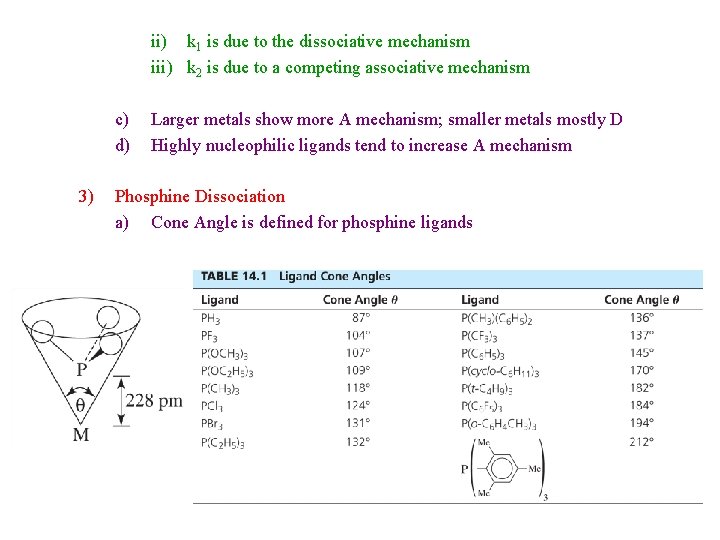
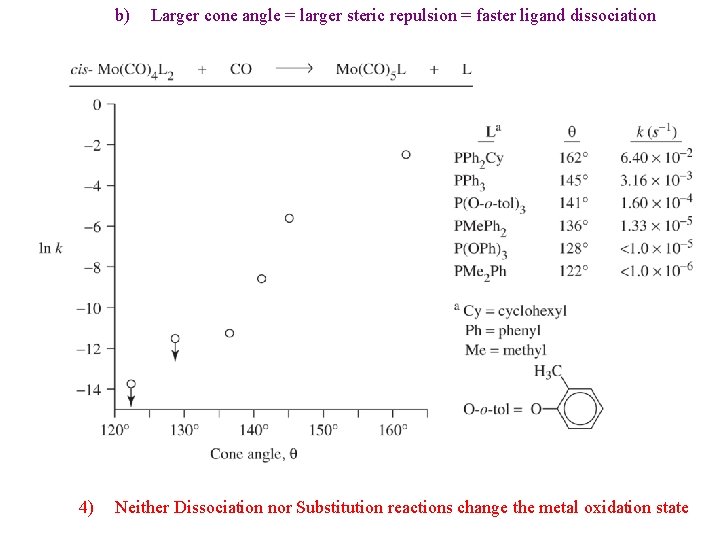
ii) k 1 is due to the dissociative mechanism iii) k 2 is due to a competing associative mechanism c) d) 3) Larger metals show more A mechanism; smaller metals mostly D Highly nucleophilic ligands tend to increase A mechanism Phosphine Dissociation a) Cone Angle is defined for phosphine ligands

b) 4) Larger cone angle = larger steric repulsion = faster ligand dissociation Neither Dissociation nor Substitution reactions change the metal oxidation state

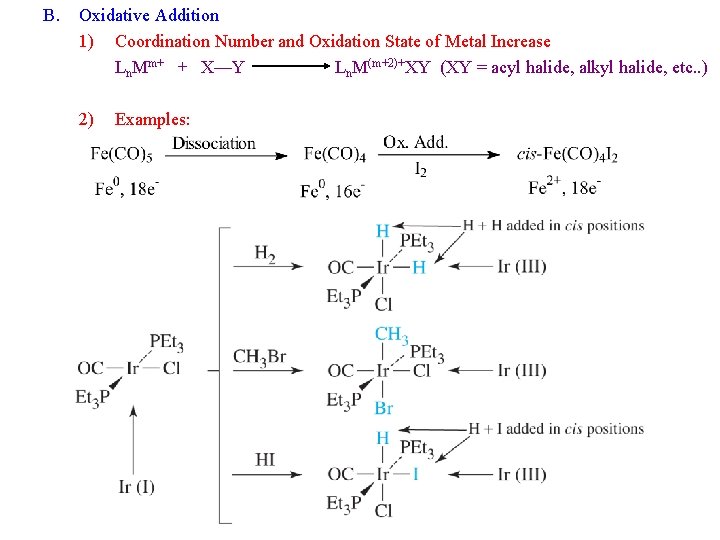
B. Oxidative Addition 1) Coordination Number and Oxidation State of Metal Increase Ln. Mm+ + X—Y Ln. M(m+2)+XY (XY = acyl halide, alkyl halide, etc. . ) 2) Examples:

