Chapter 19 The Representative Elements Groups 1 A















- Slides: 50

Chapter 19 The Representative Elements: Groups 1 A Through 4 A

19. 1 Survey of the Representative Elements n n n Elements in group 1 A through 8 A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1 A to eight in group 8 A. The valence electrons of representative elements are in s or p orbitals. Metals tend to lose their valence electrons to form cations with a configuration of the noble gas from the preceding period Nonmetals tend to gain electrons to form anins with a configuration of the noble gas in the same period

Metalloids or semi metals




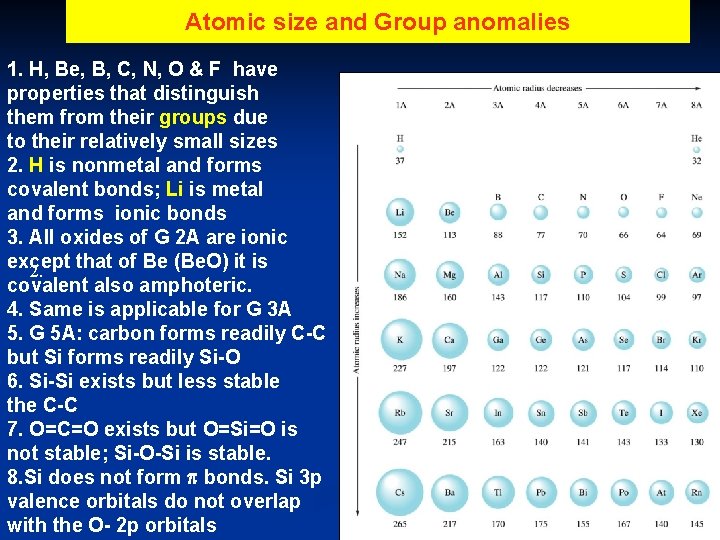
Atomic size and Group anomalies 1. H, Be, B, C, N, O & F have properties that distinguish them from their groups due to their relatively small sizes 2. H is nonmetal and forms covalent bonds; Li is metal and forms ionic bonds 3. All oxides of G 2 A are ionic except that of Be (Be. O) it is 2. covalent also amphoteric. 4. Same is applicable for G 3 A 5. G 5 A: carbon forms readily C-C but Si forms readily Si-O 6. Si-Si exists but less stable the C-C 7. O=C=O exists but O=Si=O is not stable; Si-O-Si is stable. 8. Si does not form bonds. Si 3 p valence orbitals do not overlap with the O- 2 p orbitals

n bonding is important for relatively small elements of the 2 nd period. n N exists as N≡N due to tendency to form bonds P exists as P 4; P large atoms are like Si do not form strong bonds. They prefer to achieve noble gas configuration but forming single bonds. n O (G 6 A) exists as O=O; tendency to form n n n bonding S does not form bonding thus it exists as S 8. F has smaller electron affinity than Cl (not expected) the small size of F in F-F with 6 lone pairs of electrons leads to much greater repulsion compared to Cl Abundance and Preparation (P. 917): Self study

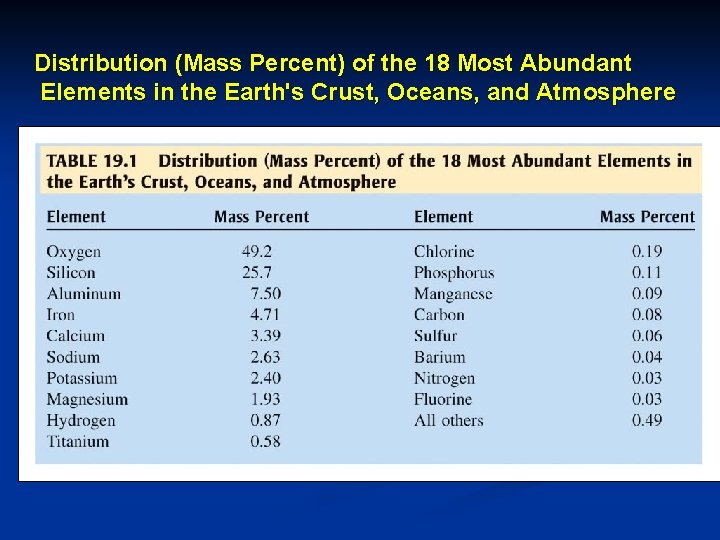
Distribution (Mass Percent) of the 18 Most Abundant Elements in the Earth's Crust, Oceans, and Atmosphere

