Chapter 2 Section 1 SCIENTIFIC METHOD The Scientific







































- Slides: 49

Chapter 2 Section 1 • SCIENTIFIC METHOD

The Scientific Method (again) • You should know the scientific method by now but here is a refresher just in case you forgot. – Make observations – Collect data – Formulate and test hypotheses by experimenting. – Interpret results and accept or reject hypothesis. Revise hypothesis and retest if necessary. – State/publish conclusions. • Make sure you know it for the test.

Units of Measurement Chapter 2 Section 2

The difference between mass and weight • Mass is a quantity of matter. • Weight is the measure of the gravitational pull on matter. • Mass does not depend on gravity.

• We do NOT ALWAYS use numbers in CHEMISTRY…we SOMETIMES use measurements. • A measurement ALWAYS includes a unit. • No unit…no credit.

Ch 2 -2 Units of Measurement

• Quantity – something that has magnitude, size or amount. • Measurements are quantitative--they represent quantities

Système International d'Unités (SI) The SI unit system consists of seven base units. 1. meter (m) - The base unit of length; determined by the length of the path traveled by light in a vacuum during a time interval of 1/299, 792, 458 of a second. 2. kilogram(kg) - The base unit of mass; equal to the mass of the international prototype of the kilogram (commissioned by the CGPM in 1889). 3. second (s) - The base unit of time; duration of 9, 192, 631, 770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state in the cesium 133 atom.

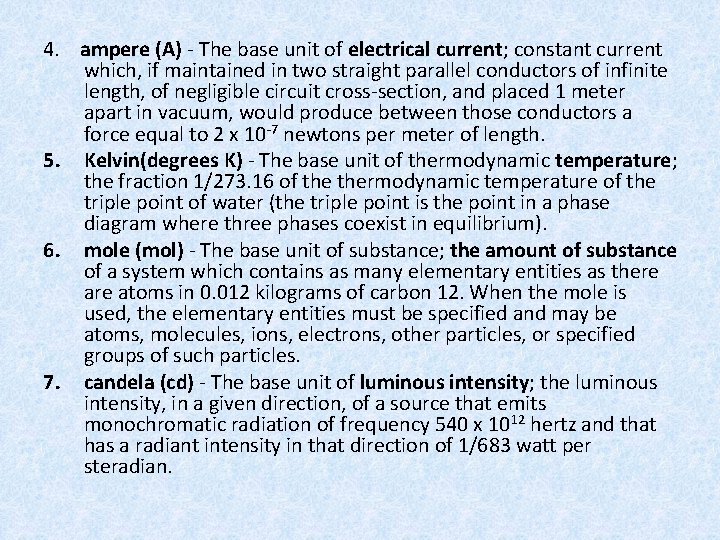
4. ampere (A) - The base unit of electrical current; constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circuit cross-section, and placed 1 meter apart in vacuum, would produce between those conductors a force equal to 2 x 10 -7 newtons per meter of length. 5. Kelvin(degrees K) - The base unit of thermodynamic temperature; the fraction 1/273. 16 of thermodynamic temperature of the triple point of water (the triple point is the point in a phase diagram where three phases coexist in equilibrium). 6. mole (mol) - The base unit of substance; the amount of substance of a system which contains as many elementary entities as there atoms in 0. 012 kilograms of carbon 12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. 7. candela (cd) - The base unit of luminous intensity; the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 x 1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian.

• From these SEVEN base units, many other units are derived. • For example: – area = meter squared = m 2 – velocity = meter per second = m / s – acceleration =meter per second squared = m/s 2 – force (newton) = N = m·kg/s 2 – pressure (pascal) = Pa = N/m 2 = kg/ms 2 – energy, work (joule) = J = N·m = m 2·kg/s 2 – molar mass = g/mole – and the list goes on and on

Density- a derived unit • D = m/V = mass/volume = g/cm 3 = g/ml = kg/L • Using the information above, what other units can you set equal to one another? • That’s right --- 1 ml = 1 cm 3

Try this: • What is the density of a block of marble that occupies 310 cm 3 and has a mass of 853 g? • D = 2. 75 g/cm 3 • Iron has a known density of 7. 87 g/cm 3. What would be the mass of a 2500 cm 3 piece of iron? • m = 19700 g • Mercury has a density of 13. 5 g/cm 3. How much space would 50. 0 g of mercury occupy? • V = 3. 70 cm 3

Prefixes In order to make many of the SI units easier to manage a system of SI prefixes is used. Example: The distance from Earth to the Sun is approximately 150, 000, 000 meters. You could write this as 1. 50 x 1011 m, 150 gigameters or 150 Gm. See TABLE 2 -2 on page 35 for what you need to know.

