Lesson 17 Heterogeneous and cloud processes Heterogeneous and



- Slides: 17

Lesson 17

Heterogeneous and cloud processes

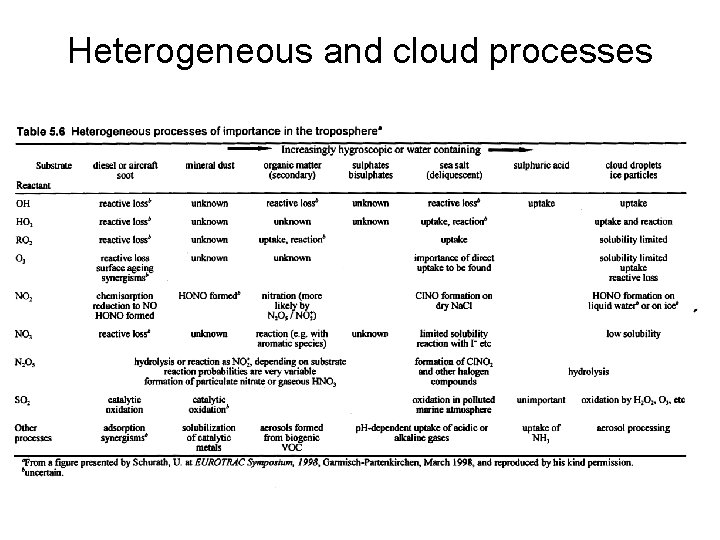
Heterogeneous and cloud processes • Wide range of physical and chemical of substrate surfaces for heterogeneous reactions to take place. • Clouds have approximately 1 billion water droplets per cubic meter. • Lots of surface area for reactions to take place. • Both ice (cirrus) and water clouds exist. • Other substrates are less abundant but are still significant e. g. sulfates, black carbon, organic compounds, sea salt.

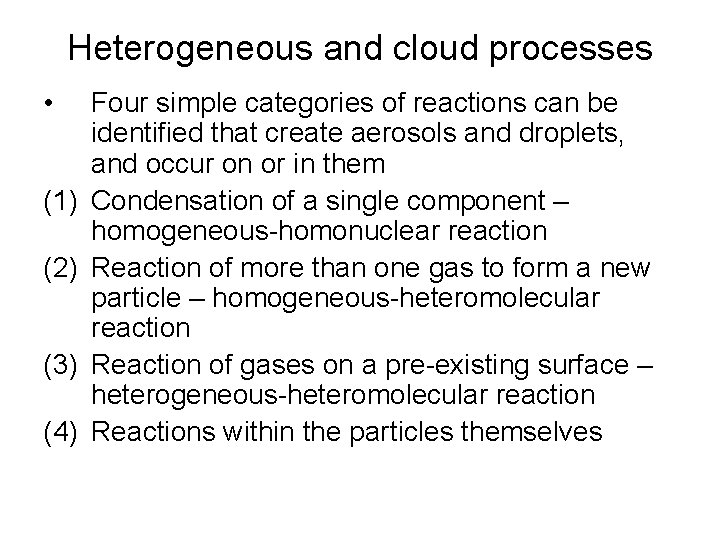
Heterogeneous and cloud processes • (1) (2) (3) (4) Four simple categories of reactions can be identified that create aerosols and droplets, and occur on or in them Condensation of a single component – homogeneous-homonuclear reaction Reaction of more than one gas to form a new particle – homogeneous-heteromolecular reaction Reaction of gases on a pre-existing surface – heterogeneous-heteromolecular reaction Reactions within the particles themselves