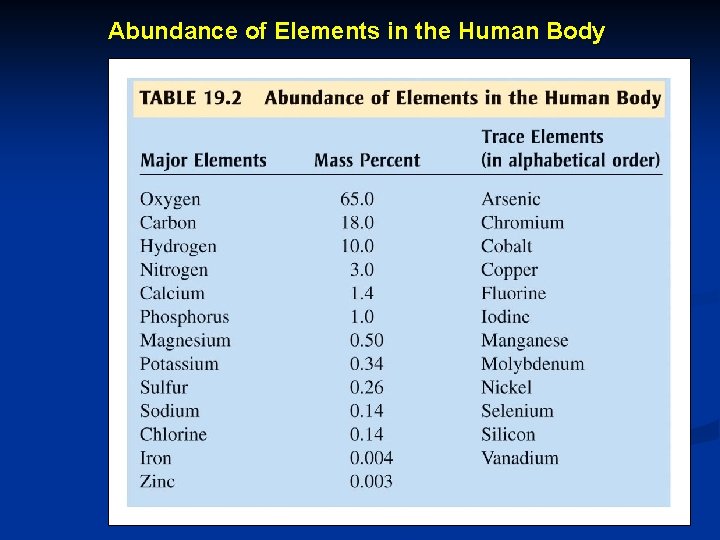
Abundance of Elements in the Human Body

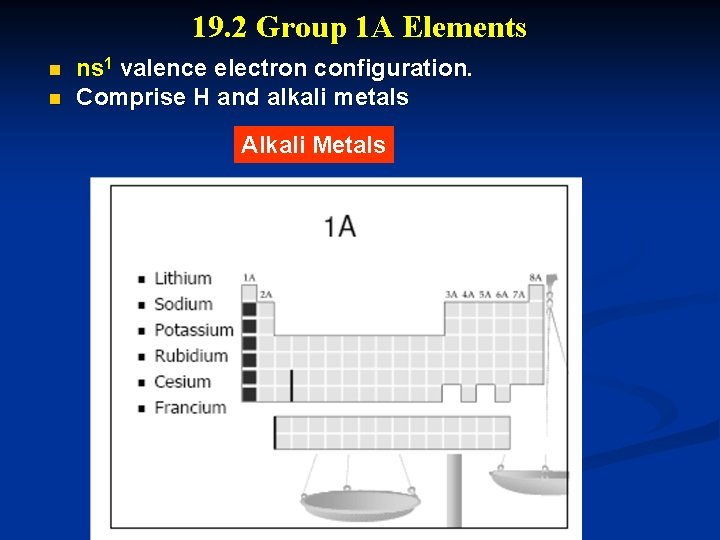
19. 2 Group 1 A Elements n n ns 1 valence electron configuration. Comprise H and alkali metals Alkali Metals

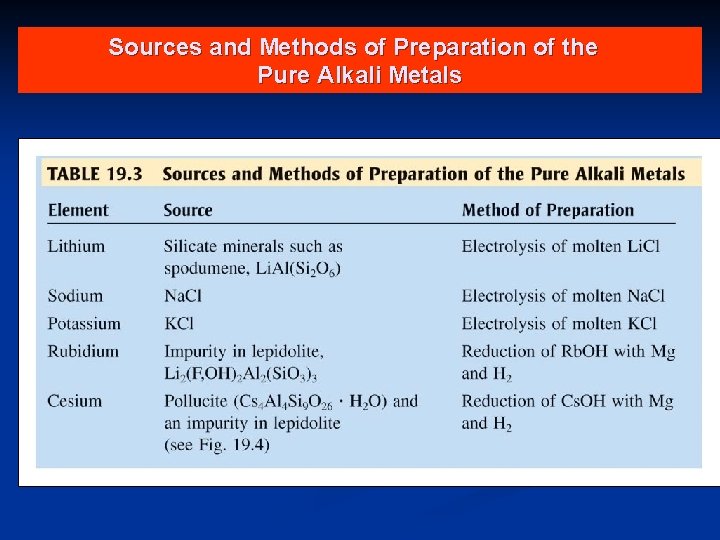
Sources and Methods of Preparation of the Pure Alkali Metals

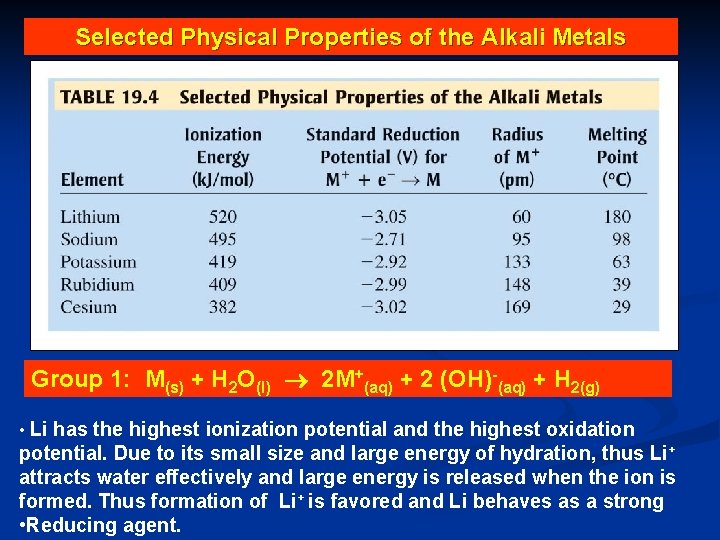
Selected Physical Properties of the Alkali Metals Group 1: M(s) + H 2 O(l) 2 M+(aq) + 2 (OH)-(aq) + H 2(g) • Li has the highest ionization potential and the highest oxidation potential. Due to its small size and large energy of hydration, thus Li + attracts water effectively and large energy is released when the ion is formed. Thus formation of Li+ is favored and Li behaves as a strong • Reducing agent.

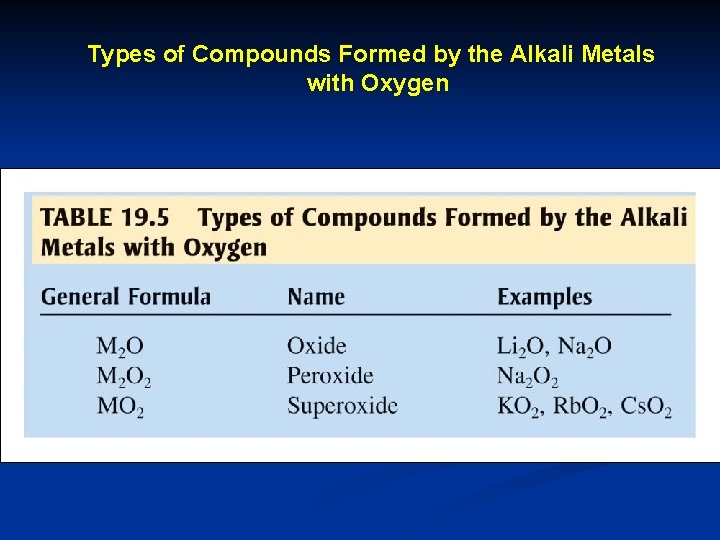
Types of Compounds Formed by the Alkali Metals with Oxygen