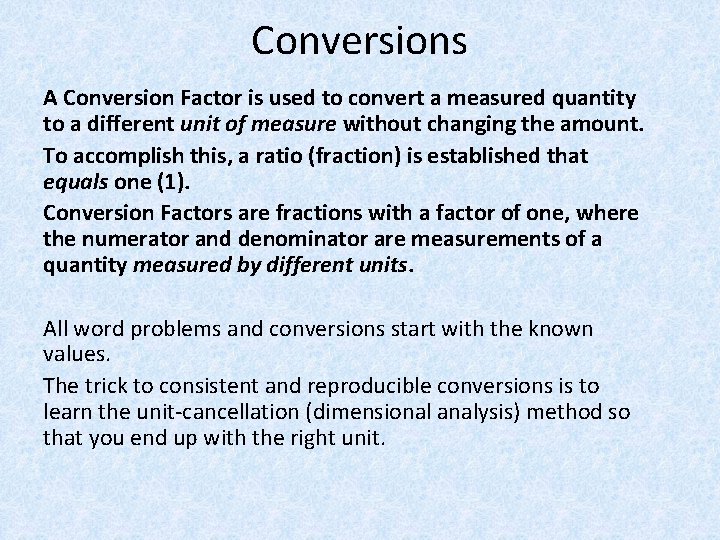
Conversions A Conversion Factor is used to convert a measured quantity to a different unit of measure without changing the amount. To accomplish this, a ratio (fraction) is established that equals one (1). Conversion Factors are fractions with a factor of one, where the numerator and denominator are measurements of a quantity measured by different units. All word problems and conversions start with the known values. The trick to consistent and reproducible conversions is to learn the unit-cancellation (dimensional analysis) method so that you end up with the right unit.

Dimensional Analysis • Dimensional analysis may be the most useful math trick you'll ever learn. • What this is all about is just conversion-converting one thing to another. • Anything you measure will have a number with some sort of "unit of measure" (the dimension) attached. A unit could be miles, gallons, miles per second, peas per pod, or pizza slices person.

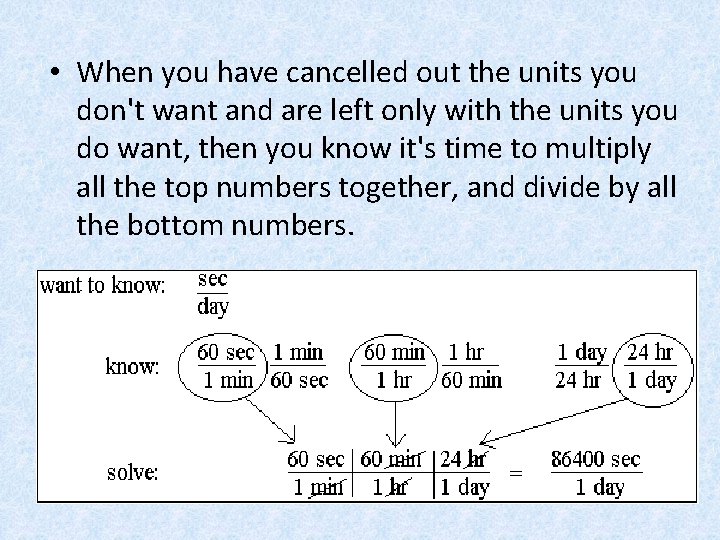
How many seconds are in a day? • Break the problem down into several small problems that you can solve. – First thing to know is "What units of measure do I want to know or have in the answer? Seconds in a day = sec/day. – So pick from the things you know a factor that has seconds on top or day(s) on the bottom. You could pick either of the following two factors as your "starting factor: ”

