Formal Charges Dr Seemal Jelani 1152020 1 Formal













- Slides: 37

Formal Charges Dr Seemal Jelani 11/5/2020 1

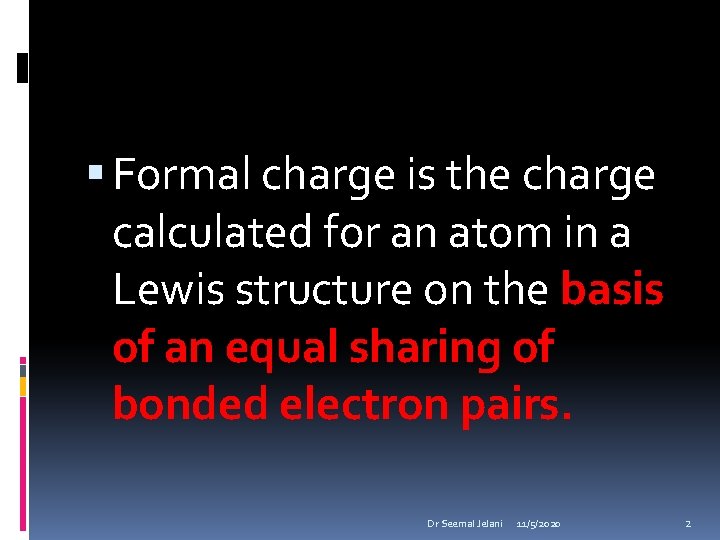
Formal charge is the charge calculated for an atom in a Lewis structure on the basis of an equal sharing of bonded electron pairs. Dr Seemal Jelani 11/5/2020 2

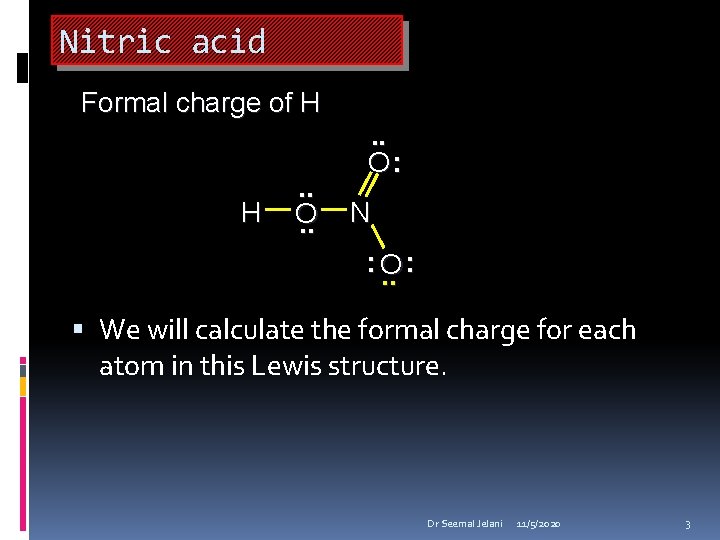
Nitric acid Formal charge of H H . . O: N : O. . : We will calculate the formal charge for each atom in this Lewis structure. Dr Seemal Jelani 11/5/2020 3

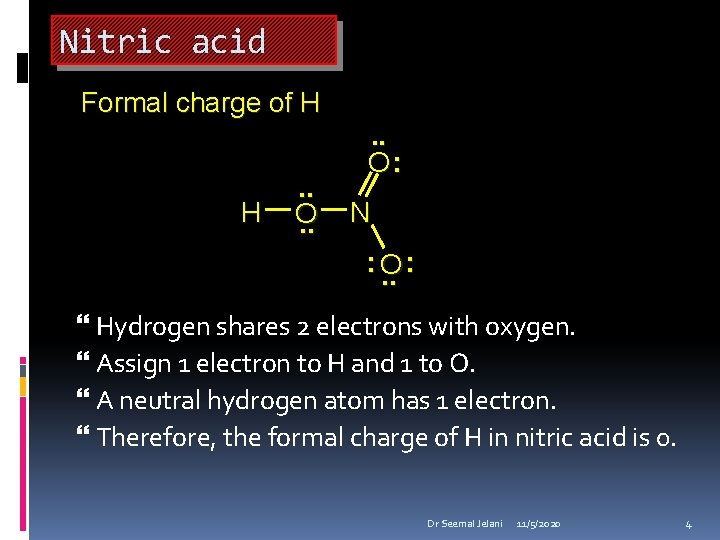
Nitric acid Formal charge of H H . . O: N : O. . : Hydrogen shares 2 electrons with oxygen. Assign 1 electron to H and 1 to O. A neutral hydrogen atom has 1 electron. Therefore, the formal charge of H in nitric acid is 0. Dr Seemal Jelani 11/5/2020 4