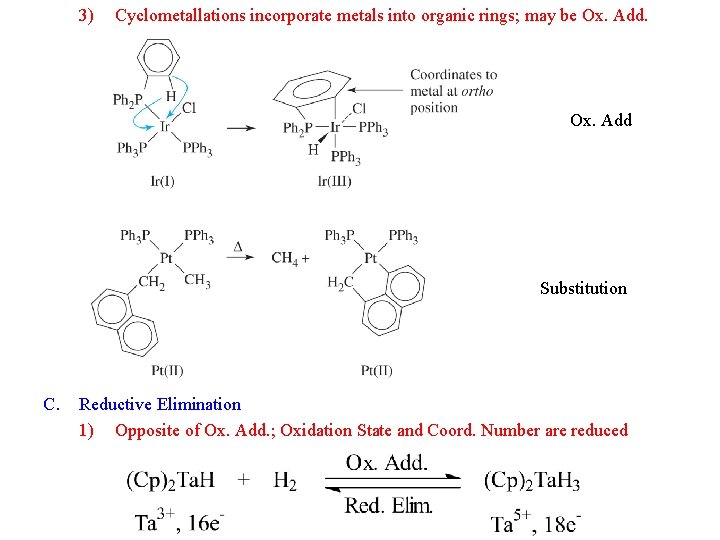
3) Cyclometallations incorporate metals into organic rings; may be Ox. Add Substitution C. Reductive Elimination 1) Opposite of Ox. Add. ; Oxidation State and Coord. Number are reduced

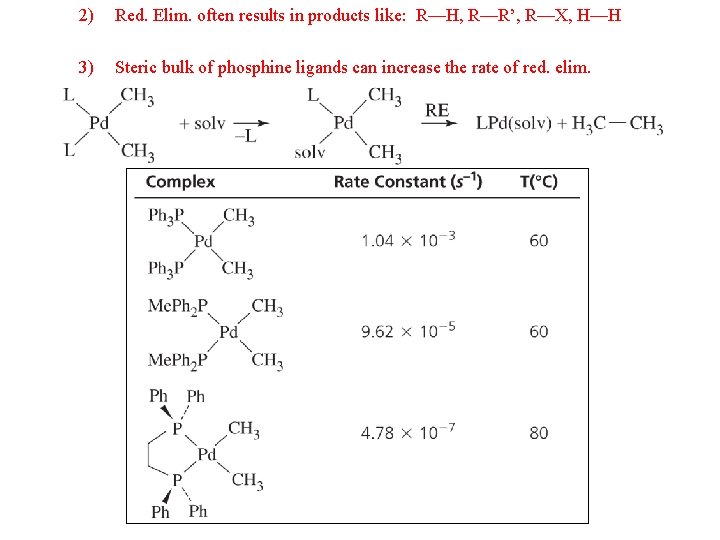
2) Red. Elim. often results in products like: R—H, R—R’, R—X, H—H 3) Steric bulk of phosphine ligands can increase the rate of red. elim.

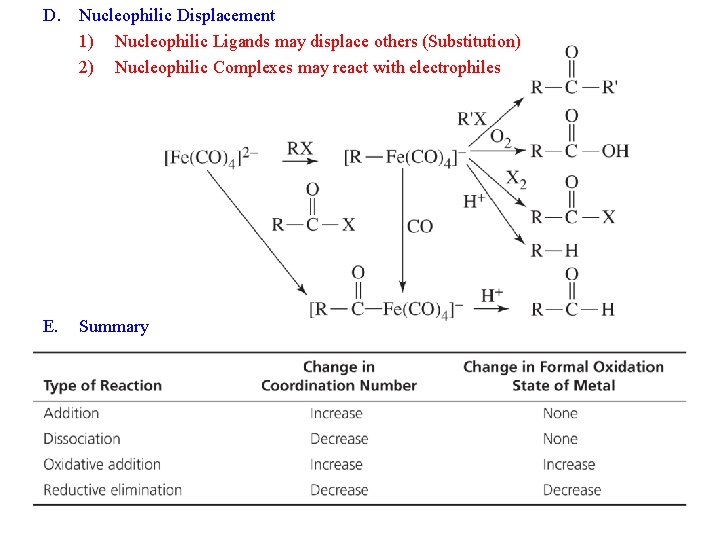
D. Nucleophilic Displacement 1) Nucleophilic Ligands may displace others (Substitution) 2) Nucleophilic Complexes may react with electrophiles E. Summary