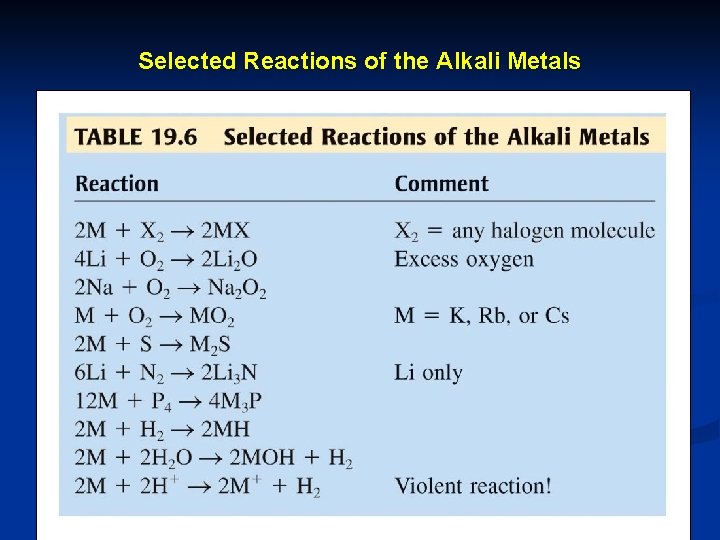
Selected Reactions of the Alkali Metals

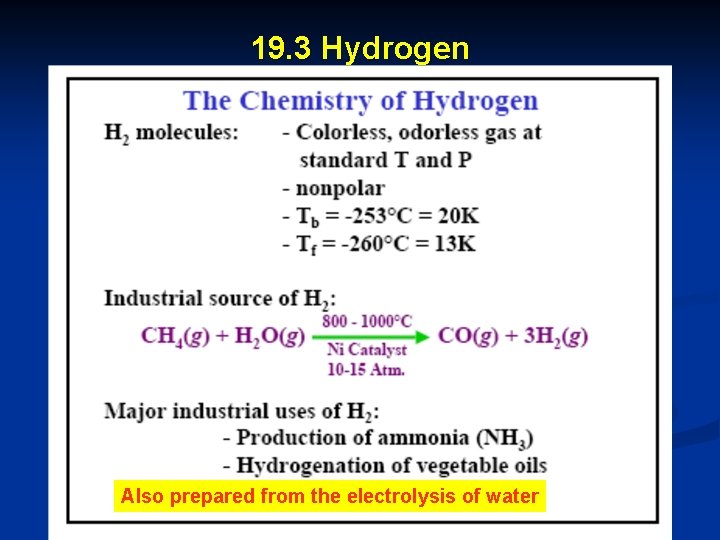
19. 3 Hydrogen Also prepared from the electrolysis of water

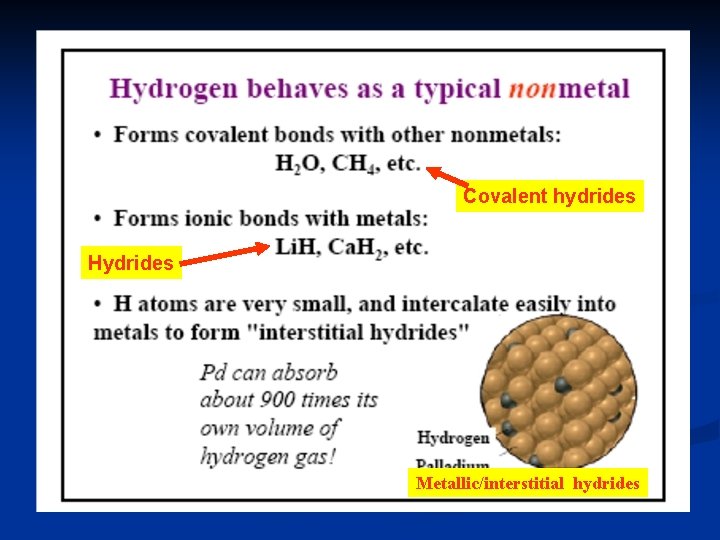
Covalent hydrides Hydrides Metallic/interstitial hydrides

Group 2 A Elements 19. 4

Group 2 A Elements n Ns 2 metals. They are called “Alkaline Earth metals” Their oxides are basic MO(s) + H 2 O M(OH)2 n The differences in reactivity among them are shown by their reaction with water: M(s) + 2 H 2 O M(OH)2 + H 2(g) n Ca, Ba, Sr react easily with cold water n Mg reacts with hot water n Be does not react with water n

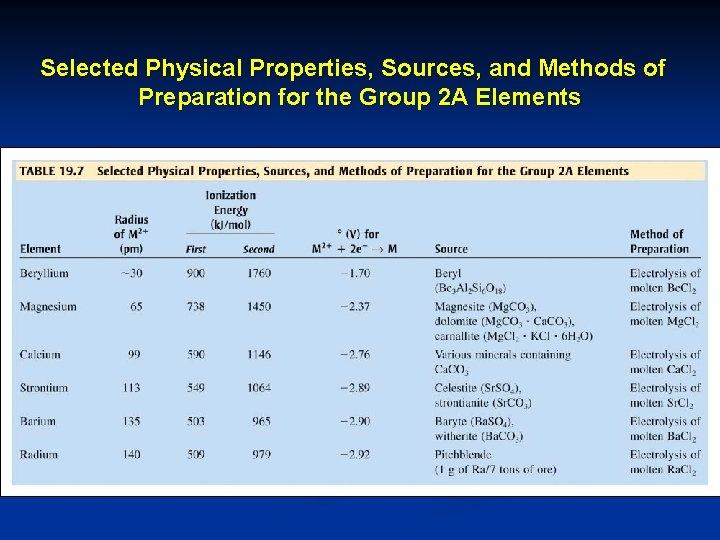
Selected Physical Properties, Sources, and Methods of Preparation for the Group 2 A Elements

