Third Year Organic Chemistry Course CHM 3 A






- Slides: 48

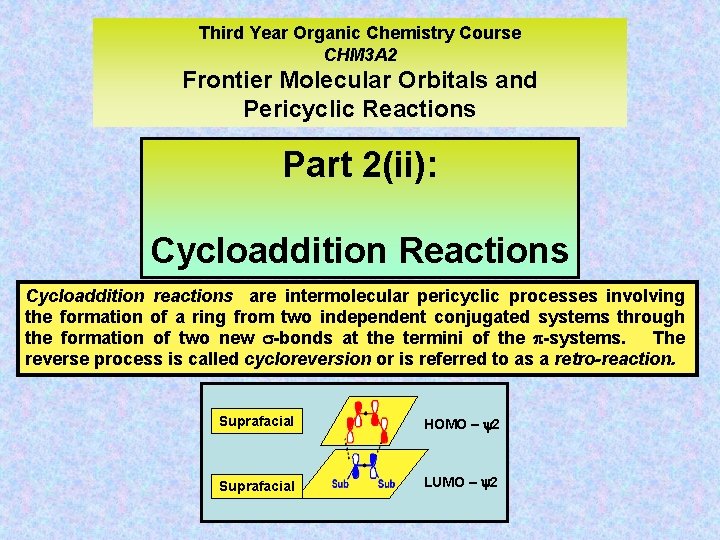
Third Year Organic Chemistry Course CHM 3 A 2 Frontier Molecular Orbitals and Pericyclic Reactions Part 2(ii): Cycloaddition Reactions Cycloaddition reactions are intermolecular pericyclic processes involving the formation of a ring from two independent conjugated systems through the formation of two new -bonds at the termini of the -systems. The reverse process is called cycloreversion or is referred to as a retro-reaction. Suprafacial HOMO – y 2 Suprafacial LUMO – y 2

CHM 3 A 2 – Introduction to FMOs – – Learning Objectives Part 2(ii) – Cycloaddition Reactions After completing PART 2(ii) of this course you should have an understanding of, and be able to demonstrate, the following terms, ideas and methods. (i) A cycloaddition reaction involves the formation of two bonds between the termini of two independent systems, resulting in ring formation - or the reverse process. (ii) Cycloaddition reactions are stereospecific (e. g. cis/trans isomers). The stereospecificity being afforded by the suprafacial or antarafacial nature of the approach of the two -units in the transition state. (iii) The suprafacial or antarafacial process involved in the bond making process is controlled by the HOMO/LUMO interactions of the two -systems in the transition state.

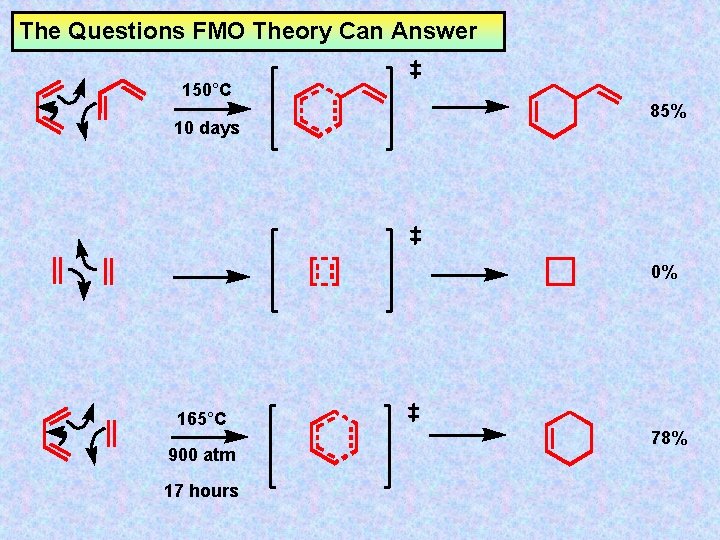
The Questions FMO Theory Can Answer 150°C 10 days 85% 0% 165°C 900 atm 17 hours 78%

