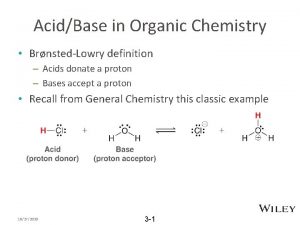
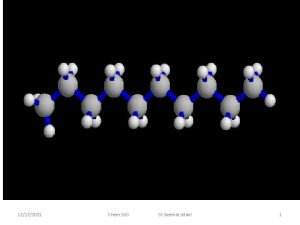
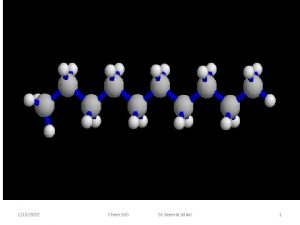
1152022 Chem160 Dr Seemal Jelani 1 Organic Chemistry











- Slides: 62

1/15/2022 Chem-160 Dr Seemal Jelani 1

Organic Chemistry • • • Introduction Functional Groups Alkanes Alkenes Alkynes Alcohols • Acids, Esters and Amides 1/15/2022 Chem-160 Dr Seemal Jelani 2

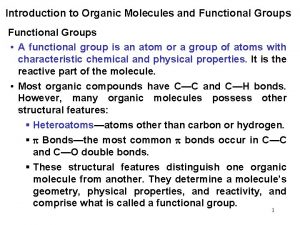
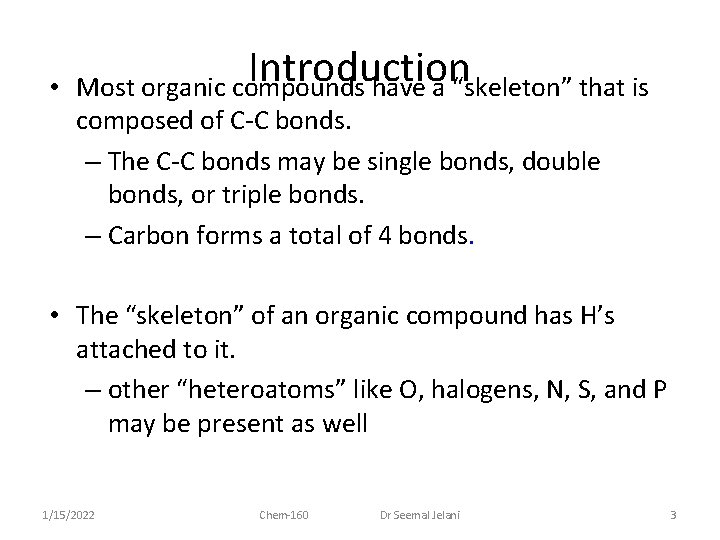
Introduction • Most organic compounds have a “skeleton” that is composed of C-C bonds. – The C-C bonds may be single bonds, double bonds, or triple bonds. – Carbon forms a total of 4 bonds. • The “skeleton” of an organic compound has H’s attached to it. – other “heteroatoms” like O, halogens, N, S, and P may be present as well 1/15/2022 Chem-160 Dr Seemal Jelani 3

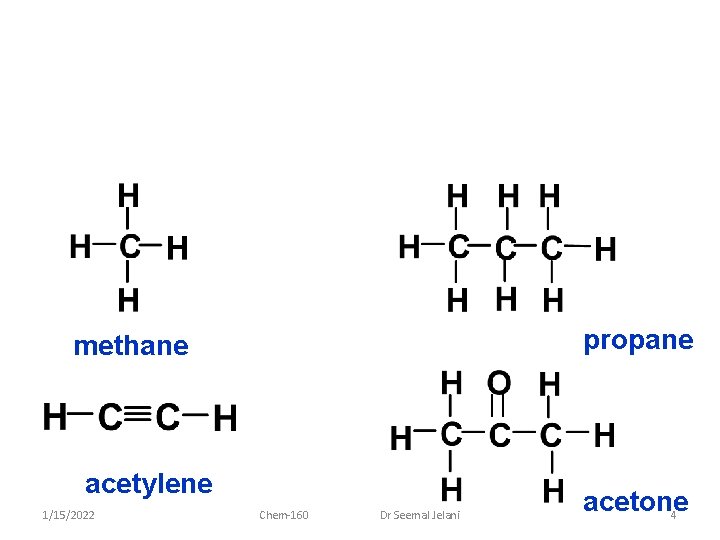
propane methane acetylene 1/15/2022 Chem-160 Dr Seemal Jelani acetone 4

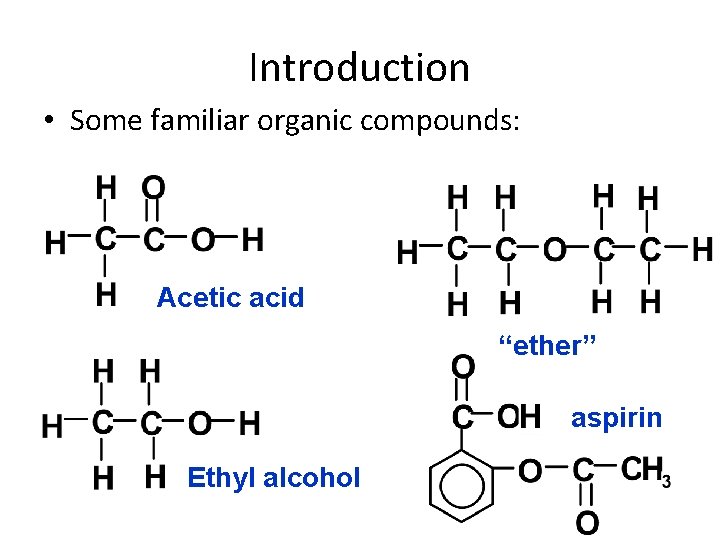
Introduction • Some familiar organic compounds: Acetic acid “ether” aspirin Ethyl alcohol 1/15/2022 Chem-160 Dr Seemal Jelani 5

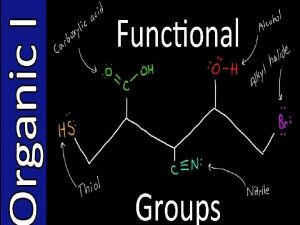
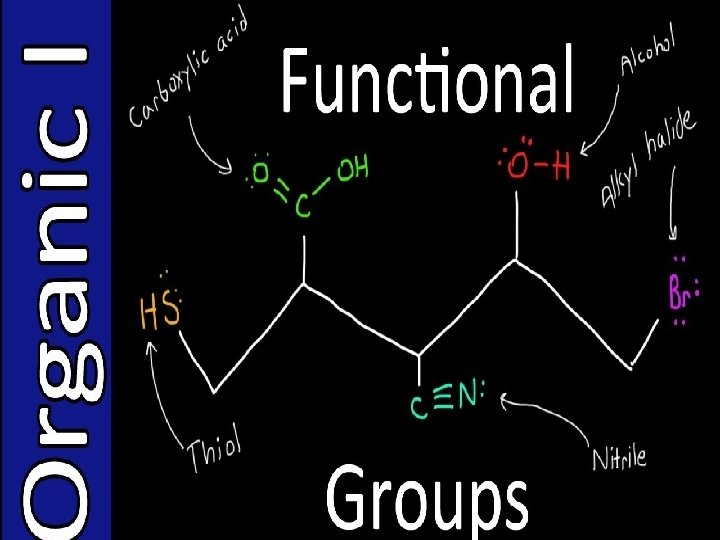
Introduction • Organic compounds are commonly classified and named based on the type of functional group present. – An atom or group of atoms that influences the way the molecule functions, reacts or behaves. – The center of reactivity in an organic compound 1/15/2022 Chem-160 Dr Seemal Jelani 6

Functional Groups • On your exam, you will be responsible for recognizing and naming the various common functional groups that are found in organic compounds: – Use Table 25. 4 and the following slides to help you study 1/15/2022 Chem-160 Dr Seemal Jelani 7

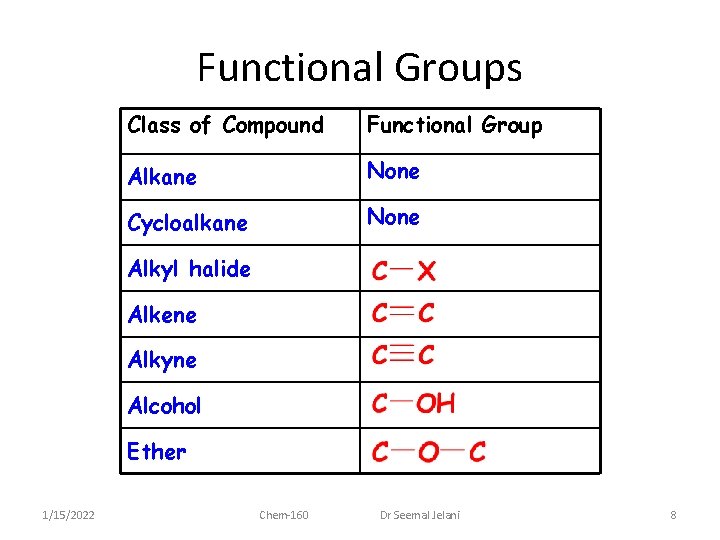
Functional Groups Class of Compound Functional Group Alkane None Cycloalkane None Alkyl halide Alkene Alkyne Alcohol Ether 1/15/2022 Chem-160 Dr Seemal Jelani 8