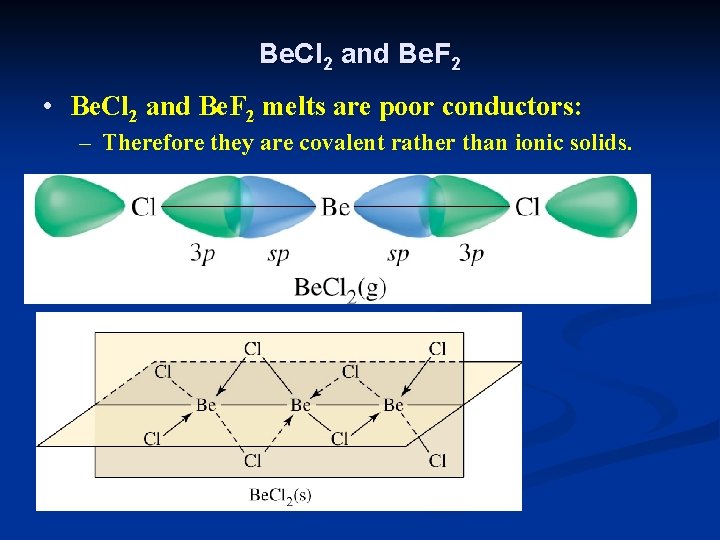
Be. Cl 2 and Be. F 2 • Be. Cl 2 and Be. F 2 melts are poor conductors: – Therefore they are covalent rather than ionic solids.

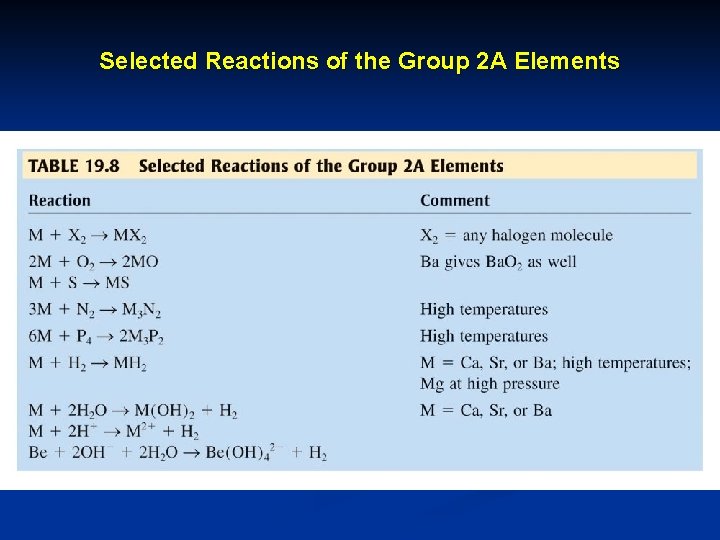
Selected Reactions of the Group 2 A Elements

Ions in Natural Waters: Hard Water n n Rainwater is not chemically pure water. n Contains dissolved atmospheric gases. n Once on the ground it may pick up a few to about 1000 ppm of dissolved substances. n If the water contains Ca 2+ and or Mg 2+ ions we say that the water is hard. Hardness may be permanent or temporary.

Temporary Hard Water n n Contains HCO 3 - ion. n When heated gives CO 32 -, CO 2 and H 2 O. n The CO 32 - reacts with multivalent ions to form precipitates. (for example Ca. CO 3, Mg. CO 3) Water softening on a large scale is carried out by precipitating the multivalent ions using slaked lime Ca(OH)2. Ca. CO 3 would be precipitated

Permanent Hard Water n Contains significant concentrations of anions other than carbonate. n For example SO 42 -, HSO 4 -. n Usually soften by precipitating the Ca 2+ and Mg 2+ using sodium carbonate leaving sodium salts in solution.

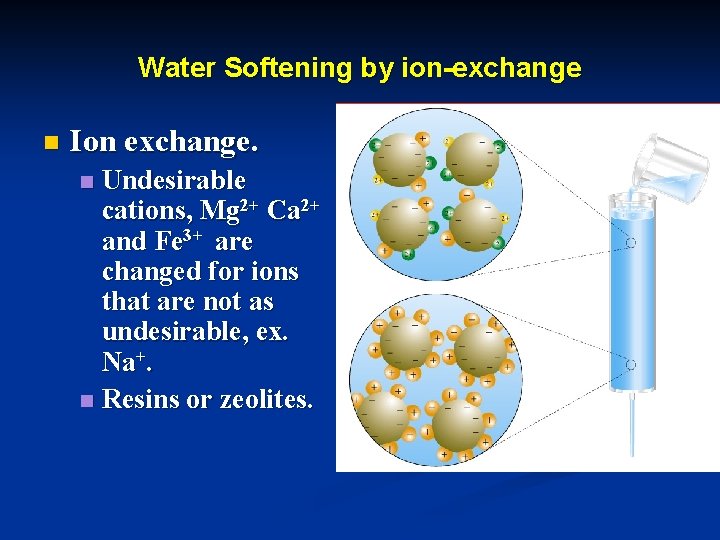
Water Softening by ion-exchange n Ion exchange. Undesirable cations, Mg 2+ Ca 2+ and Fe 3+ are changed for ions that are not as undesirable, ex. Na+. n Resins or zeolites. n

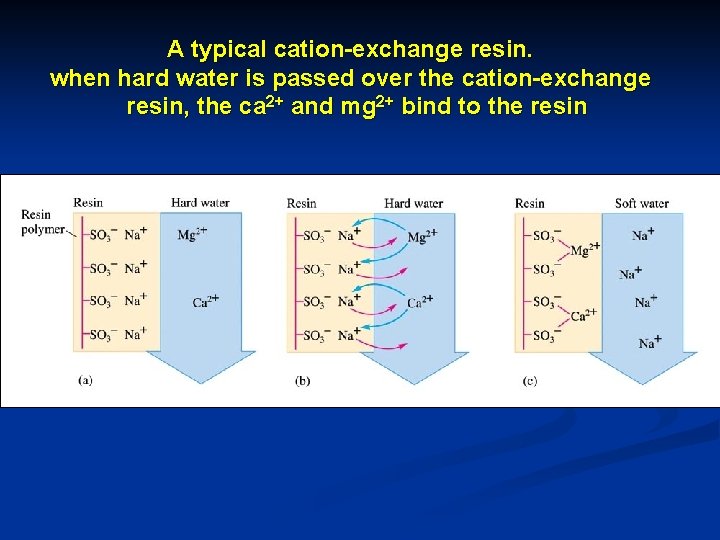
A typical cation-exchange resin. when hard water is passed over the cation-exchange resin, the ca 2+ and mg 2+ bind to the resin