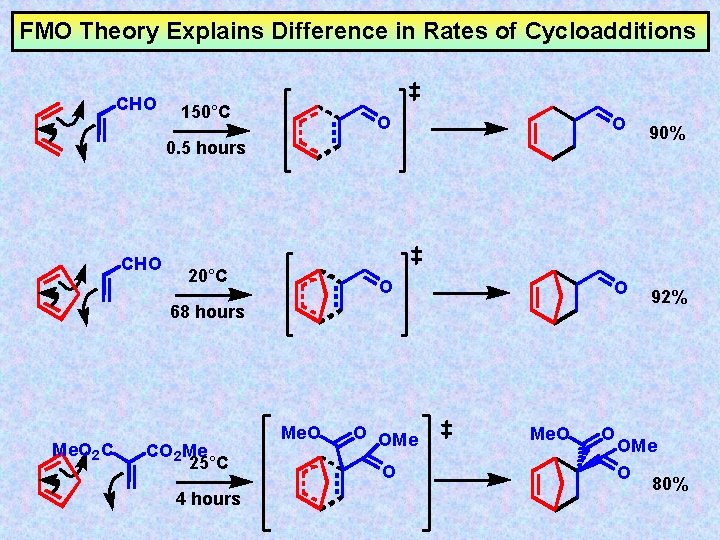
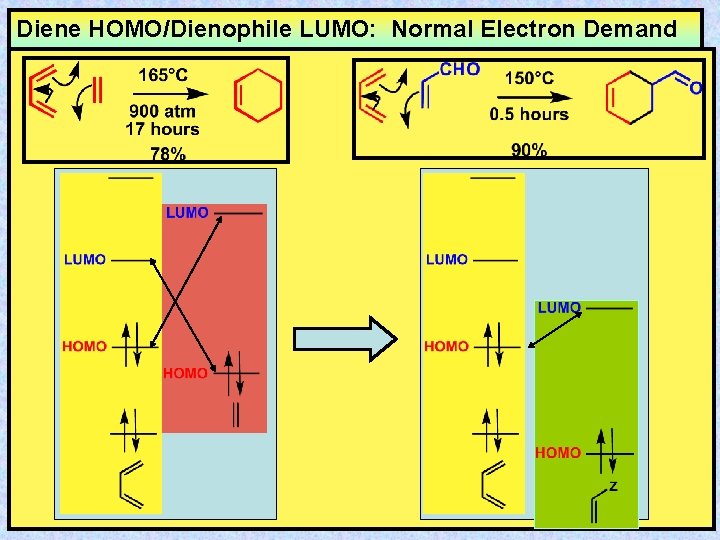
FMO Theory Explains Difference in Rates of Cycloadditions CHO 150°C O O 0. 5 hours CHO 20°C 68 hours Me. O 2 C CO 2 Me 25°C 4 hours Me. O O OMe O Me. O O 90% 92% OMe O 80%

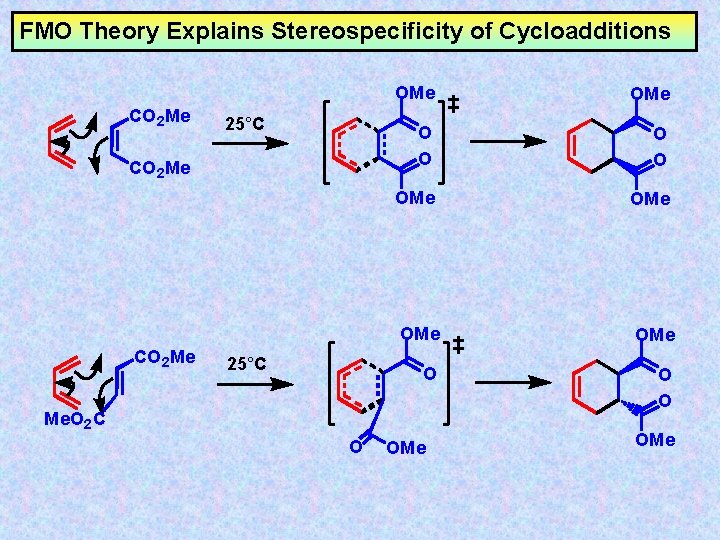
FMO Theory Explains Stereospecificity of Cycloadditions CO 2 Me 25°C OMe O O OMe OMe O O O Me. O 2 C O OMe