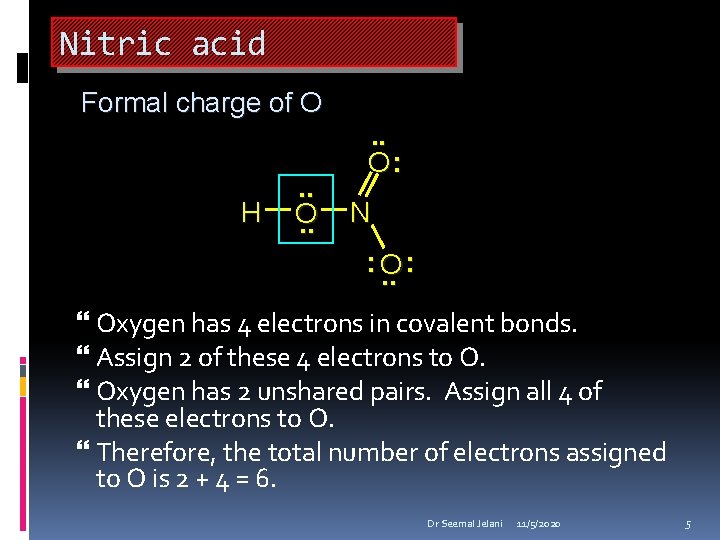
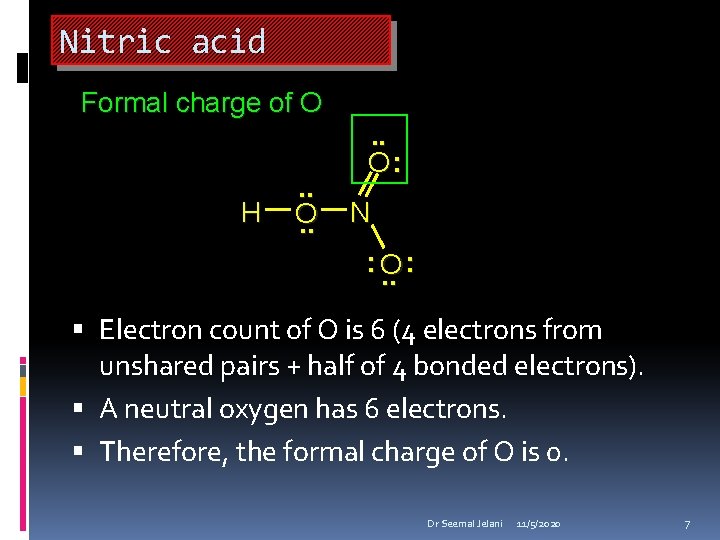
Nitric acid Formal charge of O H . . O: N : O. . : Oxygen has 4 electrons in covalent bonds. Assign 2 of these 4 electrons to O. Oxygen has 2 unshared pairs. Assign all 4 of these electrons to O. Therefore, the total number of electrons assigned to O is 2 + 4 = 6. Dr Seemal Jelani 11/5/2020 5

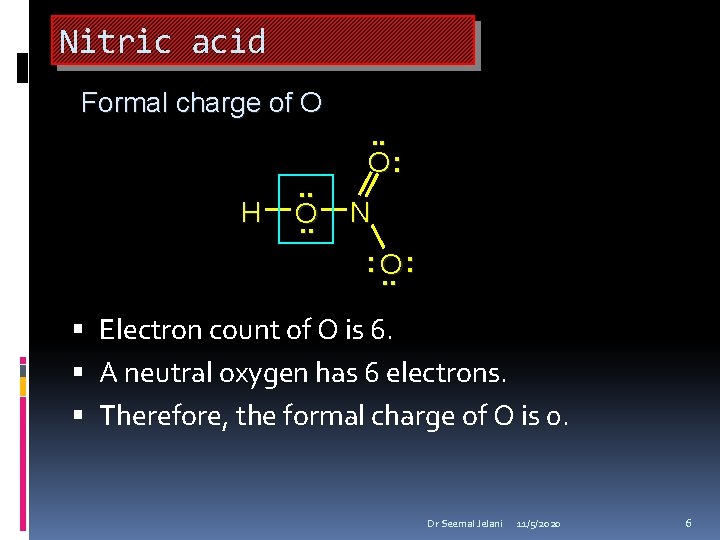
Nitric acid Formal charge of O H . . O: N : O. . : Electron count of O is 6. A neutral oxygen has 6 electrons. Therefore, the formal charge of O is 0. Dr Seemal Jelani 11/5/2020 6

Nitric acid Formal charge of O H . . O: N : O. . : Electron count of O is 6 (4 electrons from unshared pairs + half of 4 bonded electrons). A neutral oxygen has 6 electrons. Therefore, the formal charge of O is 0. Dr Seemal Jelani 11/5/2020 7