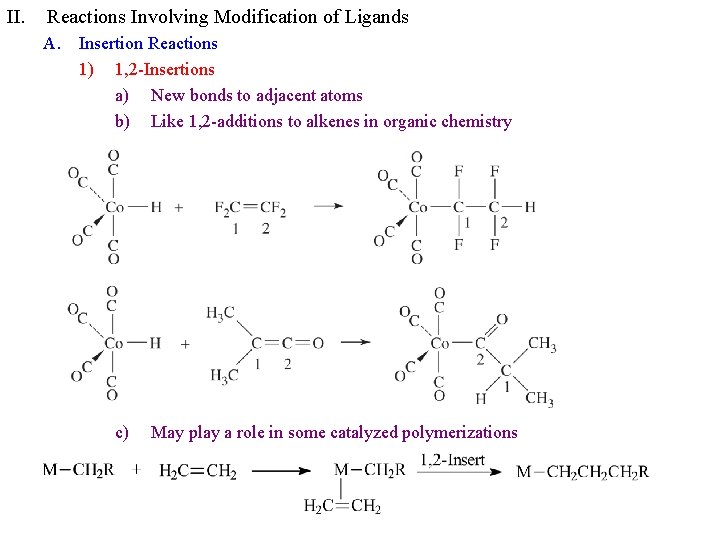
II. Reactions Involving Modification of Ligands A. Insertion Reactions 1) 1, 2 -Insertions a) New bonds to adjacent atoms b) Like 1, 2 -additions to alkenes in organic chemistry c) May play a role in some catalyzed polymerizations

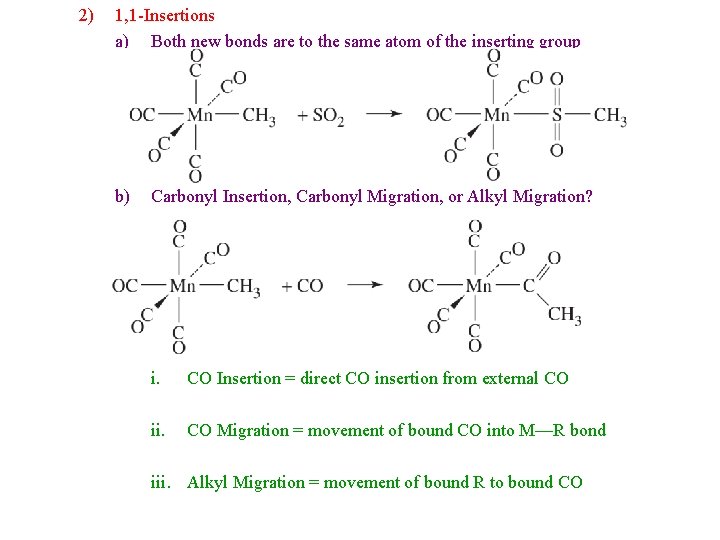
2) 1, 1 -Insertions a) Both new bonds are to the same atom of the inserting group b) Carbonyl Insertion, Carbonyl Migration, or Alkyl Migration? i. CO Insertion = direct CO insertion from external CO ii. CO Migration = movement of bound CO into M—R bond iii. Alkyl Migration = movement of bound R to bound CO