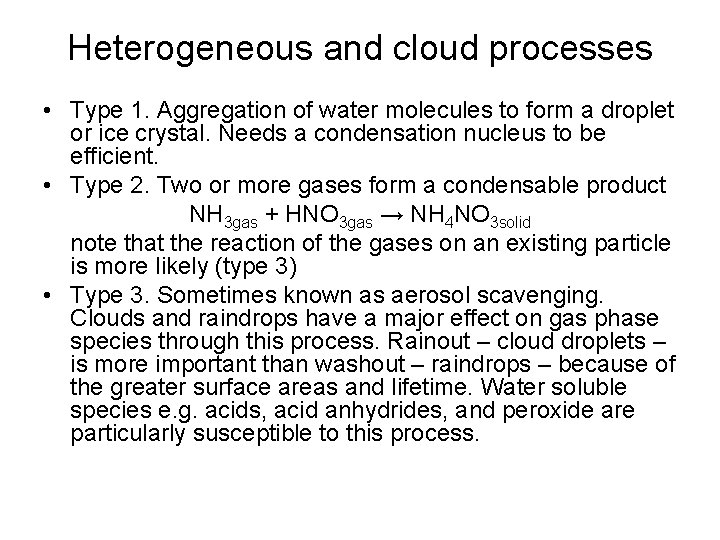
Heterogeneous and cloud processes • Type 1. Aggregation of water molecules to form a droplet or ice crystal. Needs a condensation nucleus to be efficient. • Type 2. Two or more gases form a condensable product NH 3 gas + HNO 3 gas → NH 4 NO 3 solid note that the reaction of the gases on an existing particle is more likely (type 3) • Type 3. Sometimes known as aerosol scavenging. Clouds and raindrops have a major effect on gas phase species through this process. Rainout – cloud droplets – is more important than washout – raindrops – because of the greater surface areas and lifetime. Water soluble species e. g. acids, acid anhydrides, and peroxide are particularly susceptible to this process.

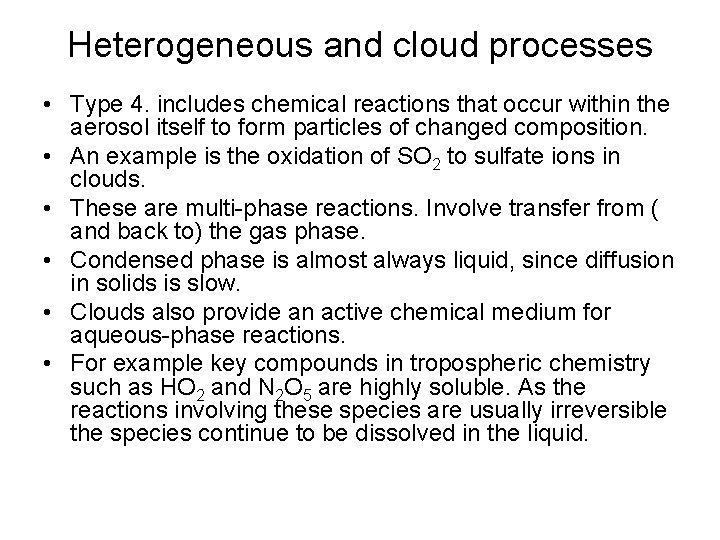
Heterogeneous and cloud processes • Type 4. includes chemical reactions that occur within the aerosol itself to form particles of changed composition. • An example is the oxidation of SO 2 to sulfate ions in clouds. • These are multi-phase reactions. Involve transfer from ( and back to) the gas phase. • Condensed phase is almost always liquid, since diffusion in solids is slow. • Clouds also provide an active chemical medium for aqueous-phase reactions. • For example key compounds in tropospheric chemistry such as HO 2 and N 2 O 5 are highly soluble. As the reactions involving these species are usually irreversible the species continue to be dissolved in the liquid.

Heterogeneous and cloud processes • The formation of HNO 3 from N 2 O 5 + H 2 O → HNO 3 + HNO 3 The gas-to-aqueous transfer of N 2 O 5 is limited by gasphase diffusion (Henry’s Law) while the reaction is so fast that the dissolution of N 2 O 5 can be considered as irreversible. • Cloud reactions further influence the oxides of nitrogen by removing the NO 3 radical. Because the radical has a long lifetime it can be incorporated into cloud water. NO 3 is not particularly soluble in pure water, but if the water contains chlorine ions (e. g. near or over the oceans) then: NO 3 + Cl- → NO 3 - + Cl Note that this reaction releases the Cl atom. • A similar sequence is also responsible for the removal of the PAN molecules.