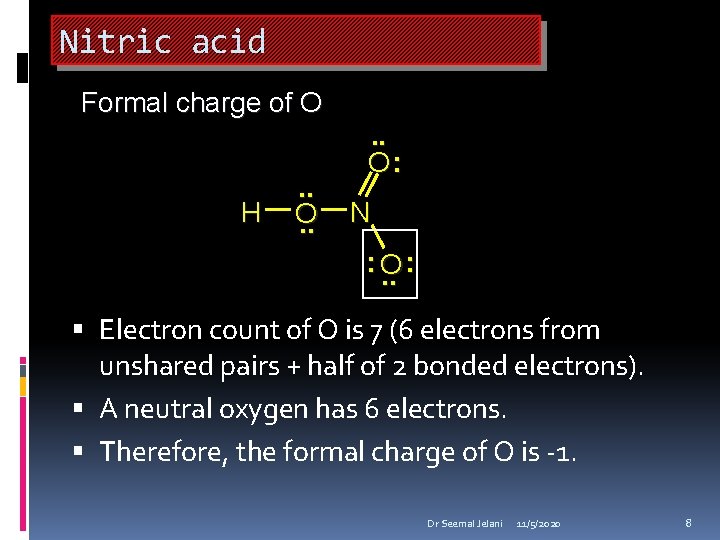
Nitric acid Formal charge of O H . . O: N : O. . : Electron count of O is 7 (6 electrons from unshared pairs + half of 2 bonded electrons). A neutral oxygen has 6 electrons. Therefore, the formal charge of O is -1. Dr Seemal Jelani 11/5/2020 8

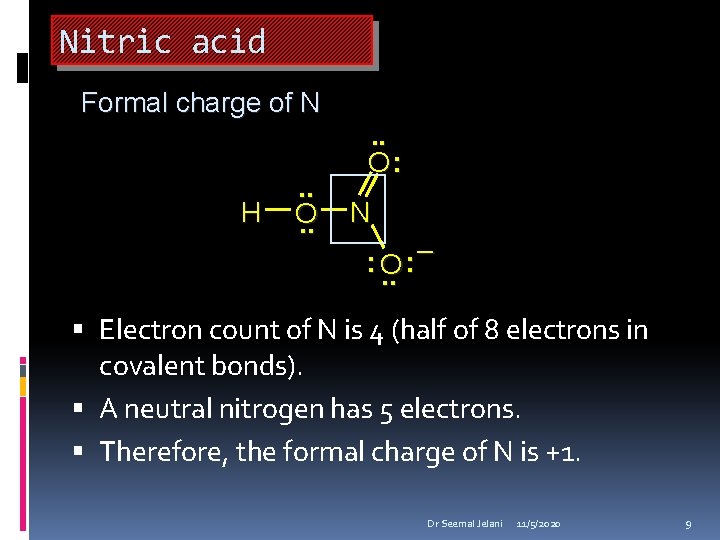
Nitric acid Formal charge of N H . . O: N : O. . – : Electron count of N is 4 (half of 8 electrons in covalent bonds). A neutral nitrogen has 5 electrons. Therefore, the formal charge of N is +1. Dr Seemal Jelani 11/5/2020 9

Nitric acid Formal charges H . . O: N+ : O. . – : A Lewis structure is not complete unless formal charges (if any) are shown. Dr Seemal Jelani 11/5/2020 10

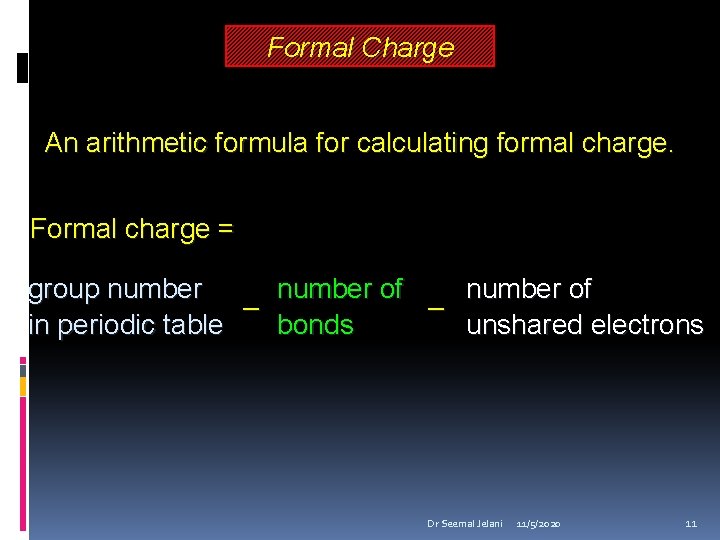
Formal Charge An arithmetic formula for calculating formal charge. Formal charge = group number of – – in periodic table bonds unshared electrons Dr Seemal Jelani 11/5/2020 11