60 sec 1 min This is where we will start. • Now we need to get rid of the units we don’t want until we get to the unit we do want. • You need to get rid of the minutes. You cancel minutes out by picking a factor that has minutes on top. So you pick 60 minutes per 1 hour as the next factor because it has minutes on top: • You now have seconds per hour, but you want seconds per day, so you need to pick a factor that cancels out hours:

• When you have cancelled out the units you don't want and are left only with the units you do want, then you know it's time to multiply all the top numbers together, and divide by all the bottom numbers.

If you are going 50 miles per hour, how many feet per second are you traveling? There are 5280 feet in a mile. • 73. 3 ft/sec

HOME FUN (and lots of it) • Pg 42 q 1 -5 • pg 59 q 1, 5, 7, 8, 11, 13, 14 • You’ll thank me later.

Chapter 2 Section 3 USING SCIENTIFIC MEASUREMENTS

Accuracy and Precision • Accuracy- The ability of a measurement to match the actual value of the quantity being measured. • Precision- The ability of a measurement to be consistently reproduced AND the number of significant digits to which a value has been reliably measured. Low accuracy High precision High accuracy Low precision High accuracy High precision

Bias (don't let precision fool you!) • Bias is a systematic (built in) error which makes all measurements wrong by a certain amount. • Examples of Bias – The scales read "1 kg" when there is no weight on them – You always measure your height wearing shoes with thick soles. – A stopwatch that takes half a second to stop when clicked • Accuracy depends on the instrument you are measuring with. As a general rule: – The degree of accuracy is half a unit each side of the unit of measure

Precision depends on the unit used to obtain a measure. The smaller the unit, the more precise the measure. • Consider measures of time, such as 12 seconds and 12 days. – A measurement of 12 seconds is precise to the nearest second, with a maximum potential error of 0. 5 seconds. – Twelve days yields a potential error of 0. 5 days, or 43, 200 seconds! – 12 seconds is much more precise.

A time of 12. 1 seconds is more precise than a time of 12 seconds; it implies a measure precise to the nearest tenth of a second with a possible error of 0. 05 s. • The measures of 12 seconds and 12. 0 seconds imply a difference in precision so you can’t just randomly add zeroes to your answers. Be sure you know what the precision of your measuring device is and then use it accurately. • If your stopwatch has increments of tenths of a second then your answer will be more accurate than a stopwatch with whole second increments.

Percent Error • T he accuracy of an experimental value can be compared to an accepted (or true) value. • If everything goes perfectly in lab (which almost never happens), the experimental value will be very close to the accepted value. • Experimental value is what is recorded/calculated based on the experiment in the lab. • The true value is the textbook/literature value.

• Percent error = Value accepted – Value experimental X 100 Value accepted • Try this: What is the percent error for a mass measurement of 17. 7 g, given that the correct value is 21. 2 g • 17%

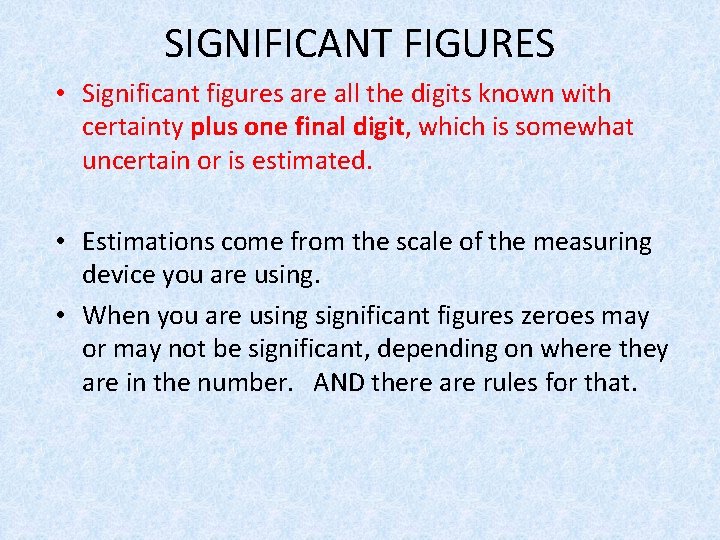
SIGNIFICANT FIGURES • Significant figures are all the digits known with certainty plus one final digit, which is somewhat uncertain or is estimated. • Estimations come from the scale of the measuring device you are using. • When you are using significant figures zeroes may or may not be significant, depending on where they are in the number. AND there are rules for that.