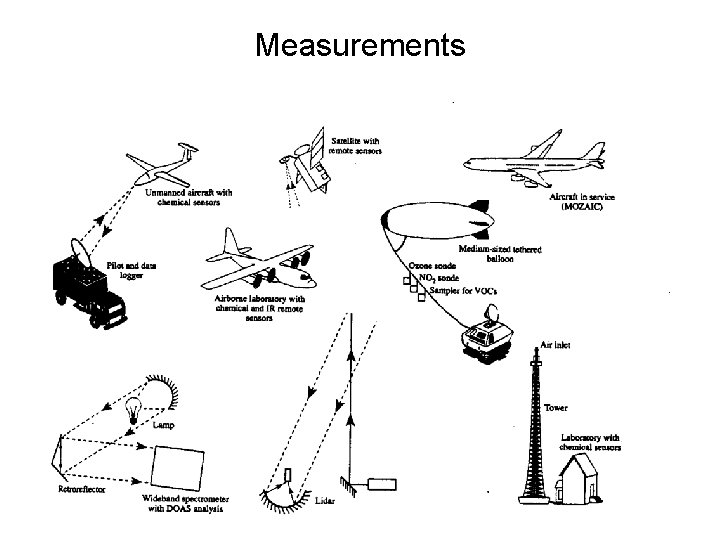
Measurements

Tropospheric measurements • Both in-situ and remote measurements are made for tropospheric species. • Earliest in-situ measurements were for ozone. Schonbeim measured ozone by noting that ozone released the iodine atom when reacting with potassium iodide. Known as a chemical sensor. • Present chemical detectors include (1) gas chromatography. (2)`Chemiluminescence (3) Resonance fluorescence (4) Mass spectroscopy (5) Absorption and emission spectroscopy

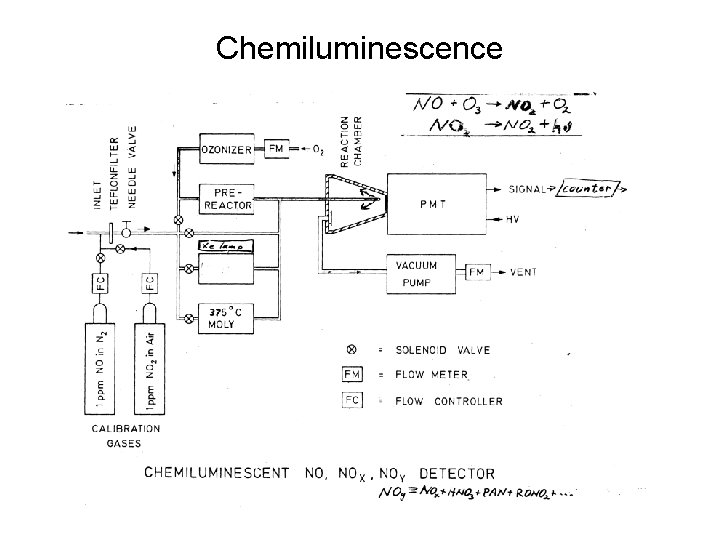
Chemiluminescence

Chemiluminescence • Certain exothermic chemical reactions lead to the emission of light. • For example: NO + O 3 → NO 2 + hν NO 2 + luminol → hν • By converting other N species to NO one can broaden the use of the chemiluminescence technique to other species, e. g. NOY, HNO 3, or PAN