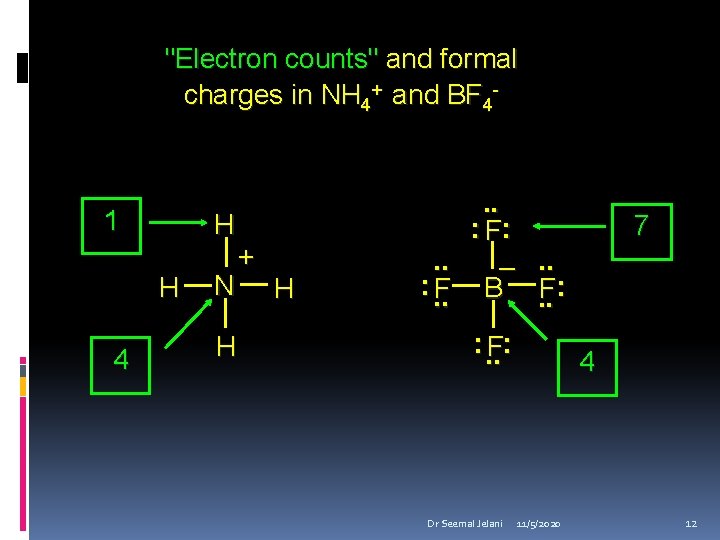
"Electron counts" and formal charges in NH 4+ and BF 4 - 1 H H 4 N H + H . . : F: . . –. . : . . F B. . F: : . . F: Dr Seemal Jelani 7 4 11/5/2020 12

CONDENSED STRUCTURAL FORMULAS Dr Seemal Jelani 11/5/2020 13

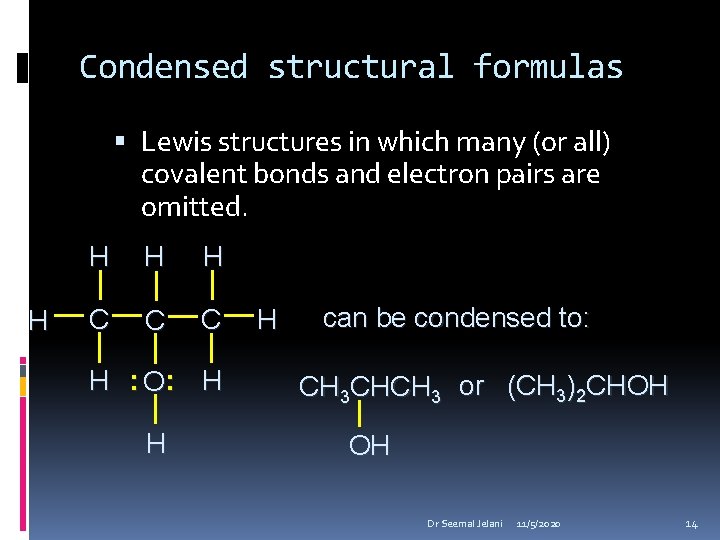
Condensed structural formulas Lewis structures in which many (or all) covalent bonds and electron pairs are omitted. H H C C C H : O: H H H can be condensed to: CH 3 CHCH 3 or (CH 3)2 CHOH OH Dr Seemal Jelani 11/5/2020 14

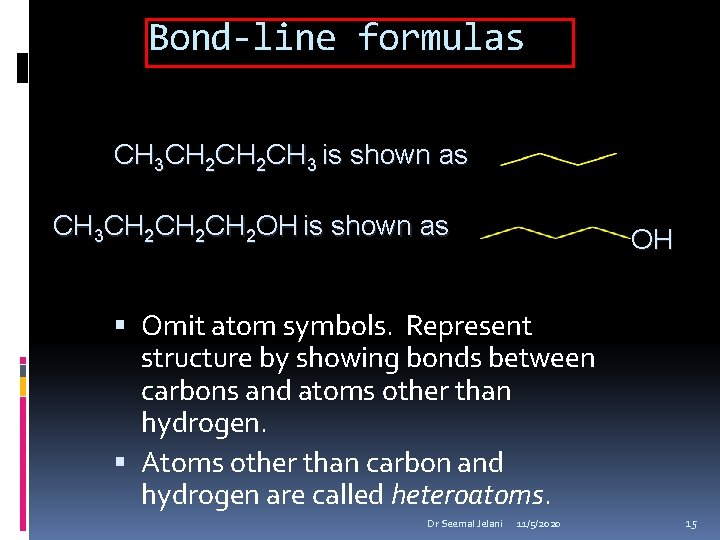
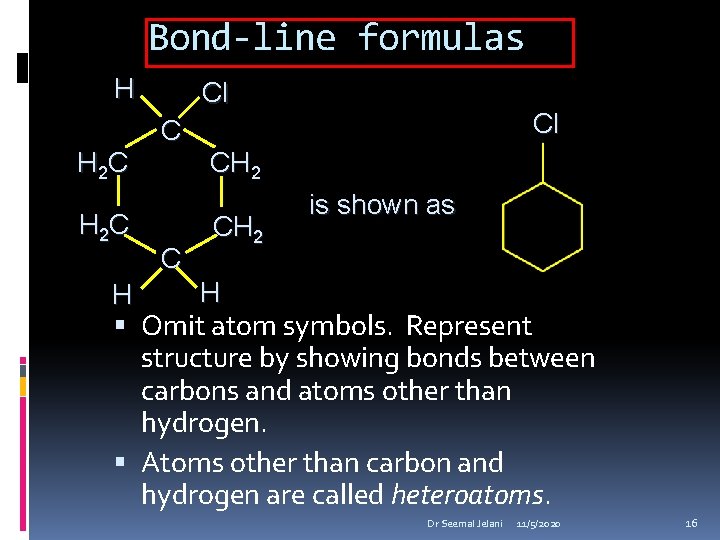
Bond-line formulas CH 3 CH 2 CH 3 is shown as CH 3 CH 2 CH 2 OH is shown as OH Omit atom symbols. Represent structure by showing bonds between carbons and atoms other than hydrogen. Atoms other than carbon and hydrogen are called heteroatoms. Dr Seemal Jelani 11/5/2020 15