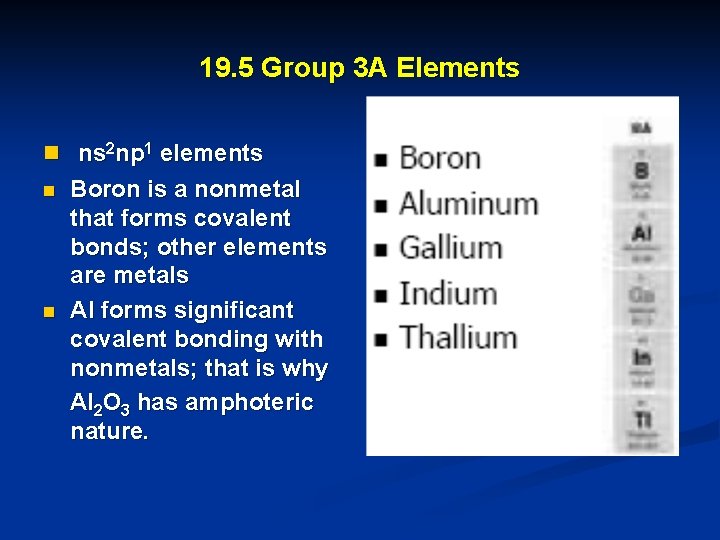
19. 5 Group 3 A Elements n ns 2 np 1 elements n n Boron is a nonmetal that forms covalent bonds; other elements are metals Al forms significant covalent bonding with nonmetals; that is why Al 2 O 3 has amphoteric nature.

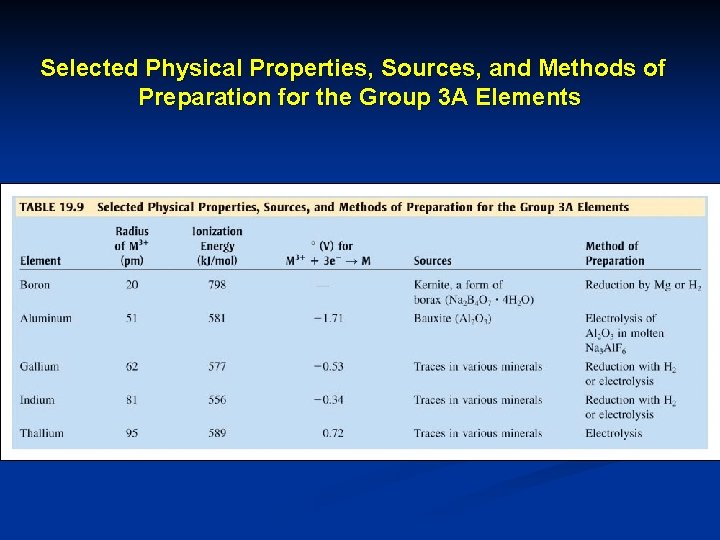
Selected Physical Properties, Sources, and Methods of Preparation for the Group 3 A Elements

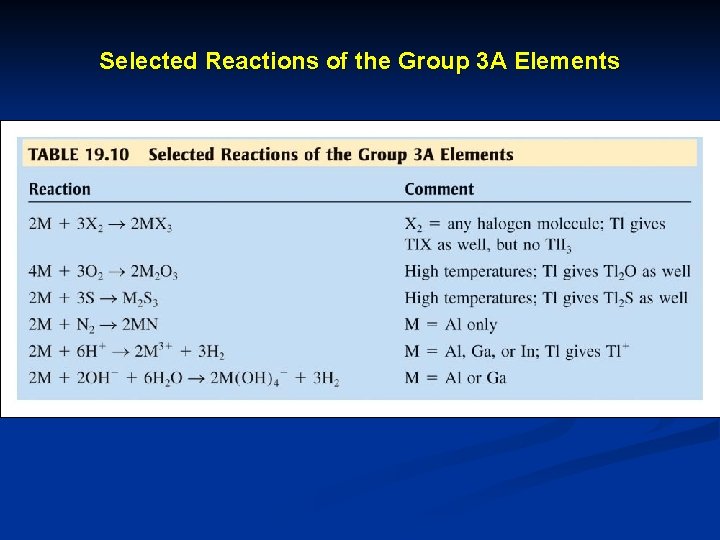
Selected Reactions of the Group 3 A Elements

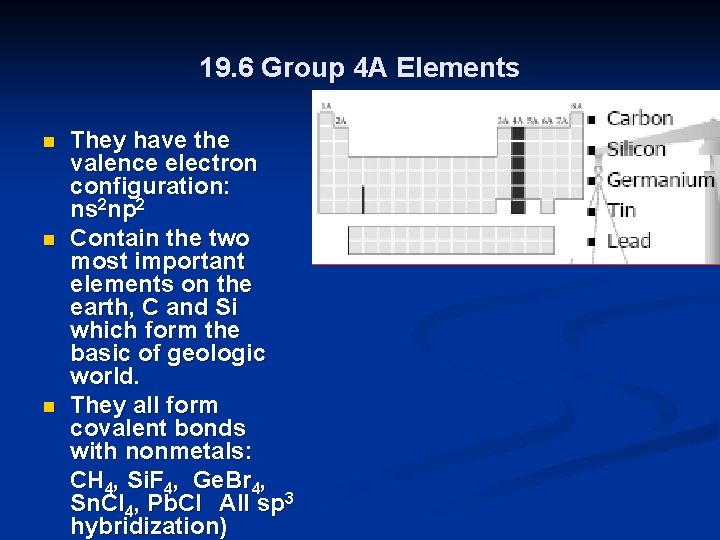
19. 6 Group 4 A Elements n n n They have the valence electron configuration: ns 2 np 2 Contain the two most important elements on the earth, C and Si which form the basic of geologic world. They all form covalent bonds with nonmetals: CH 4, Si. F 4, Ge. Br 4, Sn. Cl 4, Pb. Cl All sp 3 hybridization)