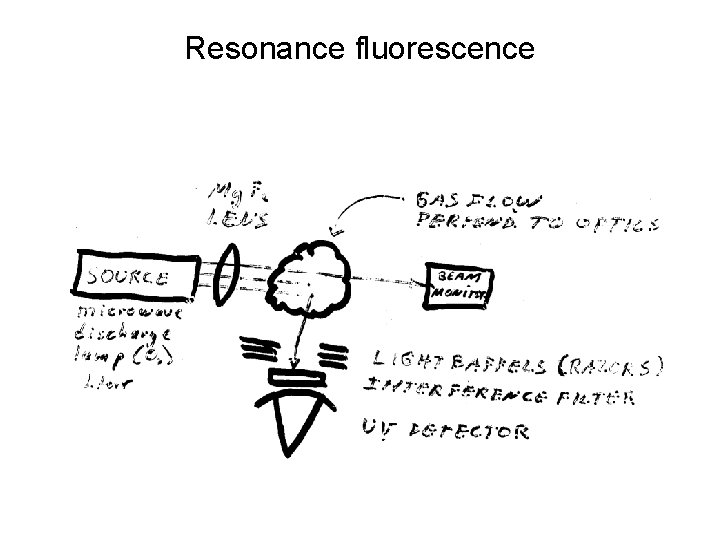
Resonance fluorescence

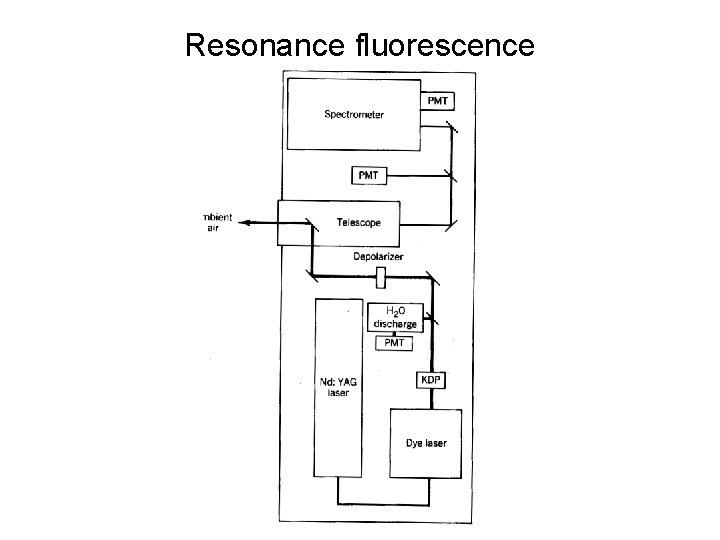
Resonance fluorescence • Spectroscopy is at the heart of all remote sensing techniques, however it can also be used as an in-situ technique. • In resonance fluorescence one excites the atom or molecule at a resonant frequency – principally to increase the absorption cross section. Has been used to detect the OH radical using a laser as the excitation source. • However this can cause a problem as the same wavelength that excites the OH also dissociates the ozone molecule O 3 + hν → O 2* + O(1Δg) + H 2 O → OH + OH • Hence the measurement can actually produce OH.

Resonance fluorescence

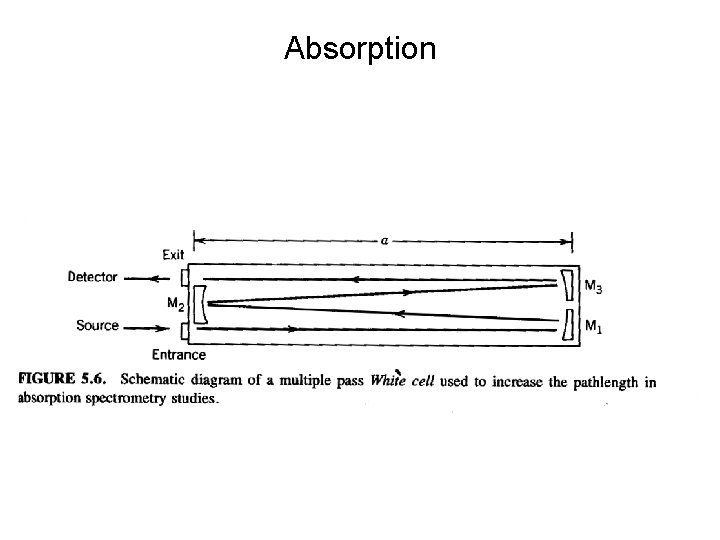
Absorption

Absorption • Ozone can be measured by passing the air through a long-pathlength chamber and measuring the absorption at 253. 7 nm. • 253. 7 nm is emitted by a quartz envelope mercury lamp. • Long path lengths are generated by placing mirrors at the ends of the chamber to produce multiple reflections • Can also be used to measure CO (in the infra-red) • If we use the sun as the source then we can measure the column amount of the gas. Ozone and sulfur dioxide are measured using this technique. • Dobson spectrophotometer measures ozone at ultraviolet wavelengths

LIDAR techniques • Light detection and ranging • Uses pulses of laser radiation. • Time of return of the pulse gives the altitude of the fluorescence. • For resonance fluorescence – the strength of the returned signal gives the density of the species at that altitude. Used to measure altitude profiles of aerosols. • For absorption the strength of the returned signal gives the optical path to and from that altitude. By using two wavelengths, one strongly absorbed by ozone, the other not, one cancel out the effect of aerosols.