Bond-line formulas H H 2 C Cl CH 2 is shown as H H Omit atom symbols. Represent structure by showing bonds between carbons and atoms other than hydrogen. Atoms other than carbon and hydrogen are called heteroatoms. Dr Seemal Jelani 11/5/2020 16

Constitutional Isomers Dr Seemal Jelani 11/5/2020 17

Constitutional isomers Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ in the order in which the atoms are connected. An older term for constitutional isomers is “structural isomers. ” Dr Seemal Jelani 11/5/2020 18

A Historical Note NH 4 OCN Ammonium cyanate O H 2 NCNH 2 Urea In 1823 Friedrich Wöhler discovered that when ammonium cyanate was dissolved in hot water, it was converted to urea. Ammonium cyanate and urea are constitutional isomers of CH 4 N 2 O. Ammonium cyanate is “inorganic. ” Urea is “organic. ” Wöhler is credited with an important early contribution that helped overturn theory of “vitalism. ” Dr Seemal Jelani 11/5/2020 19

Examples of constitutional isomers H H C H . . O: N+ – : : O. . Nitromethane H H C . . O. . N. . O: H Methyl nitrite Both have the molecular formula CH 3 NO 2 but the atoms are connected in a different order. Dr Seemal Jelani 11/5/2020 20

Shapes Dr Seemal Jelani 11/5/2020 21

Dr Seemal Jelani 11/5/2020 22

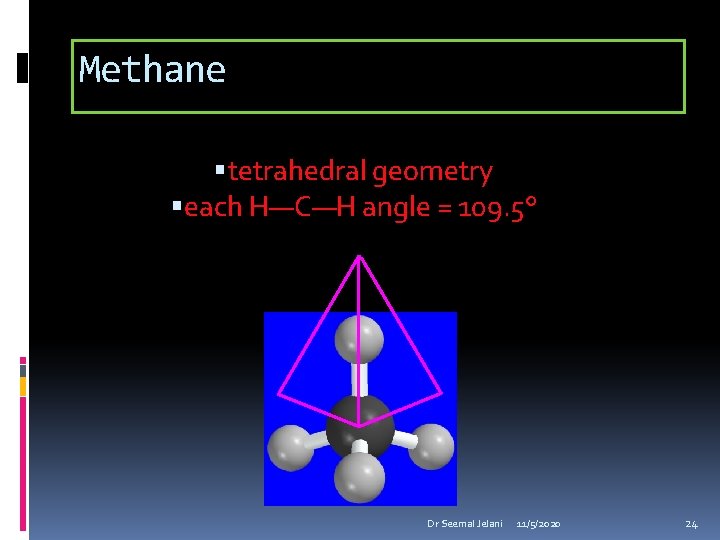
Methane tetrahedral geometry H—C—H angle = 109. 5° Dr Seemal Jelani 11/5/2020 23

Methane tetrahedral geometry each H—C—H angle = 109. 5° Dr Seemal Jelani 11/5/2020 24