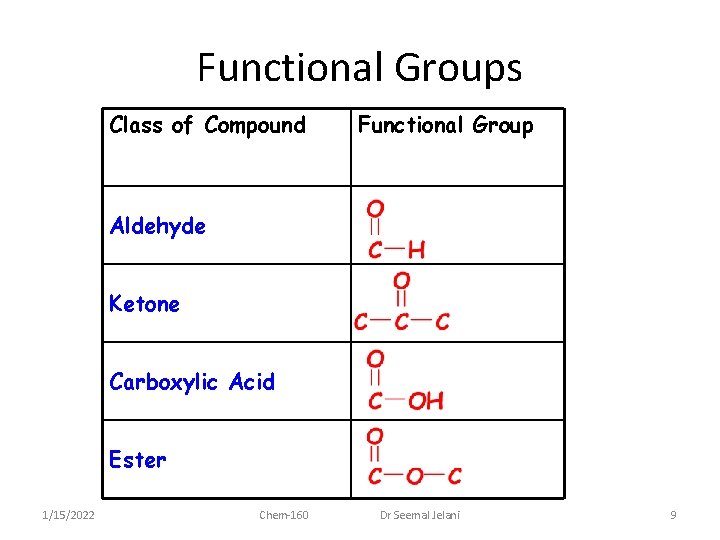
Functional Groups Class of Compound Functional Group Aldehyde Ketone Carboxylic Acid Ester 1/15/2022 Chem-160 Dr Seemal Jelani 9

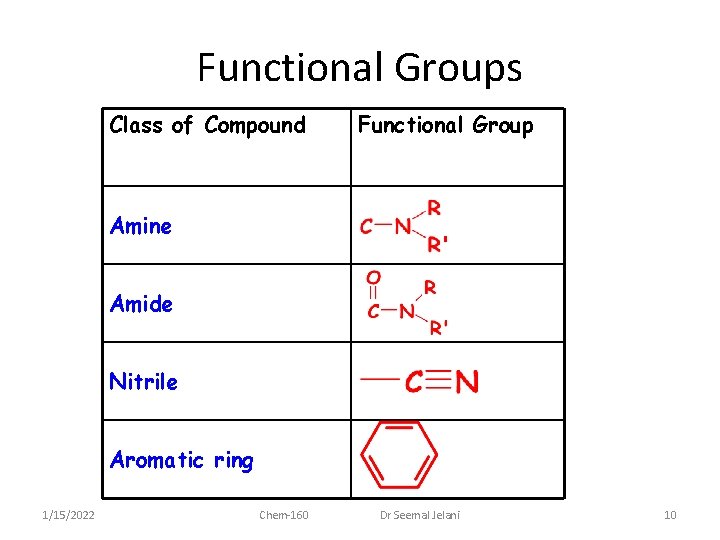
Functional Groups Class of Compound Functional Group Amine Amide Nitrile Aromatic ring 1/15/2022 Chem-160 Dr Seemal Jelani 10

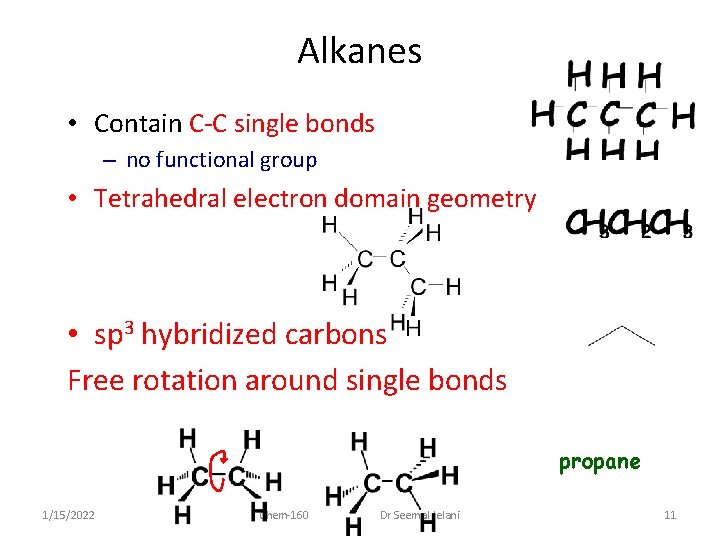
Alkanes • Contain C-C single bonds – no functional group • Tetrahedral electron domain geometry • sp 3 hybridized carbons Free rotation around single bonds propane 1/15/2022 Chem-160 Dr Seemal Jelani 11

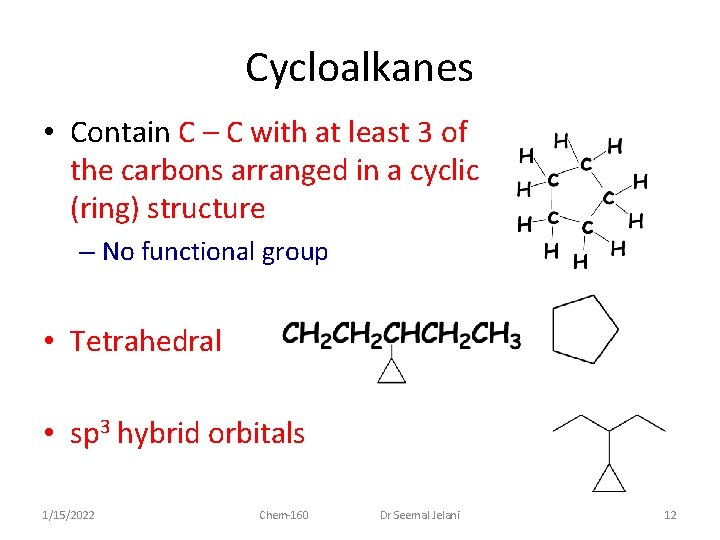
Cycloalkanes • Contain C – C with at least 3 of the carbons arranged in a cyclic (ring) structure – No functional group • Tetrahedral • sp 3 hybrid orbitals 1/15/2022 Chem-160 Dr Seemal Jelani 12

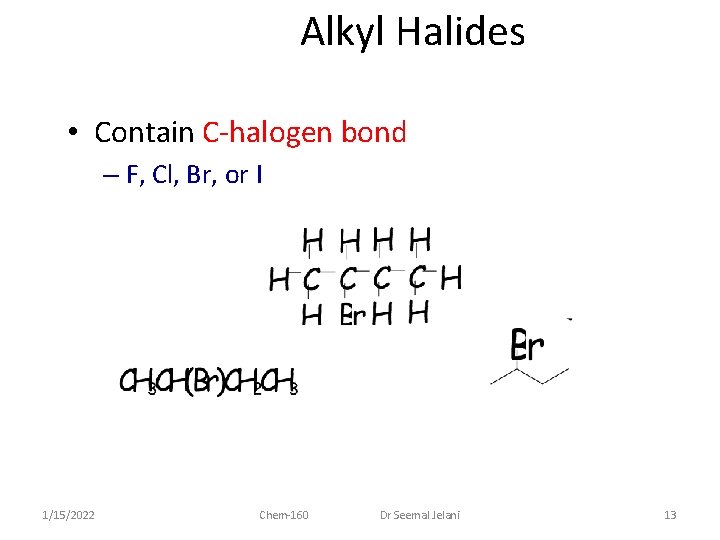
Alkyl Halides • Contain C-halogen bond – F, Cl, Br, or I 1/15/2022 Chem-160 Dr Seemal Jelani 13

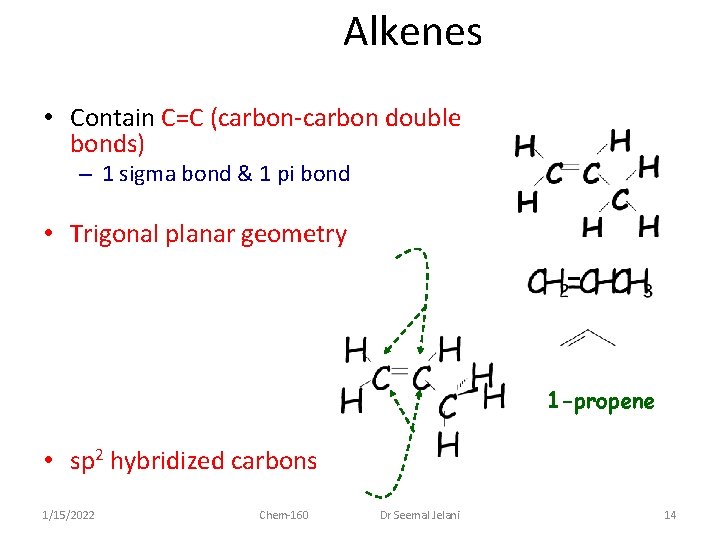
Alkenes • Contain C=C (carbon-carbon double bonds) – 1 sigma bond & 1 pi bond • Trigonal planar geometry 1 -propene • sp 2 hybridized carbons 1/15/2022 Chem-160 Dr Seemal Jelani 14