• • • Rules for Significant Figures 1. Always count nonzero digits Example: 21 has two significant figures, while 8. 926 has four 2. Never count leading zeros Example: 0. 21 and 0. 021 both have two significant figures 3. Always count zeros which fall somewhere between two nonzero digits Example: 20. 8 has three significant figures, while 0. 00104009 has six 4. Count trailing zeros if and only if the number contains a decimal point Example: 210 and 210000 both have two significant figures, while 210. has three and 210. 00 has five. (There must be a whole number in front for it to count. ) 5. For numbers expressed in scientific notation, ignore the exponent

A closer look at #4 • 210 s means "210 s, give or take a few tens of seconds” • 210. s means "210 s, give or take a few seconds” • 210. 00 s means “ 210 s, give or take a few hundredths of seconds“ • In other words, they are three different numbers.

How many significant digits are in the following: • • • 640 cm^3 200. 0 m. L 0. 5200 g 1. 005 kg 10 000 L 20. 900 cm • • • 2 4 4 4 1 5

Sig Fig in Calculations 1. When multiplying or dividing measured quantities, round the answer to as many significant figures in the answer as there are in the measurement with the least number of significant figures. 2. When adding or subtracting measured quantities, round the answer to the same number of decimal places as there are in the measurement with the least number of decimal places.

Conversion factors should be considered exact numbers. In other words, significant digits depend on the converted number not the conversion factor so do not consider them when determining significance.

Rounding Rules • A slight change to the rounding rules you have been using since 2 nd grade: • When the first digit dropped is 5 and there are no digits following or all the digits following are zeros, round to even. • Ex: 2. 35 and 2. 45 both round to 2. 4 • 2. 451 rounds as usual…to 2. 5

HOME FUN • Pg 57 q 1 -4, 7, 8 • Pg 59 q 17, 21 • Pg 60 q 35 -42 • WOOHOO

Scientific Notation • Scientific notation is the way that scientists easily handle very large numbers or very small numbers. – For example, instead of writing 0. 000056, we write 5. 6 x 10 -9

• You should all know how to use scientific notation but just in case you have forgotten: – Scientific Notation is based on powers of the base number 10. • 1. 23 X 1023 – The first number (1. 23) is called the coefficient. It must be greater than or equal to 1 and less than 10. – The second number is called the base and is always 10. The number 23 is referred to as the exponent.

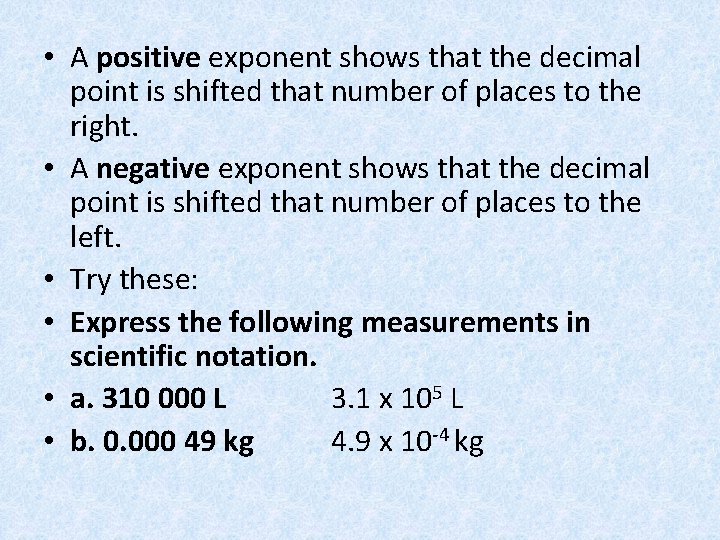
• A positive exponent shows that the decimal point is shifted that number of places to the right. • A negative exponent shows that the decimal point is shifted that number of places to the left. • Try these: • Express the following measurements in scientific notation. • a. 310 000 L 3. 1 x 105 L • b. 0. 000 49 kg 4. 9 x 10 -4 kg