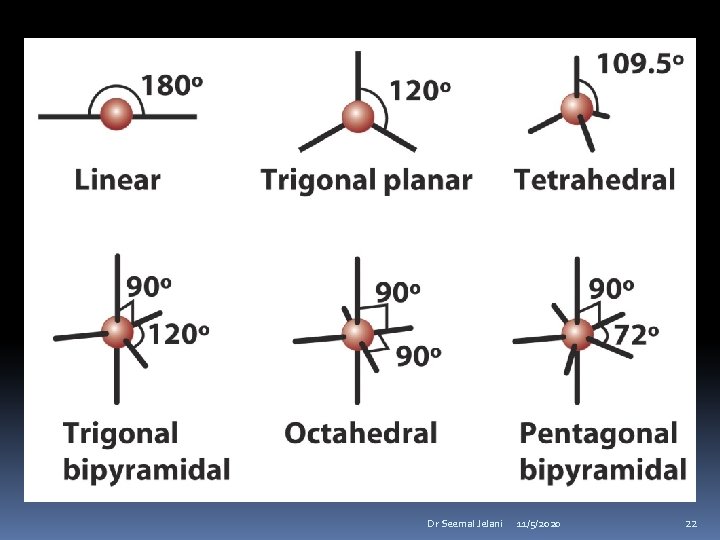
Valence Shell Electron Pair Repulsions The most stable arrangement of groups attached to a central atom is the one that has the maximum separation of electron pairs (bonded or nonbonded). Dr Seemal Jelani 11/5/2020 25

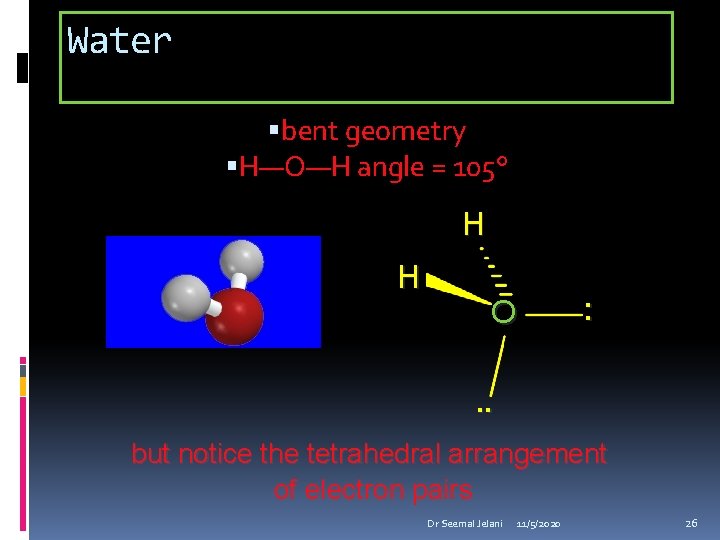
Water bent geometry H—O—H angle = 105° H H O : . . but notice the tetrahedral arrangement of electron pairs Dr Seemal Jelani 11/5/2020 26

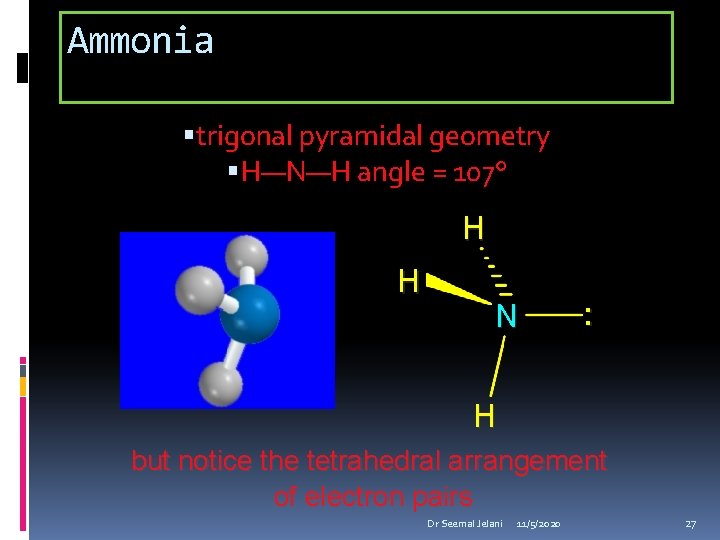
Ammonia trigonal pyramidal geometry H—N—H angle = 107° H H N : H but notice the tetrahedral arrangement of electron pairs Dr Seemal Jelani 11/5/2020 27

Boron Trifluoride F—B—F angle = 120° trigonal planar geometry allows for maximum separation of three electron pairs Dr Seemal Jelani 11/5/2020 28

Formaldehyde: CH 2=O H—C—H and H—C—O angles are close to 120° trigonal planar geometry H C O H Dr Seemal Jelani 11/5/2020 29