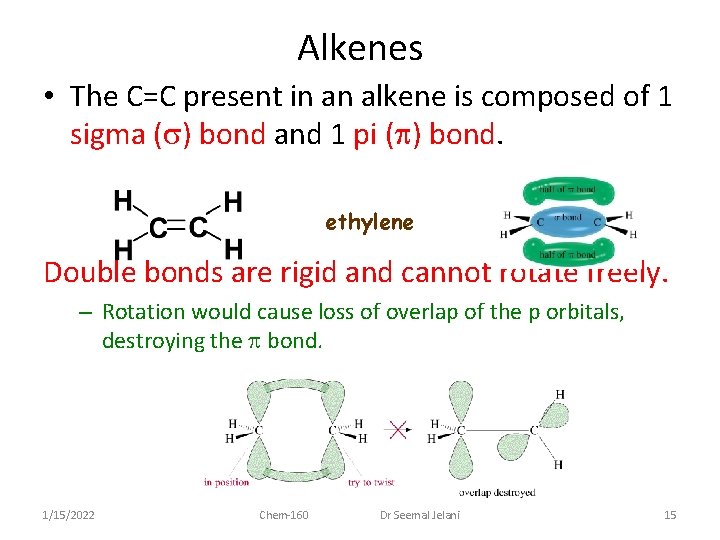
Alkenes • The C=C present in an alkene is composed of 1 sigma (s) bond and 1 pi (p) bond. ethylene Double bonds are rigid and cannot rotate freely. – Rotation would cause loss of overlap of the p orbitals, destroying the p bond. 1/15/2022 Chem-160 Dr Seemal Jelani 15

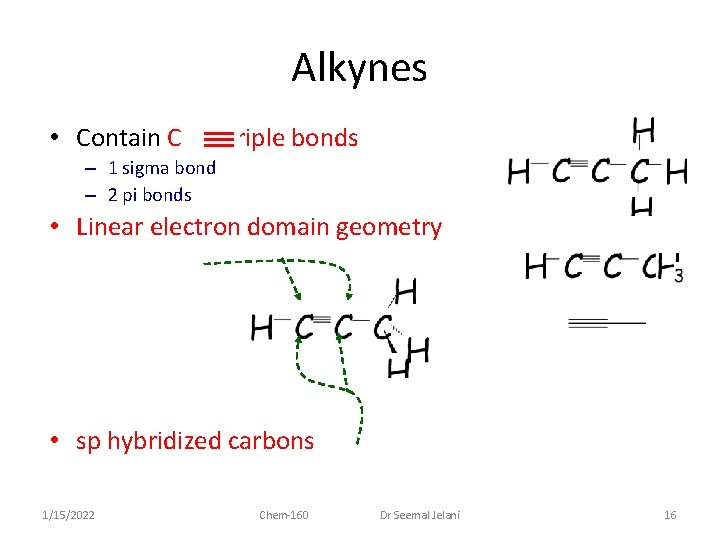
Alkynes • Contain C C triple bonds – 1 sigma bond – 2 pi bonds • Linear electron domain geometry • sp hybridized carbons 1/15/2022 Chem-160 Dr Seemal Jelani 16

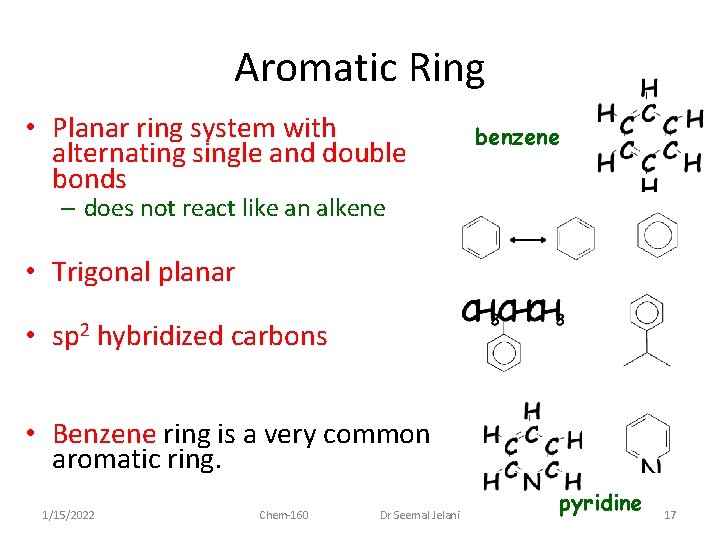
Aromatic Ring • Planar ring system with alternating single and double bonds benzene – does not react like an alkene • Trigonal planar • sp 2 hybridized carbons • Benzene ring is a very common aromatic ring. 1/15/2022 Chem-160 Dr Seemal Jelani pyridine 17

Functional Groups • Alkanes are often called saturated hydrocarbons – Organic compounds composed of carbon and hydrogen that contain the largest possible number of hydrogen atoms per carbon atom. • Alkenes, alkynes, and aromatic hydrocarbons are called unsaturated hydrocarbons – Organic compounds composed of carbon and hydrogen that contain less hydrogen than an alkane having the same number of carbon atoms 1/15/2022 Chem-160 Dr Seemal Jelani 18

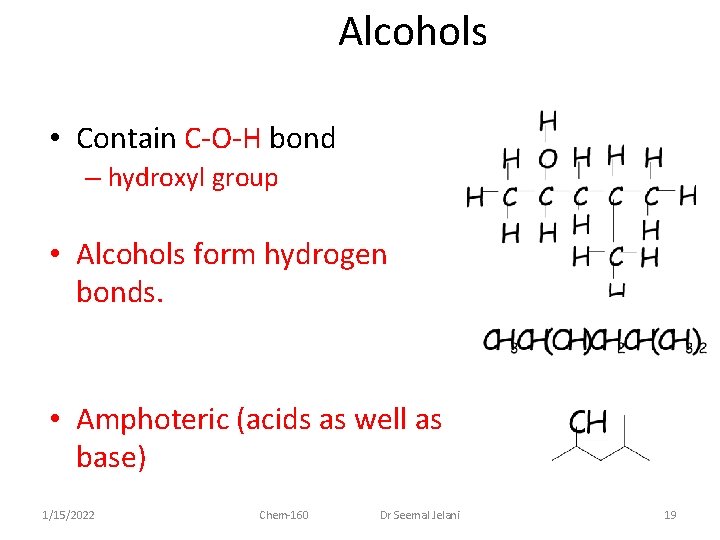
Alcohols • Contain C-O-H bond – hydroxyl group • Alcohols form hydrogen bonds. • Amphoteric (acids as well as base) 1/15/2022 Chem-160 Dr Seemal Jelani 19

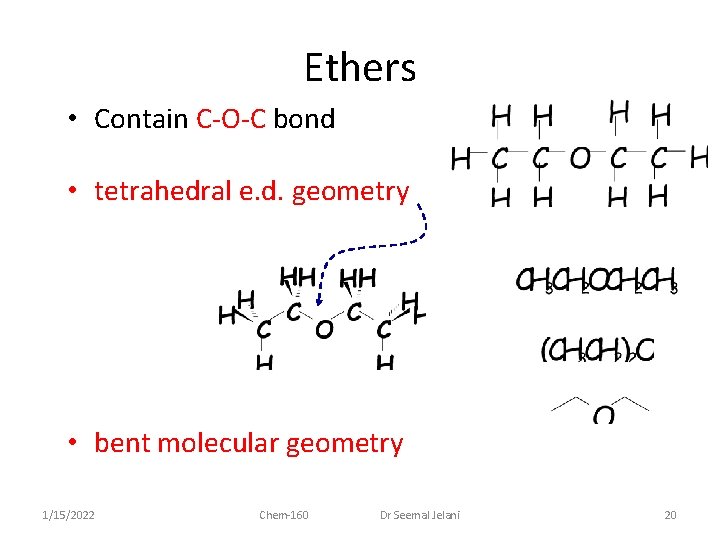
Ethers • Contain C-O-C bond • tetrahedral e. d. geometry • bent molecular geometry 1/15/2022 Chem-160 Dr Seemal Jelani 20

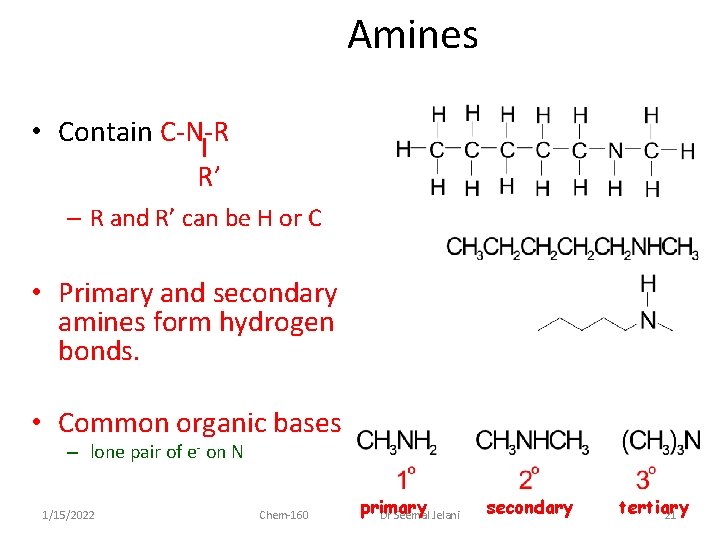
Amines • Contain C-N-R R’ – R and R’ can be H or C • Primary and secondary amines form hydrogen bonds. • Common organic bases – lone pair of e- on N 1/15/2022 Chem-160 primary Dr Seemal Jelani secondary tertiary 21