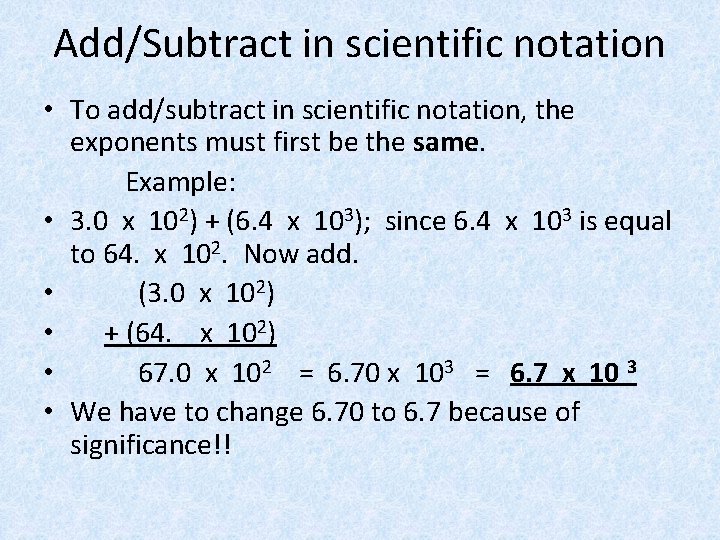
Add/Subtract in scientific notation • To add/subtract in scientific notation, the exponents must first be the same. Example: • 3. 0 x 102) + (6. 4 x 103); since 6. 4 x 103 is equal to 64. x 102. Now add. • (3. 0 x 102) • + (64. x 102) • 67. 0 x 102 = 6. 70 x 103 = 6. 7 x 10 3 • We have to change 6. 70 to 6. 7 because of significance!!

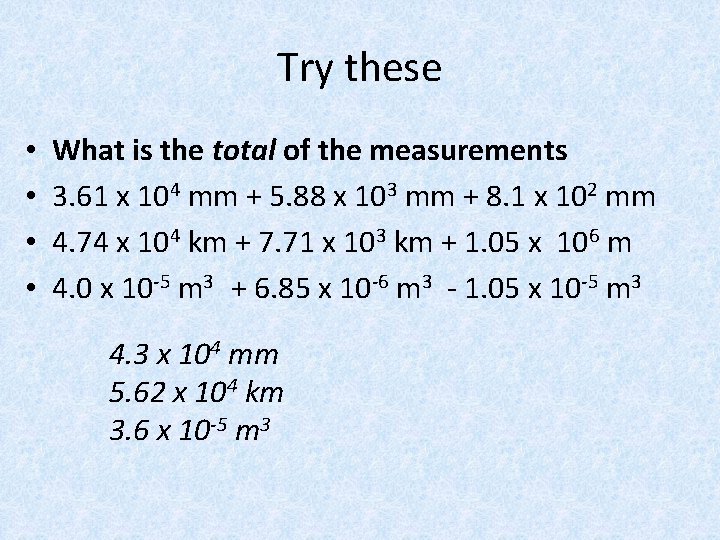
Try these • • What is the total of the measurements 3. 61 x 104 mm + 5. 88 x 103 mm + 8. 1 x 102 mm 4. 74 x 104 km + 7. 71 x 103 km + 1. 05 x 106 m 4. 0 x 10 -5 m 3 + 6. 85 x 10 -6 m 3 - 1. 05 x 10 -5 m 3 4. 3 x 104 mm 5. 62 x 104 km 3. 6 x 10 -5 m 3

Multiplying in scientific notation • To multiply, find the product of the numbers, then add the exponents. • Example: (2. 4 x 102) (5. 5 x 10 – 4) = [2. 4 x 5. 5 = 13. 2] and [2 + -4 = -2], so (2. 4 x 102) (5. 5 x 10 – 4) = 13. 2 x 10 – 2 = 1. 3 x 10 – 1