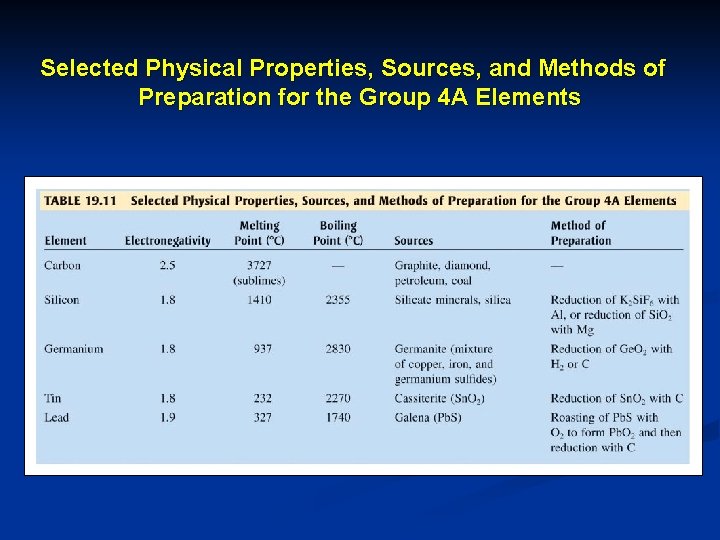
Selected Physical Properties, Sources, and Methods of Preparation for the Group 4 A Elements

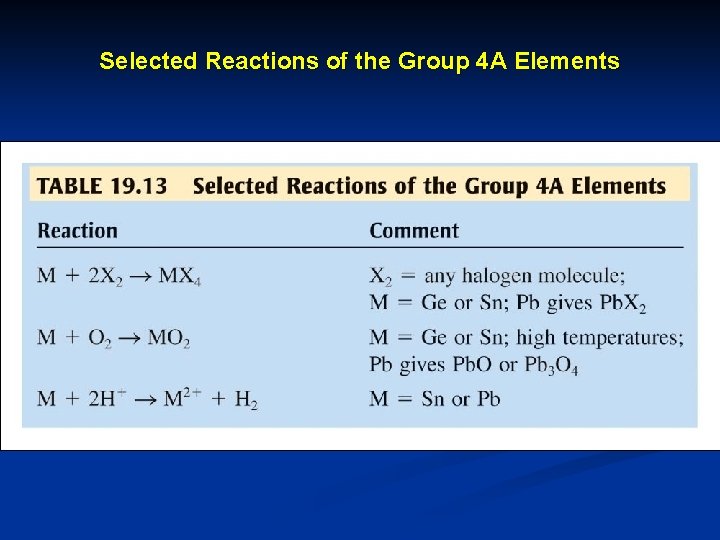
Selected Reactions of the Group 4 A Elements

Chapter 20 The Representative Elements: Groups 5 A Through 8 A

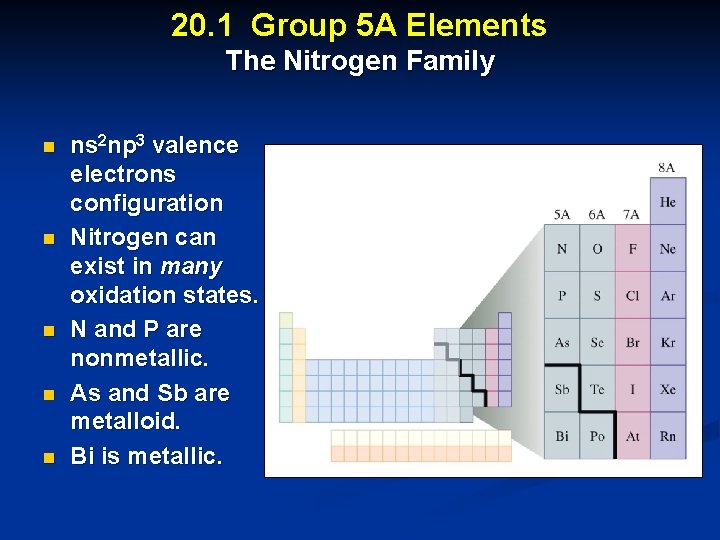
20. 1 Group 5 A Elements The Nitrogen Family n n ns 2 np 3 valence electrons configuration Nitrogen can exist in many oxidation states. N and P are nonmetallic. As and Sb are metalloid. Bi is metallic.

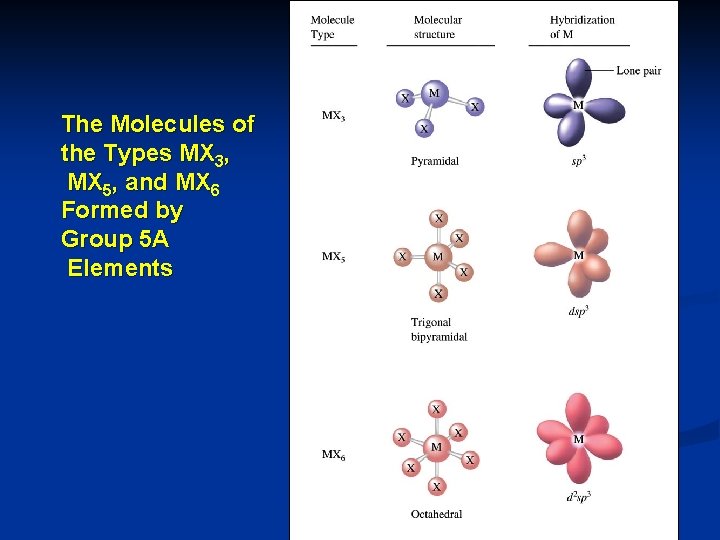
n n n n Bi and Sb tend to be metallic But no ionic compounds containing Bi 5+ and Sb 5+ are known Bi. F 5, Sb. F 5 and Sb. Cl 5 are molecular rather than ionic G 5 A elements can form molecules or ions that involve 3, 5 or 6 covalent bonds to the G 5 A atom NH 3, PH 3, NF 3, and As. Cl 3. They all behave as Lewis base. All G 5 A elements except N can form molecules (MX 5) with 5 covalent bonds. The ability of G 5 A elements to form bonds decreases dramatically after N. This is why N exists as N 2 molecules; while other elements in the group exist as larger aggregates containing single bonds: P 4, As 4, Sb 4