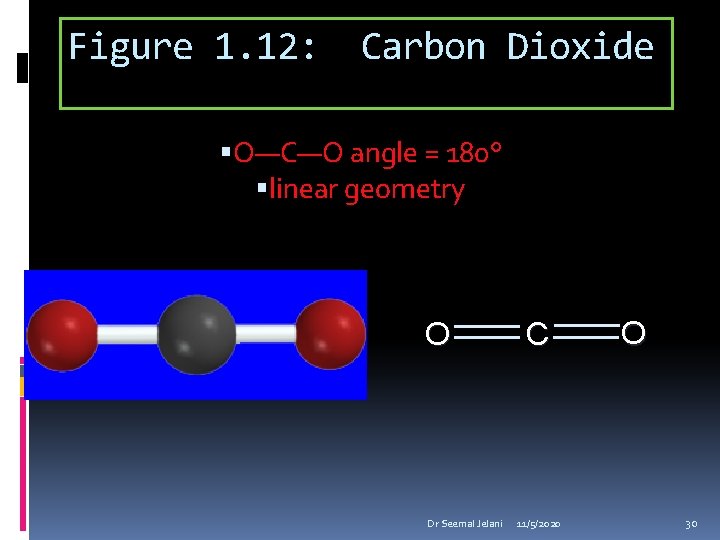
Figure 1. 12: Carbon Dioxide O—C—O angle = 180° linear geometry O Dr Seemal Jelani C 11/5/2020 O 30

Polar Covalent Bonds and Electronegativity Dr Seemal Jelani 11/5/2020 31

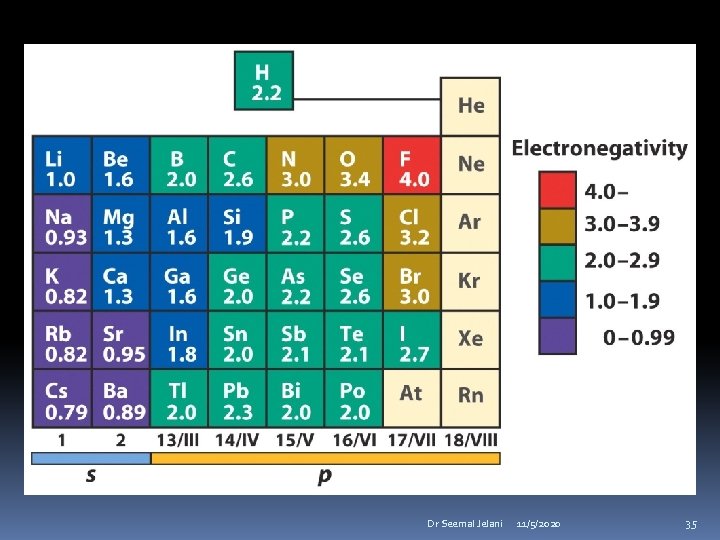
Electronegativity is a measure of an element to attract electrons toward itself when bonded to another element. An electronegative element attracts electrons. An electropositive element releases electrons. Dr Seemal Jelani 11/5/2020 32

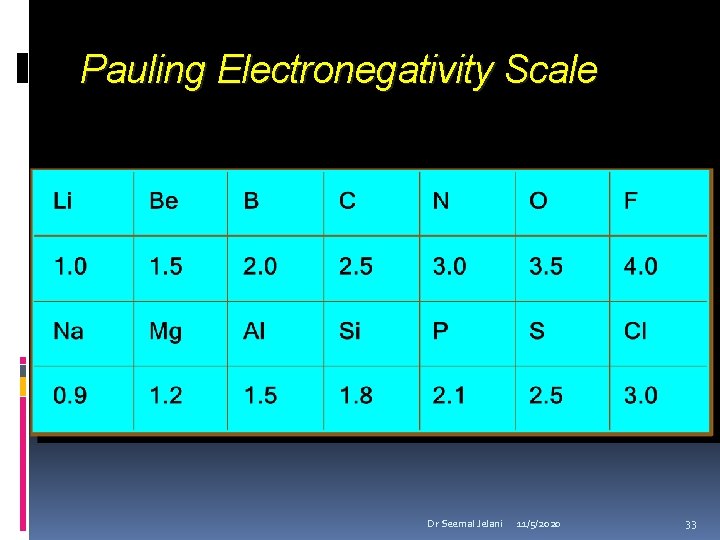
Pauling Electronegativity Scale Dr Seemal Jelani 11/5/2020 33

Electronegativity increases from left to right in the periodic table Electronegativity decreases going down a group. Dr Seemal Jelani 11/5/2020 34