Try These • 7. 20 x 103 cm x 8. 08 x 103 cm • 4. 44 x 10 -35 m x 5. 55 x 1019 m x 7. 69 x 10 -12 kg 5. 82 x 107 cm 2 1. 89 x 10 -26 kg m 2

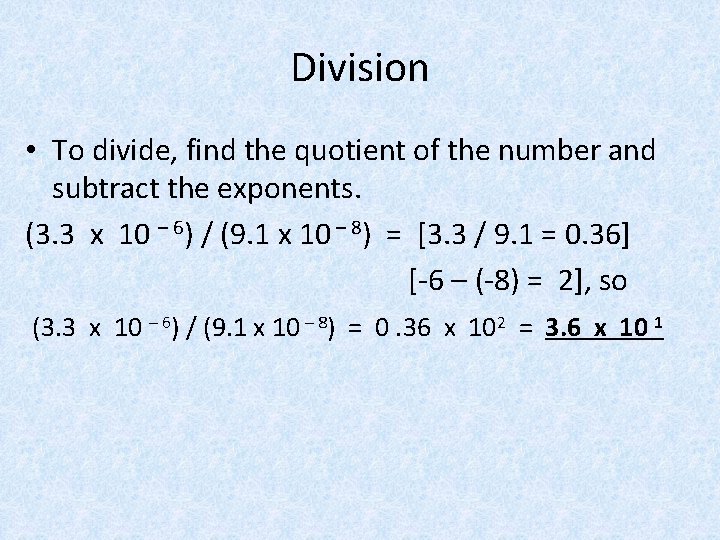
Division • To divide, find the quotient of the number and subtract the exponents. (3. 3 x 10 – 6) / (9. 1 x 10 – 8) = [3. 3 / 9. 1 = 0. 36] [-6 – (-8) = 2], so (3. 3 x 10 – 6) / (9. 1 x 10 – 8) = 0. 36 x 102 = 3. 6 x 10 1

Try these: • 2. 290 x 107 cm/ 4. 33 x 103 s • 1. 788 x 105 L / 7. 111 103 m 2 • 4. 73 x 10 -4 g / (2. 08 x 10 -3 km x 5. 60 x 10 -4 km) 5. 29 x 103 cm/s 2. 514 x 101 L/m 2 4. 06 x 102 g/km 2

Direct and Inverse Proportions • A change in one quantity can cause a change, or is linked to a change, in another. • If these changes are related through equal factors, then the quantities are said to be in direct proportion. – For example, suppose that you are buying cans of soup at the store. Let us imagine that they cost 50 cents, or $0. 50, each. – Case #1: – Suppose that you buy 4 cans. You would pay $2. 00. – Case #2: – Suppose that you buy 8 cans. You would pay $4. 00. • Notice that the number of cans changed by a factor of 2, and the amount of money that you must pay also changed by a factor of 2.

• Two quantities are directly proportional to each other if dividing one by the other gives a constant value. – Example- Mass and volume are directly proportional. If the mass changes, the volume must change at the same rate. Whenever you have a direct proportion you can change it into an equation by using a proportionality constant. y = kx k is the proportionality constant.

Inverse Proportions • Inverse proportions relate two quantities through factors that are multiplicative inverses (through factors that are reciprocals, such as 3 and 1/3). – For example, let us say that you are driving a car and you are going to travel 60 miles. Consider this to be a constant distance throughout the following discussion. – Case #1: – Suppose that you spent 1 hour driving. Your average speed would be 60 mph. – Case #2: – Suppose that you spent 2 hours driving. Your average speed would be 30 mph. • Notice that the number of hours is changed by a factor of 2, and the speed at which you were traveling changed by a factor of ½. • The two quantities, time and speed, changed by reciprocal factors.

• Two quantities are inversely proportional if their product is constant xy = k The graph of inversely proportional variables is a hyperbola.

HOME FUN • Pg 57 q 5, 6, 9 • Pg 59 q 24, 25 • Pg 60 q 43 -46