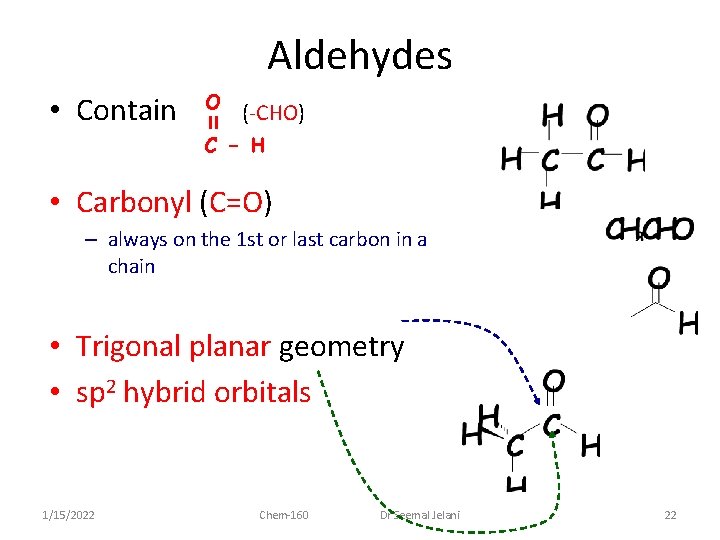
Aldehydes • Contain O C (-CHO) -H • Carbonyl (C=O) – always on the 1 st or last carbon in a chain • Trigonal planar geometry • sp 2 hybrid orbitals 1/15/2022 Chem-160 Dr Seemal Jelani 22

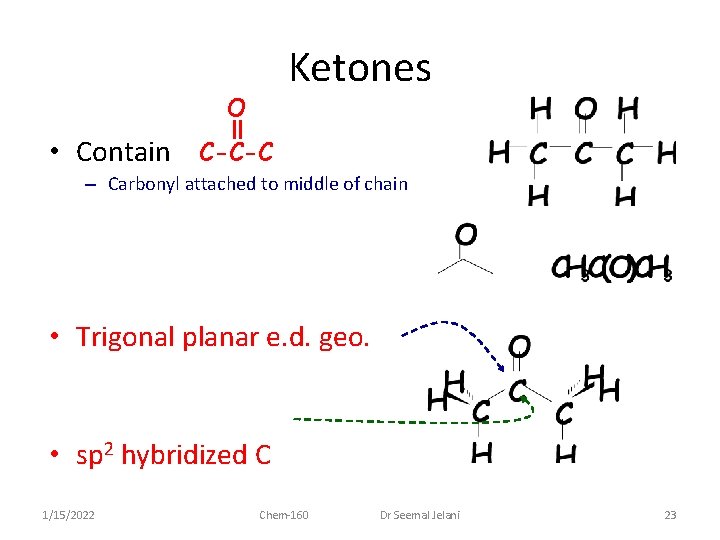
Ketones O • Contain C-C-C – Carbonyl attached to middle of chain • Trigonal planar e. d. geo. • sp 2 hybridized C 1/15/2022 Chem-160 Dr Seemal Jelani 23

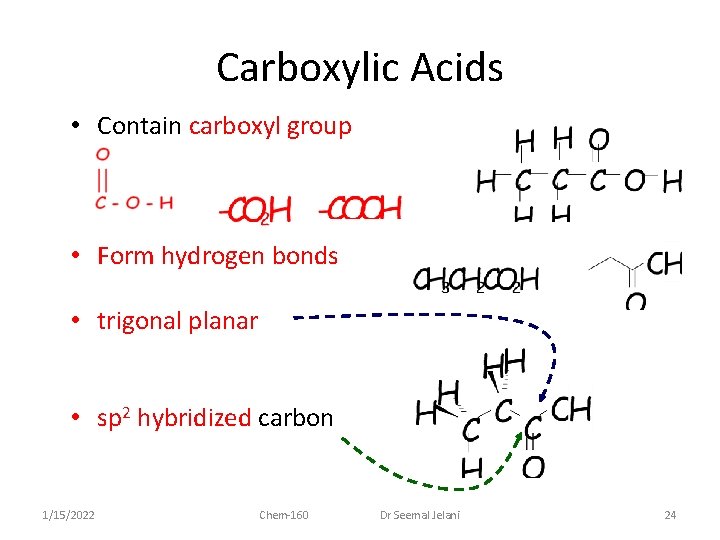
Carboxylic Acids • Contain carboxyl group • Form hydrogen bonds • trigonal planar • sp 2 hybridized carbon 1/15/2022 Chem-160 Dr Seemal Jelani 24

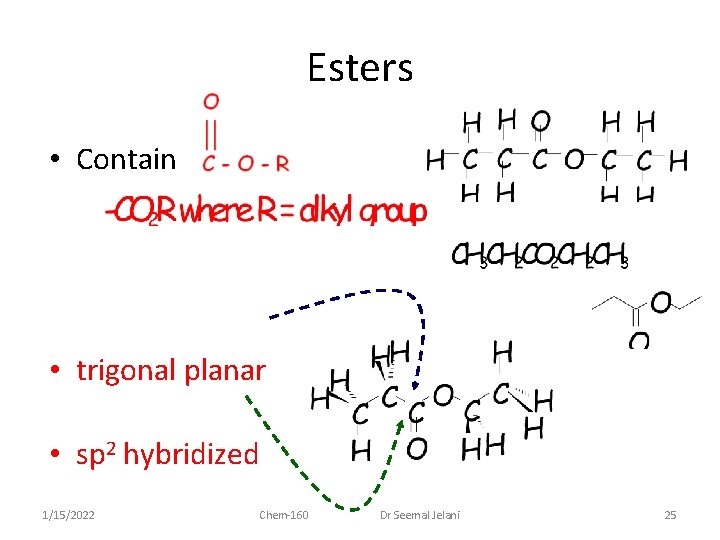
Esters • Contain • trigonal planar • sp 2 hybridized 1/15/2022 Chem-160 Dr Seemal Jelani 25

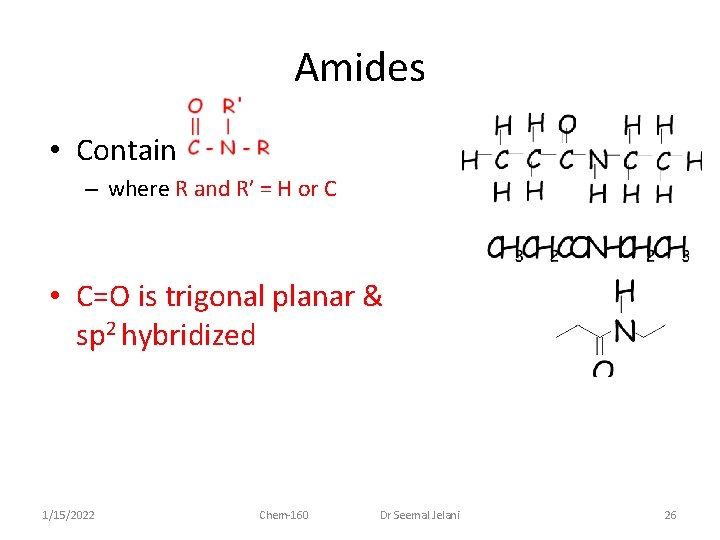
Amides • Contain – where R and R’ = H or C • C=O is trigonal planar & sp 2 hybridized 1/15/2022 Chem-160 Dr Seemal Jelani 26

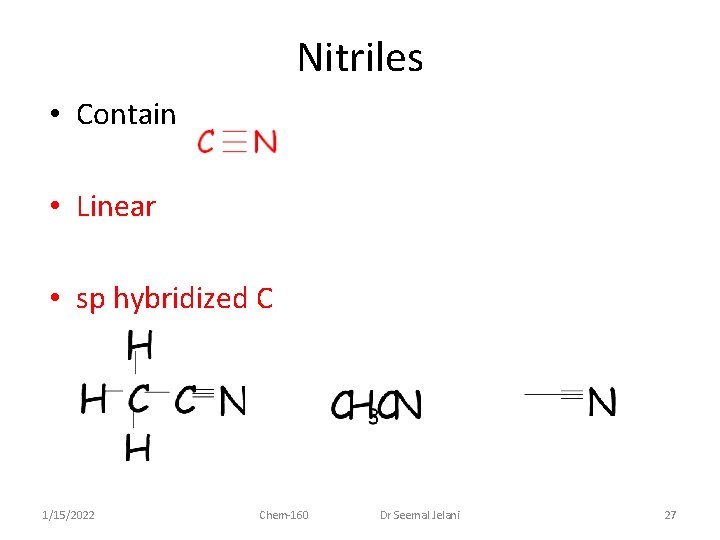
Nitriles • Contain • Linear • sp hybridized C 1/15/2022 Chem-160 Dr Seemal Jelani 27

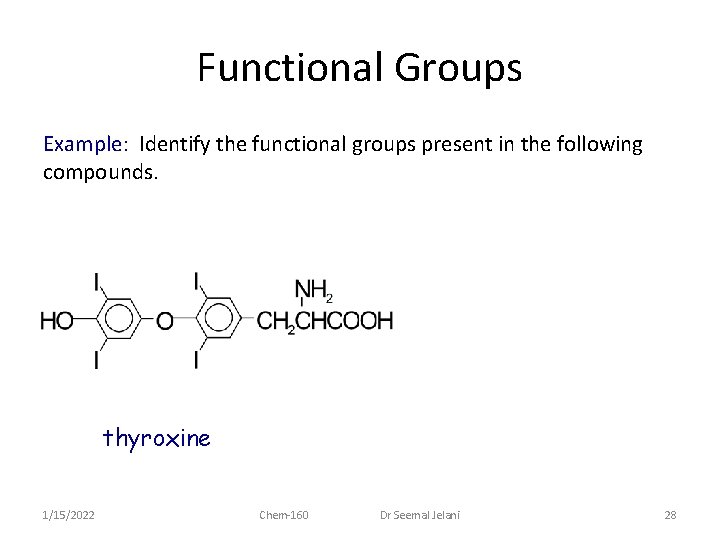
Functional Groups Example: Identify the functional groups present in the following compounds. thyroxine 1/15/2022 Chem-160 Dr Seemal Jelani 28