The Molecules of the Types MX 3, MX 5, and MX 6 Formed by Group 5 A Elements

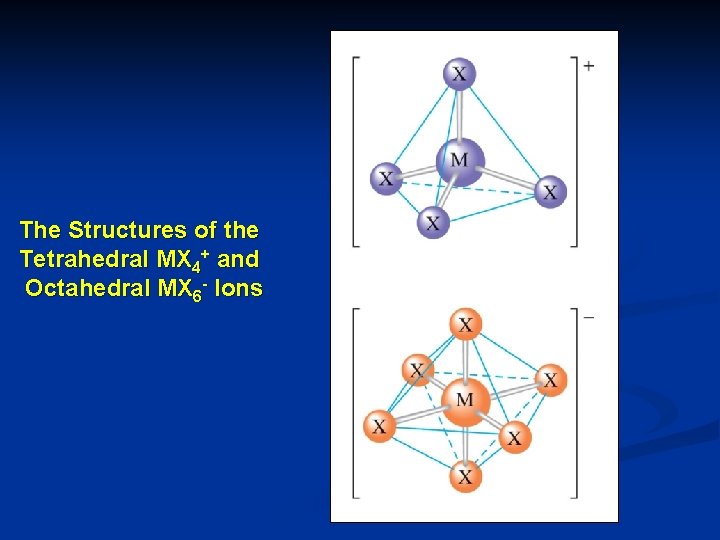
The Structures of the Tetrahedral MX 4+ and Octahedral MX 6 - Ions

20. 2 The Chemistry of Nitrogen n n Since N 2 molecule contains a triple bond, most binary compounds (except NH 3) containing N decompose exothermically to the elements In the preparation of NH 3 from N 2 and H 2, too much energy is needed to disrupt the N≡N bond. Thus, though K (106) is high the reaction is very slow at room temperature. Haber process is used to prepare NH 3 (high pressure, high temperature and a catalyst are needed) Nitrogen fixation: The process of transforming N 2 to other nitrogen containing compounds

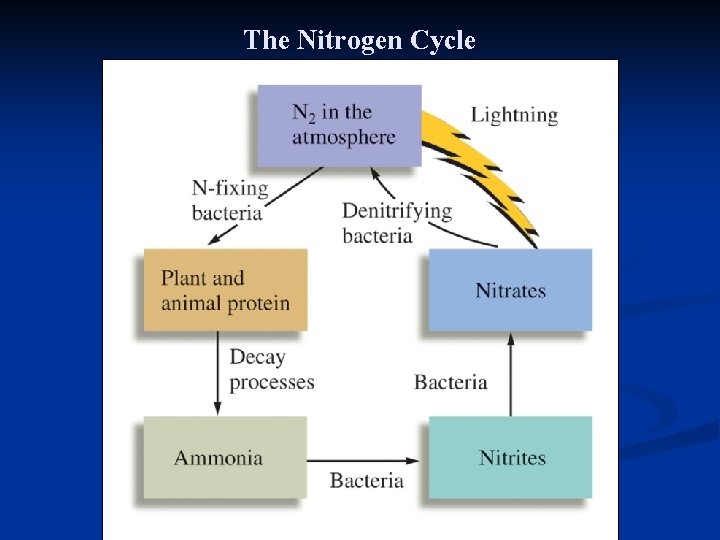
n n Nitrogen fixation can be carried out by: n Haber process (ammonia can be applied to the soil as a fertilizer) n High temperature combustion process in automobile engines. NO produced is converted into NO 2 that with moisture is concerted into NO 3 that reaches soil. n Natural. Lightning produces the energy that disrupt N 2 and O 2 molecules producing reactive N and O atoms that attack other molecules to form nitrogen oxides that convert eventually to NO 3 n Nitrogen-fixing bacteria that reside on the root of nodules of plants such as beans and peas. This converts N 2 to ammonia and other nitrogen containing compounds. Denitrification: return of N element to the atmosphere as N 2 gas. Bacteria changes NO 3 - to N 2