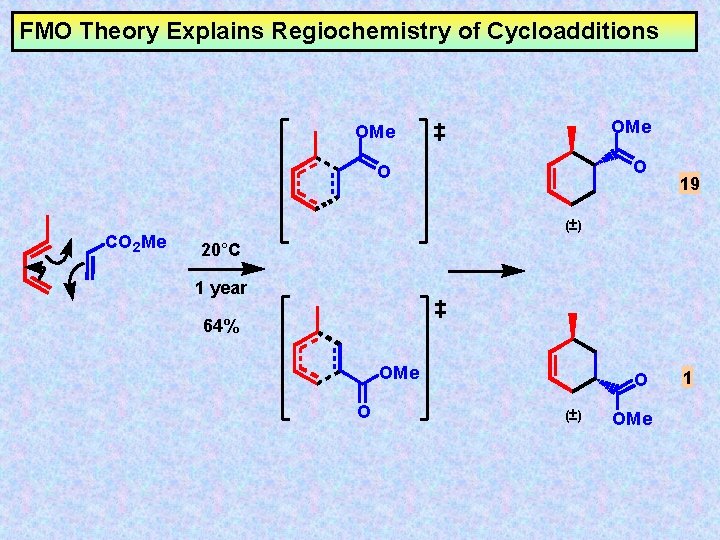
FMO Theory Explains Regiochemistry of Cycloadditions CO 2 Me OMe O O 19 (±) 20°C 1 year 64% OMe O O (±) OMe 1

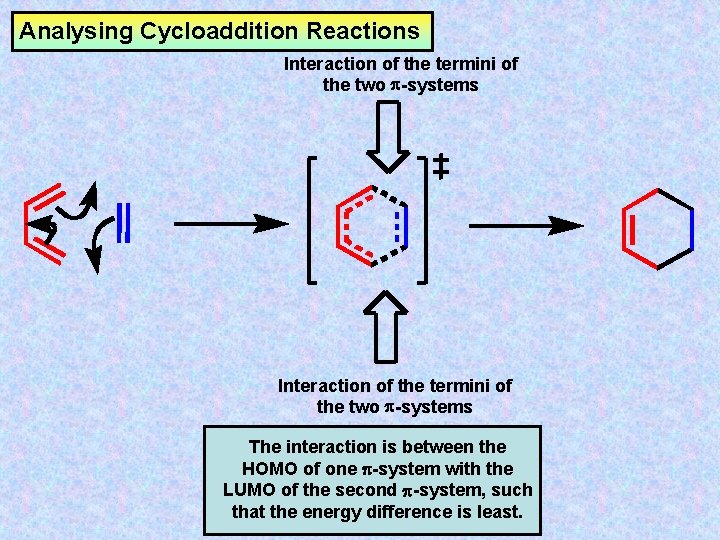
Analysing Cycloaddition Reactions Interaction of the termini of the two -systems The interaction is between the HOMO of one -system with the LUMO of the second -system, such that the energy difference is least.

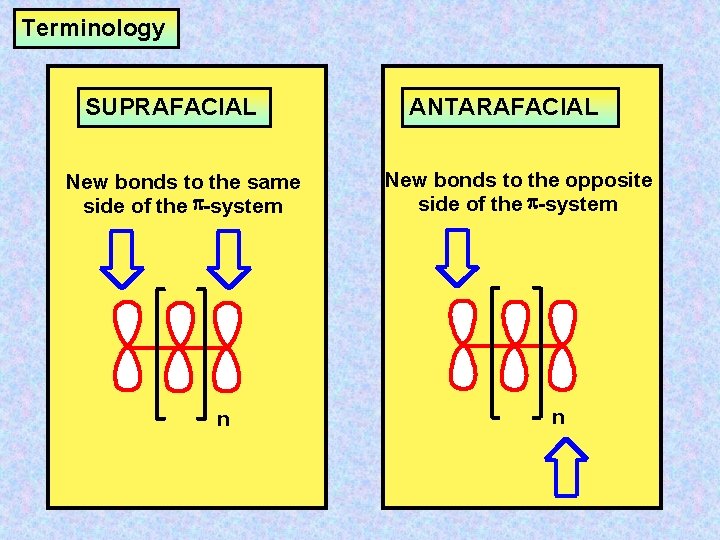
Terminology SUPRAFACIAL New bonds to the same side of the -system n ANTARAFACIAL New bonds to the opposite side of the -system n