Dr Seemal Jelani 11/5/2020 35

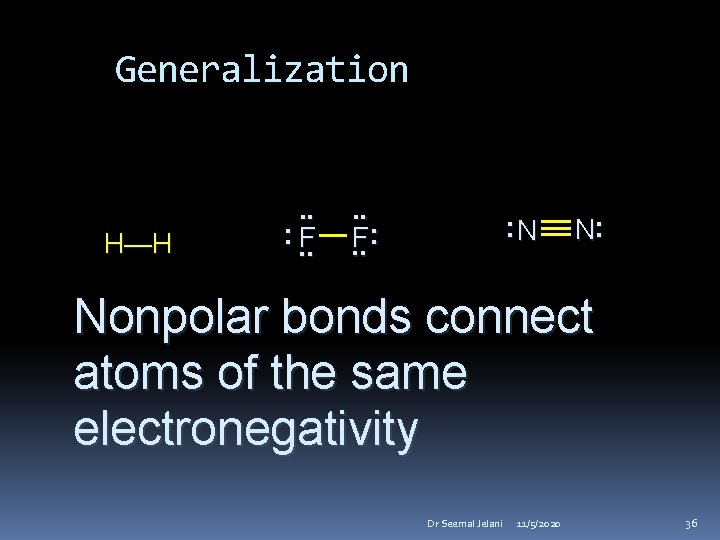
Generalization H—H . . : . . F: . . : N N: Nonpolar bonds connect atoms of the same electronegativity Dr Seemal Jelani 11/5/2020 36

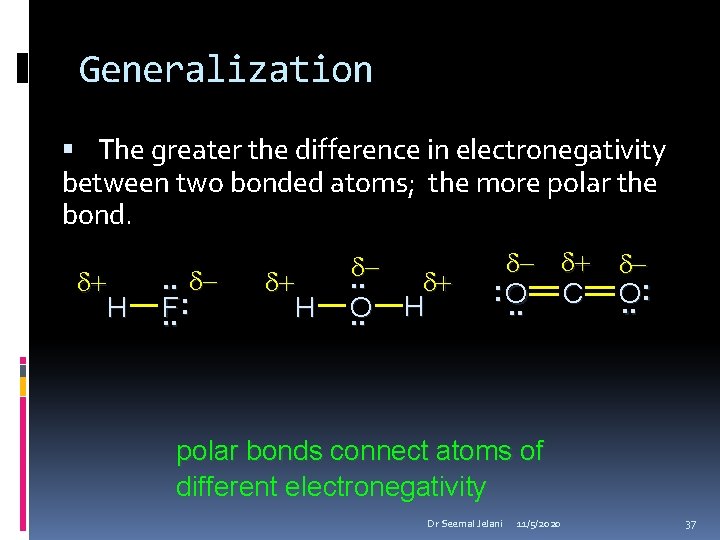
Generalization The greater the difference in electronegativity between two bonded atoms; the more polar the bond. d+ H . . d. F: . . d+ H d. . d+ O H. . d: O. . d+ d. C O. . : polar bonds connect atoms of different electronegativity Dr Seemal Jelani 11/5/2020 37
 Seemal desai
Seemal desai 1152020
1152020 1152020
1152020 Carpal tunnel syndrome differential diagnosis
Carpal tunnel syndrome differential diagnosis Like charges blank and opposite charges blank
Like charges blank and opposite charges blank Resonance and formal charge
Resonance and formal charge Formula for formal charge
Formula for formal charge Calculate formal charge
Calculate formal charge Electrostatic potential energy
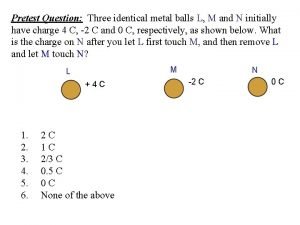
Electrostatic potential energy Two identical metal balls with charges
Two identical metal balls with charges Gr 47 towing charges
Gr 47 towing charges How to write polyatomic ions
How to write polyatomic ions A point p is equidistant from both point charges
A point p is equidistant from both point charges Opposite charges attract.
Opposite charges attract. Like charges attract or repel
Like charges attract or repel Nnn charges
Nnn charges Hmda dpms land use
Hmda dpms land use Like charges attract or repel
Like charges attract or repel 2004
2004 Electric charges
Electric charges Electron configuration na
Electron configuration na Continuous flow of electric charges
Continuous flow of electric charges City of ottawa development charges
City of ottawa development charges Cahier des charges recrutement
Cahier des charges recrutement Partial positive and negative charges
Partial positive and negative charges Electric charges and electric forces lesson outline
Electric charges and electric forces lesson outline Multiple charge cations
Multiple charge cations Late charges in front office
Late charges in front office Like charges attract or repel
Like charges attract or repel Caiso transmission access charges
Caiso transmission access charges Three point charges are arranged along the x-axis
Three point charges are arranged along the x-axis Charges and countercharges mean nothing
Charges and countercharges mean nothing Tung shin tcm
Tung shin tcm The figure shows three electric charges labeled
The figure shows three electric charges labeled What is the study of stationary electric charges
What is the study of stationary electric charges Phet charges and charged objects investigation
Phet charges and charged objects investigation Cahier des charges robot suiveur de ligne
Cahier des charges robot suiveur de ligne Pertes de charges
Pertes de charges