The Nitrogen Cycle

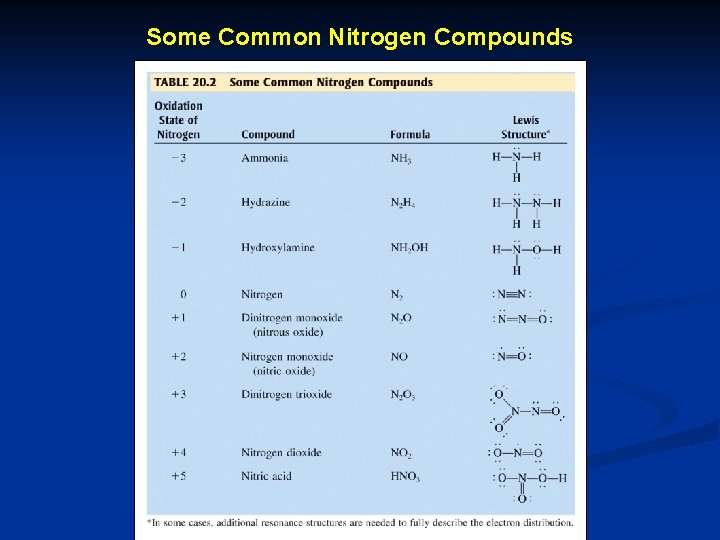
Some Common Nitrogen Compounds

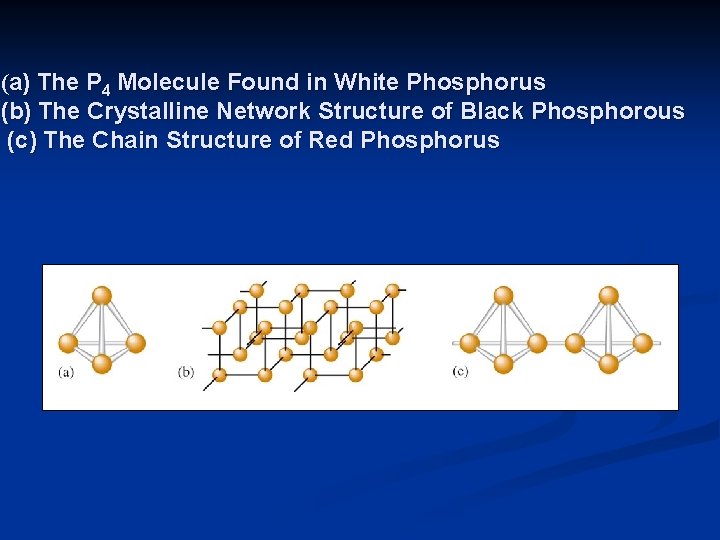
20. 3 The Chemistry of Phosphorus n Chemical properties of P are significantly different from N for the following reasons: n Nitrogen’s stability to form much stronger bonds n Grater electronegativity of N n Larger size of P atom n Availability of empty valence d orbitals on P n White phosphorus exists as P 4: very reactive and bursts into flames on contact with air. n n It is commonly stored under water Black P and Red P are network solids

(a) The P 4 Molecule Found in White Phosphorus (b) The Crystalline Network Structure of Black Phosphorous (c) The Chain Structure of Red Phosphorus

P is essential for plant growth n Soluble phosphate fertilizer is made by treating phosphate rock with sulfuric acid to make superphosphate of lime, that is a mixture of : Ca. SO 4. 2 H 2 O and Ca(H 2 PO 4)2. H 2 O n A reaction of NH 3 and P produces NH 4 H 2 PO 4 a very efficient fertilizer n

20. 4 The Group 6 A Elements n n n The valence electron configuration is ns 2 np 4 Non of these elements behaves as a metal They achive the noble gas configuration by adding 2 electrons to become 2 anion G 6 A elements can form covalent bonds with other nonmetals Due to the presence of empty d orbitals (except O), they form molecules in which central atom is surrounded by more than 8 electrons: SF 4 and SF 6

Group 7 A n n n ns 2 np 5 valence electron configuration. All nonmetals Reactive. Not free in nature. Found as halide (X-) ions. Astatine radioactive with t 1/2 = 8. 3 hrs for its longest living isotope Very high electronegativities (4, 3, 2. 8, 2. 5 and 2). Ionic bonds with metals and covalent bonds with nonmetals in low oxidation states & polar covalent in metals in high oxidation states.

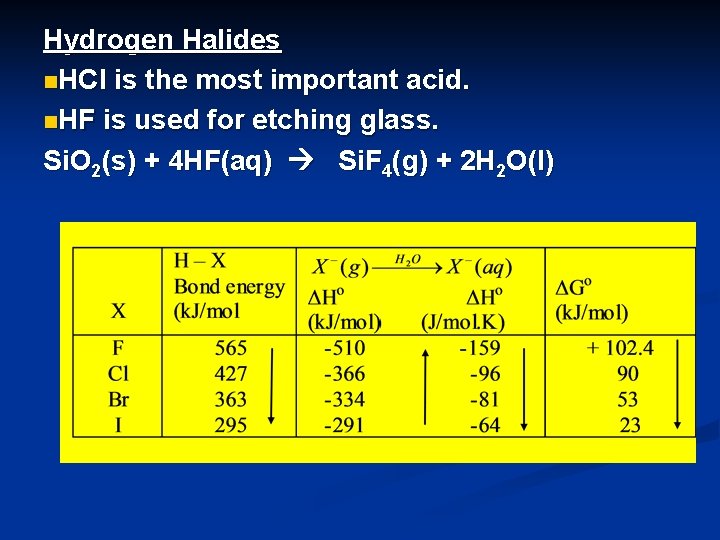
Hydrogen Halides n. HCl is the most important acid. n. HF is used for etching glass. Si. O 2(s) + 4 HF(aq) Si. F 4(g) + 2 H 2 O(l)

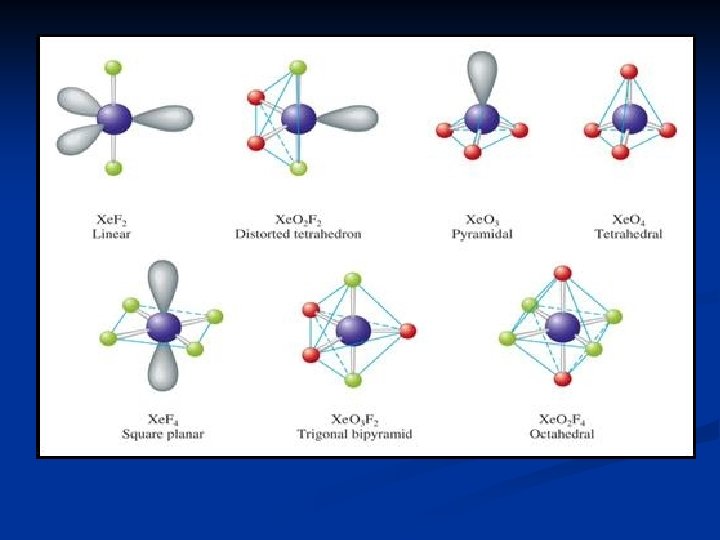
Group 8 A ns 2 p 6 configuration; Un-reactive. n He. Component of the sun. n Present in natural gas (from decay of radioactive elements). n Used as Coolant and a rocket pressurizing gas. n Ne. Used in Luminescent lighting. n Ar. Used as a non-corrosive atmosphere in light bulbs. n Xe & Kr form compounds with O and F. n