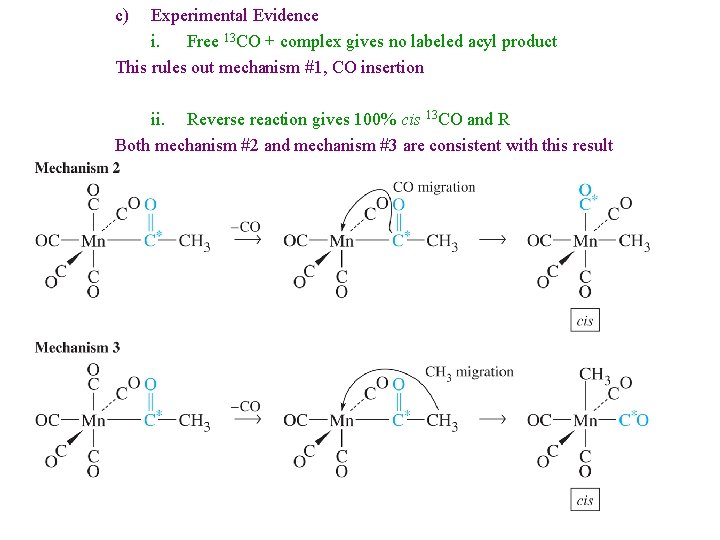
c) Experimental Evidence i. Free 13 CO + complex gives no labeled acyl product This rules out mechanism #1, CO insertion ii. Reverse reaction gives 100% cis 13 CO and R Both mechanism #2 and mechanism #3 are consistent with this result

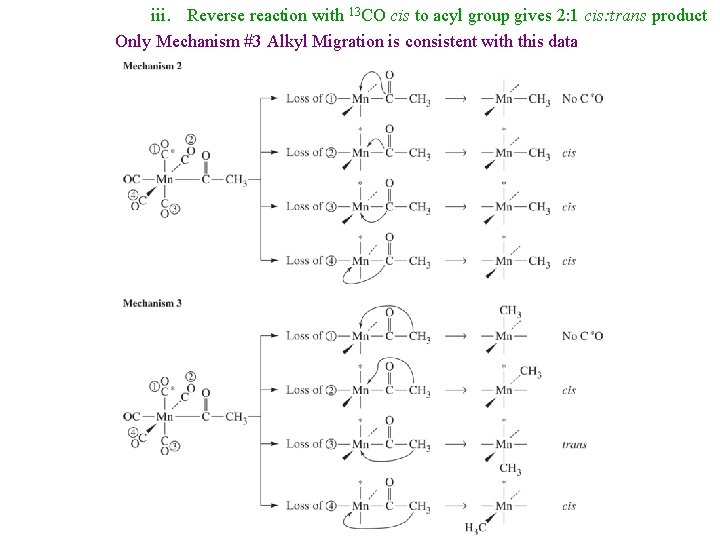
iii. Reverse reaction with 13 CO cis to acyl group gives 2: 1 cis: trans product Only Mechanism #3 Alkyl Migration is consistent with this data

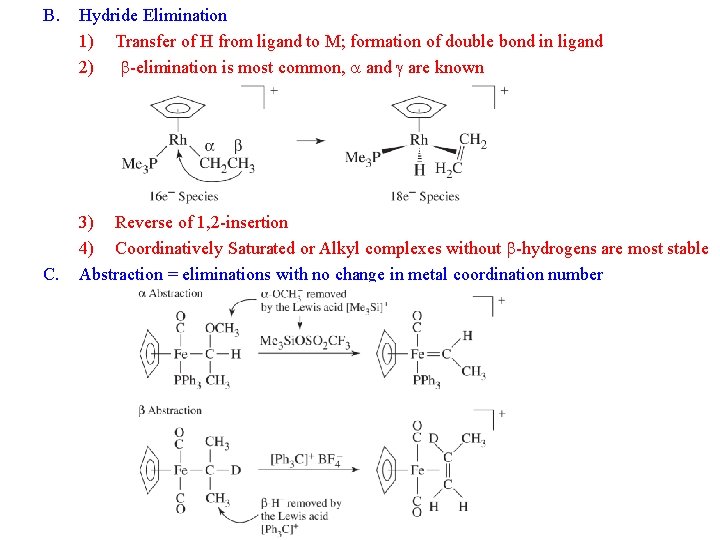
B. C. Hydride Elimination 1) Transfer of H from ligand to M; formation of double bond in ligand 2) b-elimination is most common, a and g are known 3) Reverse of 1, 2 -insertion 4) Coordinatively Saturated or Alkyl complexes without b-hydrogens are most stable Abstraction = eliminations with no change in metal coordination number
 What variables affect enzyme activity in each of the graphs
What variables affect enzyme activity in each of the graphs Organometallic
Organometallic Classification of organometallic compounds
Classification of organometallic compounds 오비탈
오비탈 Ean rule organometallic compounds
Ean rule organometallic compounds Organometallic
Organometallic Gain / loss
Gain / loss Semiconductor
Semiconductor Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet 10 examples of redox reaction
10 examples of redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Scrap account
Scrap account