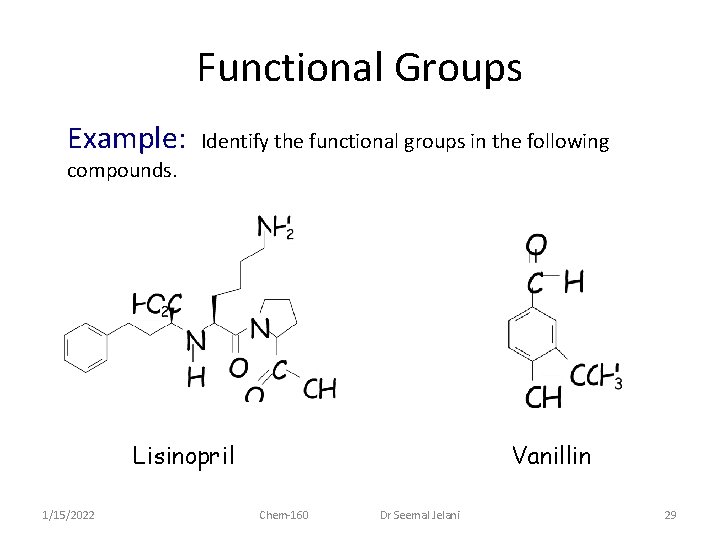
Functional Groups Example: Identify the functional groups in the following compounds. Lisinopril 1/15/2022 Vanillin Chem-160 Dr Seemal Jelani 29

Depicting Structures of Organic Compounds • Organic compounds can be depicted using a variety of formulas: – – – – Empirical formula Molecular formula Lewis structure Full structural formula Three dimensional drawings Condensed structural formula Line angle drawings 1/15/2022 Chem-160 Dr Seemal Jelani 30

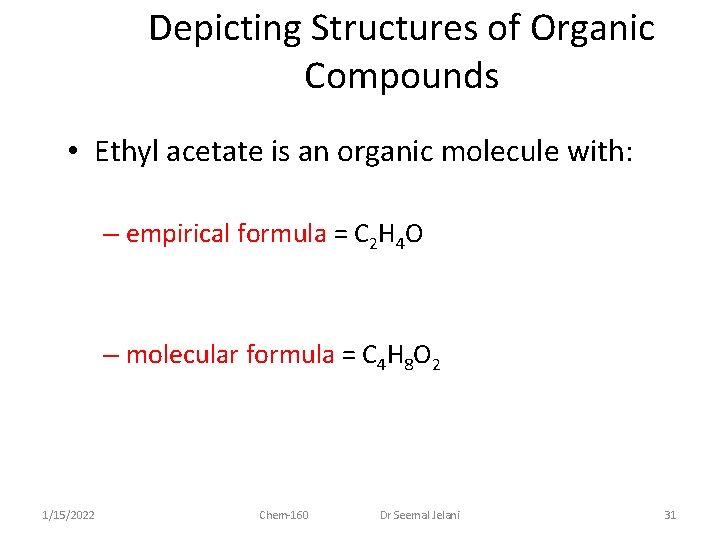
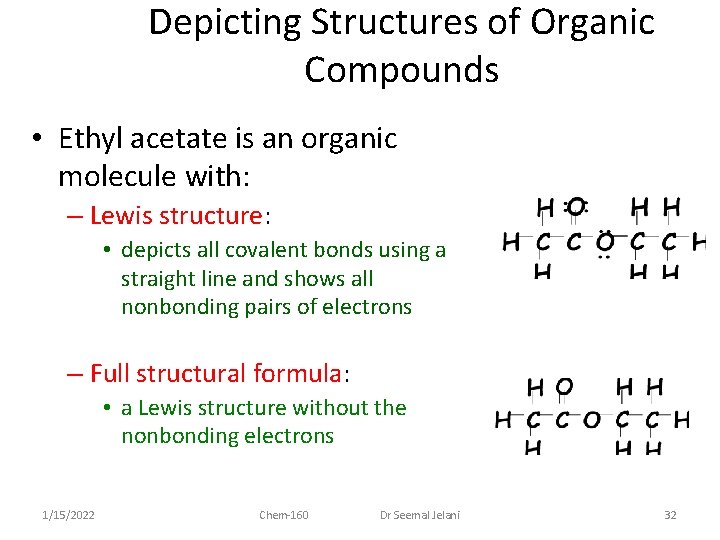
Depicting Structures of Organic Compounds • Ethyl acetate is an organic molecule with: – empirical formula = C 2 H 4 O – molecular formula = C 4 H 8 O 2 1/15/2022 Chem-160 Dr Seemal Jelani 31

Depicting Structures of Organic Compounds • Ethyl acetate is an organic molecule with: – Lewis structure: • depicts all covalent bonds using a straight line and shows all nonbonding pairs of electrons – Full structural formula: • a Lewis structure without the nonbonding electrons 1/15/2022 Chem-160 Dr Seemal Jelani 32

Depicting Structures of Organic Compounds • Ethyl acetate is an organic molecule with: – 3 -d drawing: – Condensed structural formula – Line angle drawing 1/15/2022 Chem-160 Dr Seemal Jelani 33

Alkanes Some of the simplest alkanes: 1/15/2022 You must know. Drthese!!! Chem-160 Seemal Jelani 34

Alkanes Some of the simplest alkanes: 1/15/2022 You must know these!!! Chem-160 Dr Seemal Jelani 35

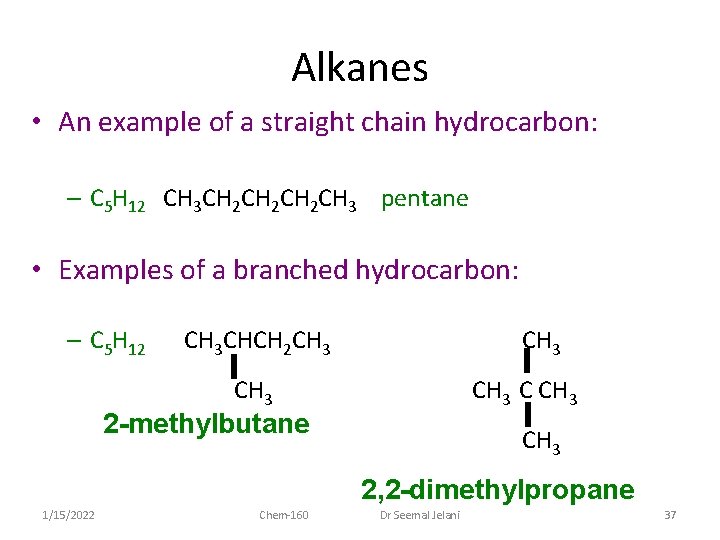
Alkanes • The previous alkanes are also called straightchain hydrocarbons: – all of the carbon atoms are joined in a continuous chain • Alkanes containing 4 or more carbons can also form branched-chain hydrocarbons (branched hydrocarbons) – some of the carbon atoms form a “branch” or sidechain off of the main chain 1/15/2022 Chem-160 Dr Seemal Jelani 36

Alkanes • An example of a straight chain hydrocarbon: – C 5 H 12 CH 3 CH 2 CH 2 CH 3 pentane • Examples of a branched hydrocarbon: – C 5 H 12 CH 3 CHCH 2 CH 3 C CH 3 2 -methylbutane CH 3 2, 2 -dimethylpropane 1/15/2022 Chem-160 Dr Seemal Jelani 37

Alkanes • The three structures shown previously for C 5 H 12 are structural isomers: – compounds with the same molecular formula but different bonding arrangements • Structural isomers generally have different properties: – different melting points – different boiling points – often different chemical reactivity 1/15/2022 Chem-160 Dr Seemal Jelani 38

Alkanes • Organic compounds can be named either using common names or IUPAC names. • You must be able to name alkanes, alkenes, alkynes, and alcohols with 10 or fewer carbons in the main chain using the IUPAC naming system. 1/15/2022 Chem-160 Dr Seemal Jelani 39

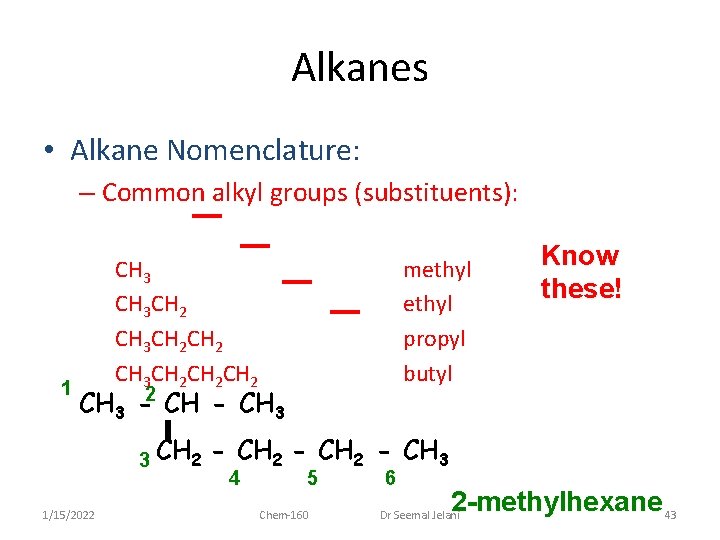
Alkanes • Alkane Nomenclature: – Find the longest continuous chain of carbon atoms and use the name of the chain for the base name of the compound: • longest chain may not always be written in a straight line 1 CH 3 -2 CH - CH 3 3 CH 2 1/15/2022 - CH 3 4 5 Chem-160 Base name: hexane 6 Dr Seemal Jelani 40

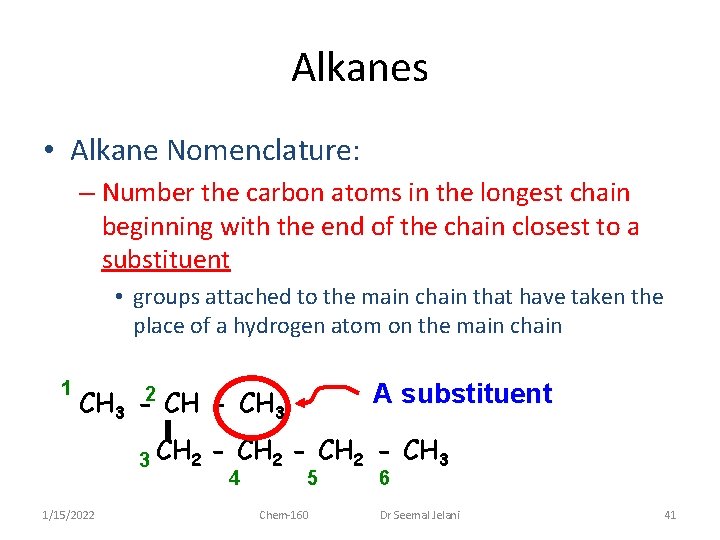
Alkanes • Alkane Nomenclature: – Number the carbon atoms in the longest chain beginning with the end of the chain closest to a substituent • groups attached to the main chain that have taken the place of a hydrogen atom on the main chain 1 A substituent CH 3 -2 CH - CH 3 3 CH 2 1/15/2022 - CH 3 4 5 Chem-160 6 Dr Seemal Jelani 41

Alkanes • Alkane Nomenclature: – Name and give the location of each substituent group • A substituent group that is formed by removing an H atom from an alkane is called an alkyl group: – Name alkyl groups by dropping the “ane” ending of the parent alkane and adding “yl” 1/15/2022 Chem-160 Dr Seemal Jelani 42

Alkanes • Alkane Nomenclature: – Common alkyl groups (substituents): 1 CH 3 CH 2 CH 2 methyl propyl butyl CH 3 -2 CH - CH 3 3 CH 2 1/15/2022 Know these! - CH 2 - CH 3 4 5 Chem-160 6 2 -methylhexane 43 Dr Seemal Jelani

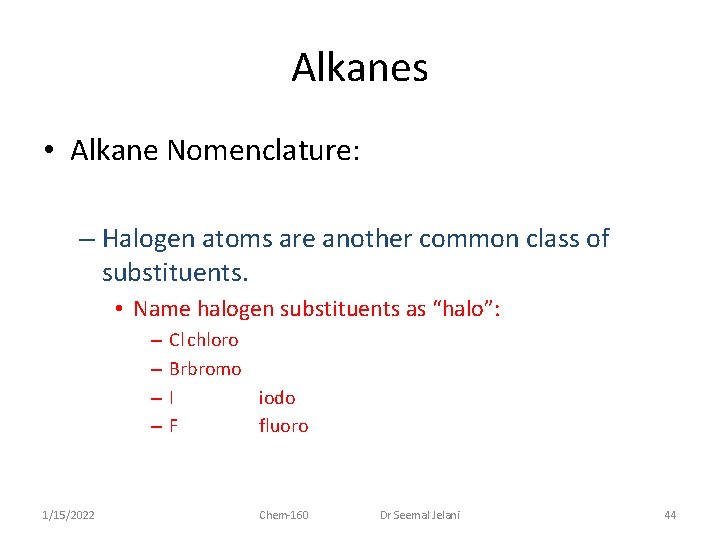
Alkanes • Alkane Nomenclature: – Halogen atoms are another common class of substituents. • Name halogen substituents as “halo”: – – 1/15/2022 Cl chloro Brbromo I iodo F fluoro Chem-160 Dr Seemal Jelani 44

Alkanes • Alkane Nomenclature: – When two or more substituents are present, list them in alphabetical order: • Butyl vs. ethyl vs. methyl vs. propyl – When more than one of the same substituent is present (i. e. two methyl groups), use prefixes to indicate the number: • • 1/15/2022 Di = two Tri = three Tetra = four Penta = five Know these. Chem-160 Dr Seemal Jelani 45

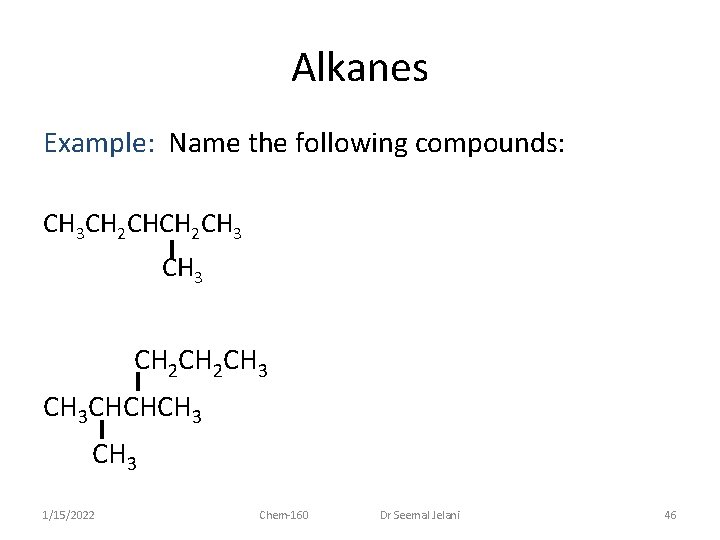
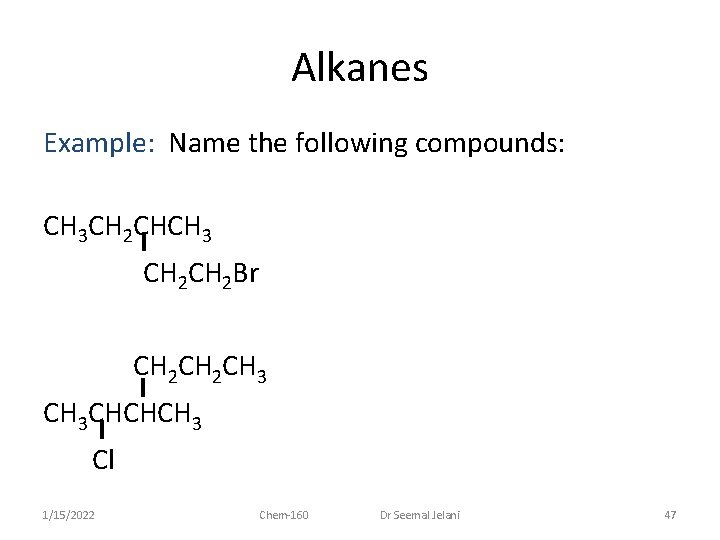
Alkanes Example: Name the following compounds: CH 3 CH 2 CH 3 CH 3 CHCHCH 3 1/15/2022 Chem-160 Dr Seemal Jelani 46

Alkanes Example: Name the following compounds: CH 3 CH 2 CHCH 3 CH 2 Br CH 2 CH 3 CHCHCH 3 Cl 1/15/2022 Chem-160 Dr Seemal Jelani 47

Alkanes • You must also be able to write the structure of an alkane when given the IUPAC name. • To do so: – Identify the main chain and draw the carbons in it – Identify the substituents (type and #) and attach them to the appropriate carbon atoms on the main chain. – Add hydrogen atoms to the carbons to make a total of 4 bonds to each carbon 1/15/2022 Chem-160 Dr Seemal Jelani 48

Alkanes Example: Write the condensed structure for the following compounds: 3, 3 -dimethylpentane 3 -ethyl-2 -methylhexane 2 -methyl-4 -propyloctane 1, 2 -dichloro-3 -methylheptane 1/15/2022 Chem-160 Dr Seemal Jelani 49

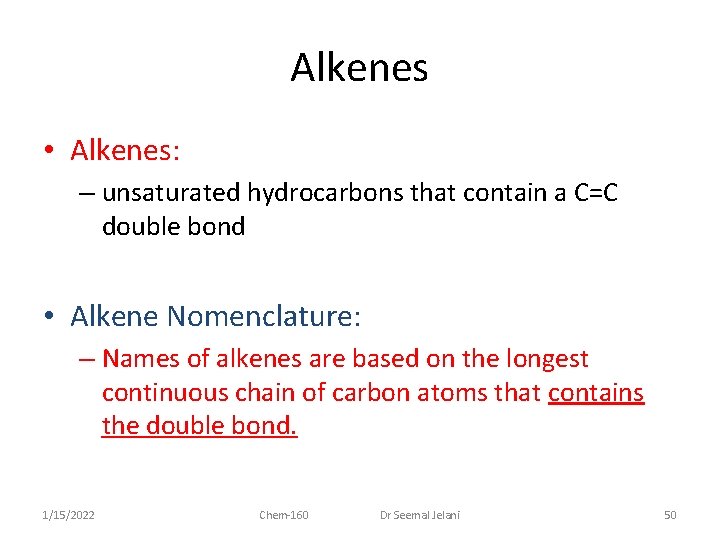
Alkenes • Alkenes: – unsaturated hydrocarbons that contain a C=C double bond • Alkene Nomenclature: – Names of alkenes are based on the longest continuous chain of carbon atoms that contains the double bond. 1/15/2022 Chem-160 Dr Seemal Jelani 50

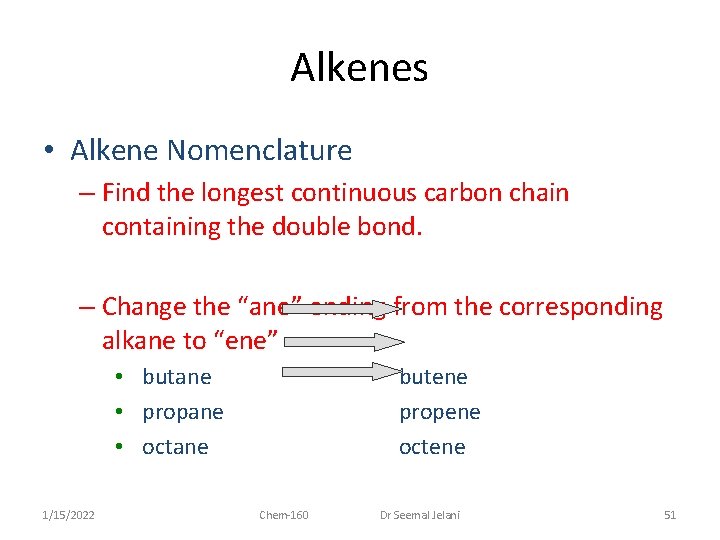
Alkenes • Alkene Nomenclature – Find the longest continuous carbon chain containing the double bond. – Change the “ane” ending from the corresponding alkane to “ene” • butane • propane • octane 1/15/2022 butene propene octene Chem-160 Dr Seemal Jelani 51

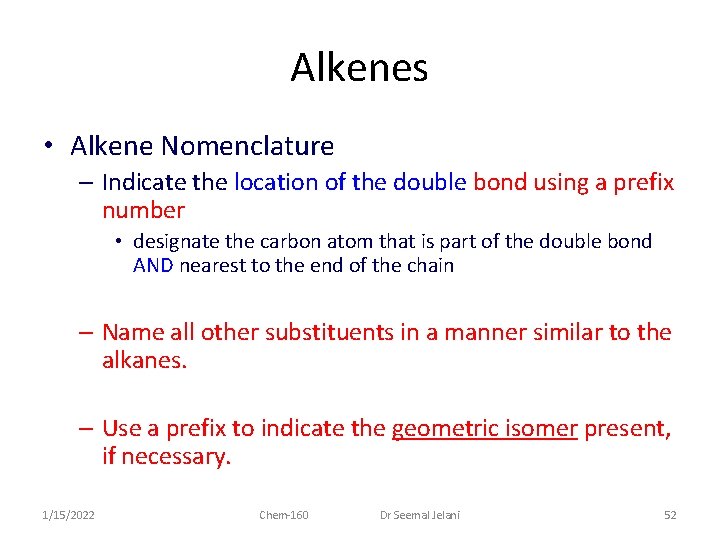
Alkenes • Alkene Nomenclature – Indicate the location of the double bond using a prefix number • designate the carbon atom that is part of the double bond AND nearest to the end of the chain – Name all other substituents in a manner similar to the alkanes. – Use a prefix to indicate the geometric isomer present, if necessary. 1/15/2022 Chem-160 Dr Seemal Jelani 52

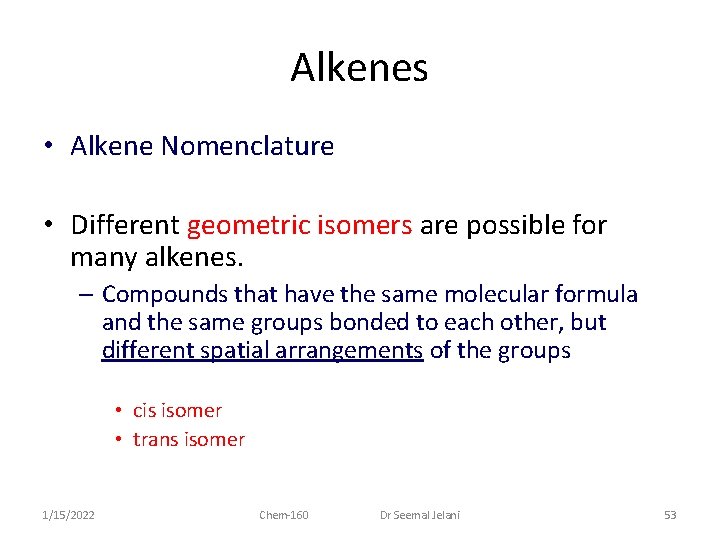
Alkenes • Alkene Nomenclature • Different geometric isomers are possible for many alkenes. – Compounds that have the same molecular formula and the same groups bonded to each other, but different spatial arrangements of the groups • cis isomer • trans isomer 1/15/2022 Chem-160 Dr Seemal Jelani 53

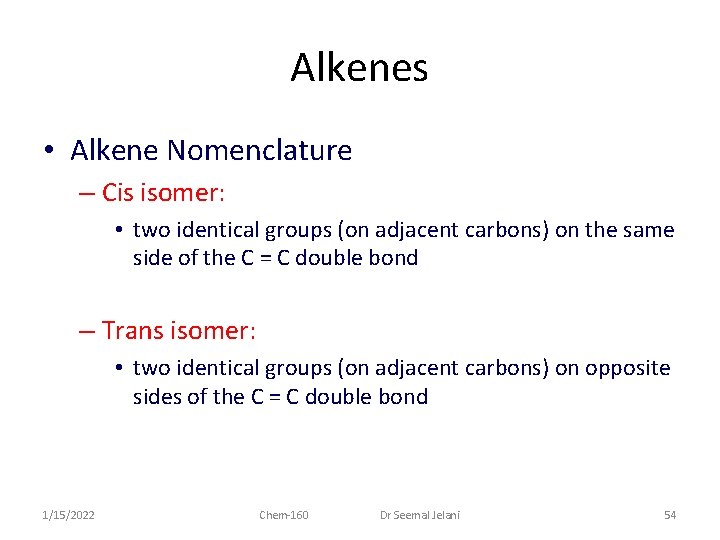
Alkenes • Alkene Nomenclature – Cis isomer: • two identical groups (on adjacent carbons) on the same side of the C = C double bond – Trans isomer: • two identical groups (on adjacent carbons) on opposite sides of the C = C double bond 1/15/2022 Chem-160 Dr Seemal Jelani 54

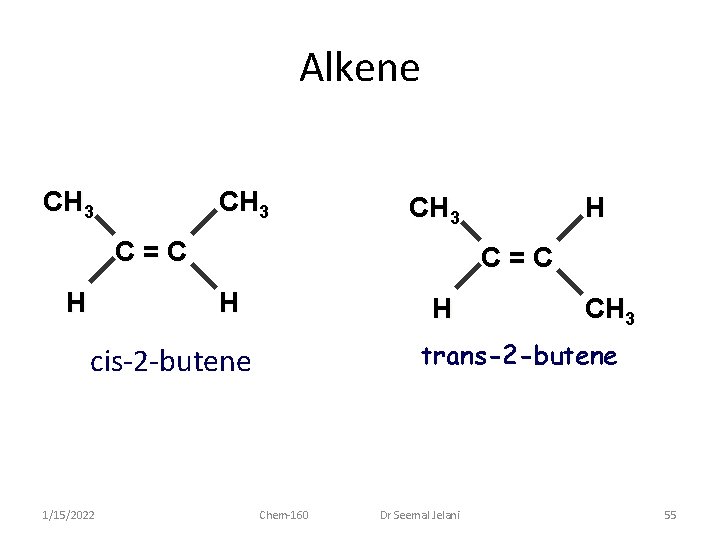
Alkene CH 3 C=C H H CH 3 trans-2 -butene cis-2 -butene 1/15/2022 H Chem-160 Dr Seemal Jelani 55

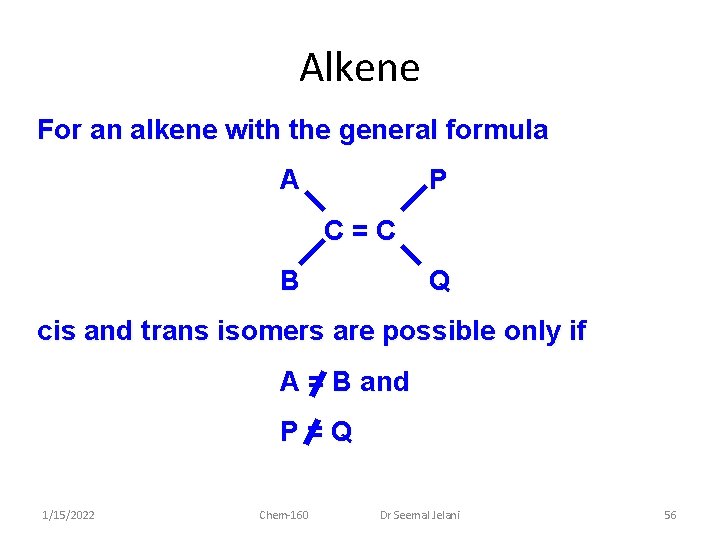
Alkene For an alkene with the general formula A P C=C B Q cis and trans isomers are possible only if A = B and P=Q 1/15/2022 Chem-160 Dr Seemal Jelani 56

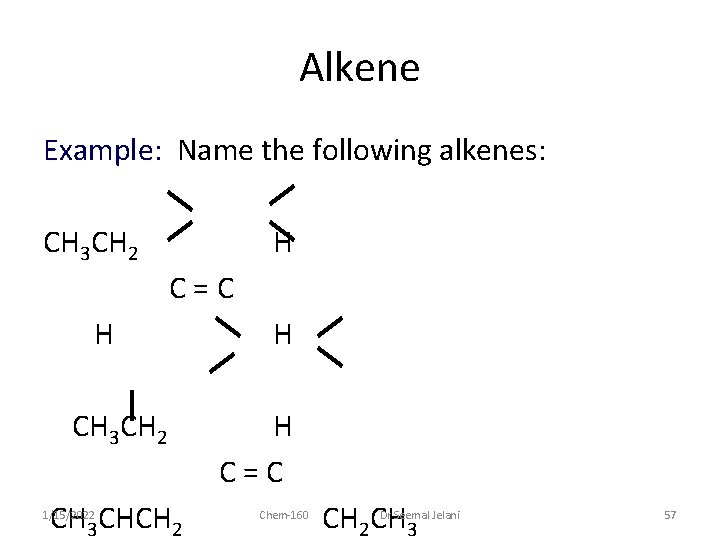
Alkene Example: Name the following alkenes: CH 3 CH 2 H C=C H CH 3 CH 2 CH 3 CHCH 2 1/15/2022 H H C=C Chem-160 CH 2 CH 3 Dr Seemal Jelani 57

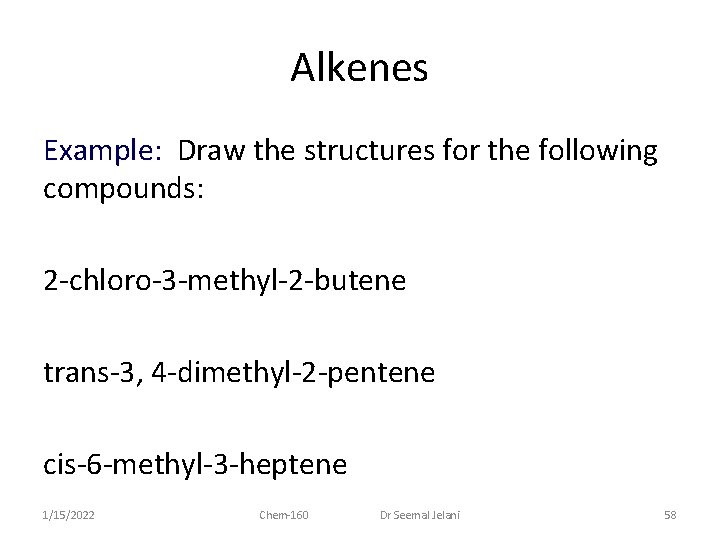
Alkenes Example: Draw the structures for the following compounds: 2 -chloro-3 -methyl-2 -butene trans-3, 4 -dimethyl-2 -pentene cis-6 -methyl-3 -heptene 1/15/2022 Chem-160 Dr Seemal Jelani 58

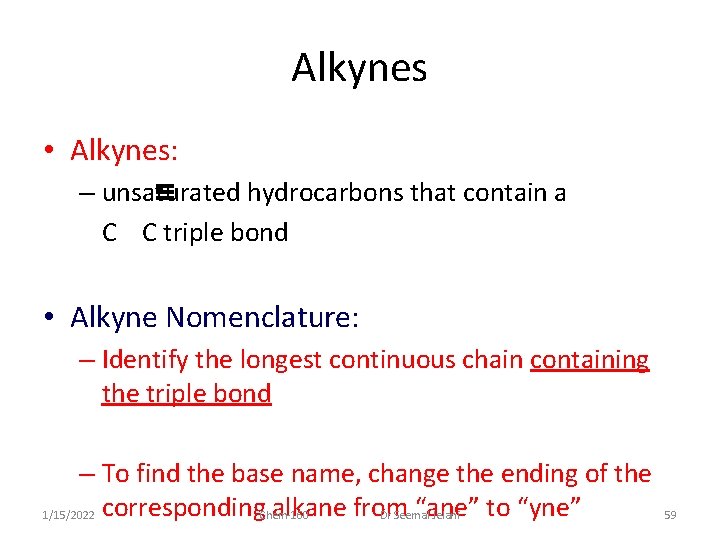
Alkynes • Alkynes: – unsaturated hydrocarbons that contain a C C triple bond • Alkyne Nomenclature: – Identify the longest continuous chain containing the triple bond – To find the base name, change the ending of the alkane from “ane” to “yne” 1/15/2022 corresponding Chem-160 Dr Seemal Jelani 59

Alkynes • Alkyne Nomenclature: – Use a number to designate the position of the triple bond • number from the end of the chain closest to the triple bond – just like with alkenes – Name substituents like you do with alkanes and alkenes 1/15/2022 Chem-160 Dr Seemal Jelani 60

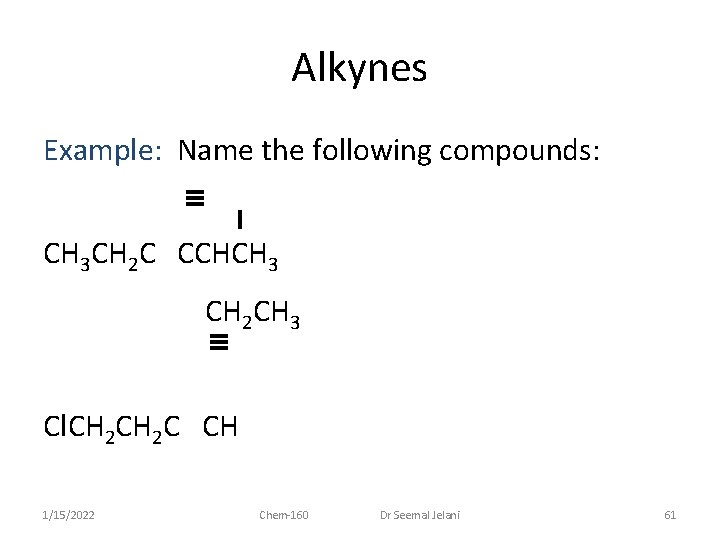
Alkynes Example: Name the following compounds: CH 3 CH 2 C CCHCH 3 CH 2 CH 3 Cl. CH 2 C CH 1/15/2022 Chem-160 Dr Seemal Jelani 61

Alkynes Example: Draw the following alkynes. 4 -chloro-2 -pentyne 3 -propyl-1 -hexyne 1/15/2022 Chem-160 Dr Seemal Jelani 62
 Seemal desai
Seemal desai Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic chemistry
Organic chemistry Macromolecule cheat sheet
Macromolecule cheat sheet Organic chemistry chapter 1
Organic chemistry chapter 1 Chapter 3 organic chemistry
Chapter 3 organic chemistry Meth eth prop but
Meth eth prop but Organic synthesis via enolates
Organic synthesis via enolates Hybridisation
Hybridisation Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry
Organic chemistry David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition What is organic chemistry like
What is organic chemistry like Organic chemistry
Organic chemistry Organic and biochemistry
Organic and biochemistry Chemistry of soap making
Chemistry of soap making Organic chemistry conversion chart
Organic chemistry conversion chart Carbohydrates organic chemistry
Carbohydrates organic chemistry Iupac
Iupac Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry
Organic chemistry Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual Hono organic chemistry
Hono organic chemistry Ee organic chemistry
Ee organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? Kiliani fischer synthesis
Kiliani fischer synthesis Organic chemistry
Organic chemistry Mind map organic chemistry
Mind map organic chemistry Organic chemistry
Organic chemistry Analytical chemistry chapter 1
Analytical chemistry chapter 1 Silver nitrate test
Silver nitrate test How to calculate yield in chemistry
How to calculate yield in chemistry Condensed formula
Condensed formula Is ch4o organic or inorganic
Is ch4o organic or inorganic Wiley
Wiley Organic chemistry
Organic chemistry Is alkane an organic compound
Is alkane an organic compound Rhodopsin cgmp
Rhodopsin cgmp Organic chemistry myanmar
Organic chemistry myanmar Ester organic chemistry
Ester organic chemistry Organic chemistry grade 10
Organic chemistry grade 10 Resonance in benzyl carbocation
Resonance in benzyl carbocation Organic chemistry case studies
Organic chemistry case studies -oate functional group
-oate functional group Where is lysine found
Where is lysine found A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Propyl bromide
Propyl bromide Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Halohydrin formation
Halohydrin formation Prop but pent hex hept oct
Prop but pent hex hept oct Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Ethos
Ethos Brooklyn college organic chemistry
Brooklyn college organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Polarimetry organic chemistry
Polarimetry organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Iodine test for starch
Iodine test for starch Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry vocabulary
Organic chemistry vocabulary