4 n+2 Electron Cycloaddition Transition States


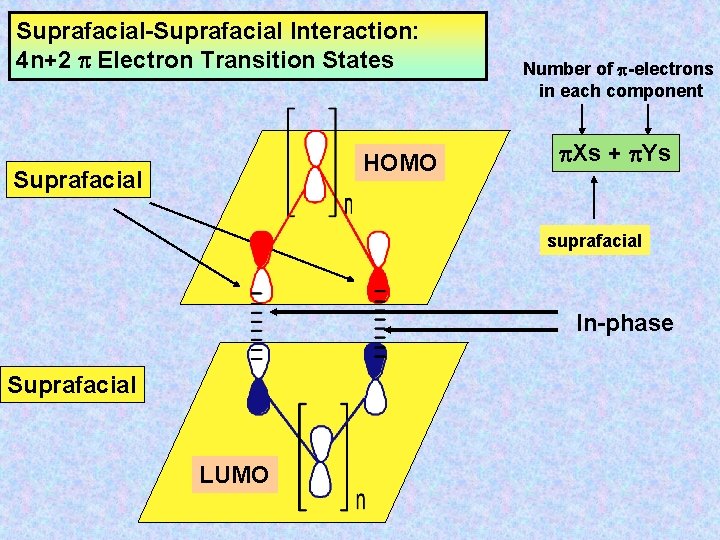
Suprafacial-Suprafacial Interaction: 4 n+2 Electron Transition States HOMO Suprafacial Number of -electrons in each component Xs + Ys suprafacial In-phase Suprafacial LUMO

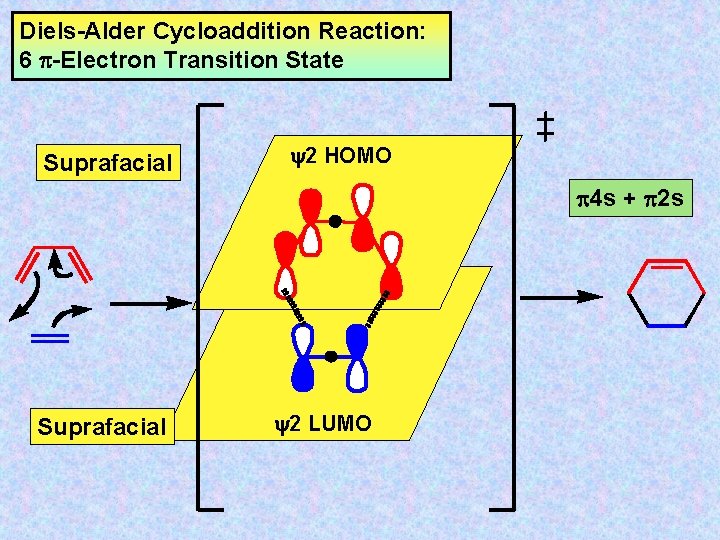
Diels-Alder Cycloaddition Reaction: 6 -Electron Transition State Suprafacial y 2 HOMO 4 s + 2 s Suprafacial y 2 LUMO

4 n Electron Cycloaddition Transition States


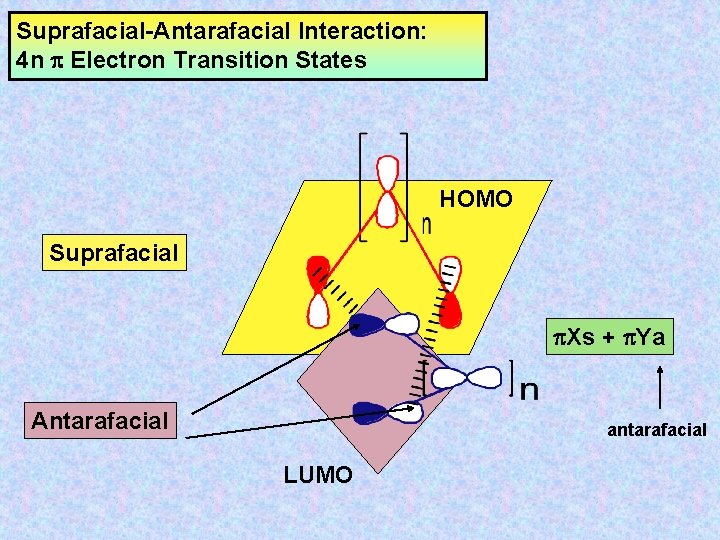
Suprafacial-Antarafacial Interaction: 4 n Electron Transition States HOMO Suprafacial Xs + Ya Antarafacial antarafacial LUMO

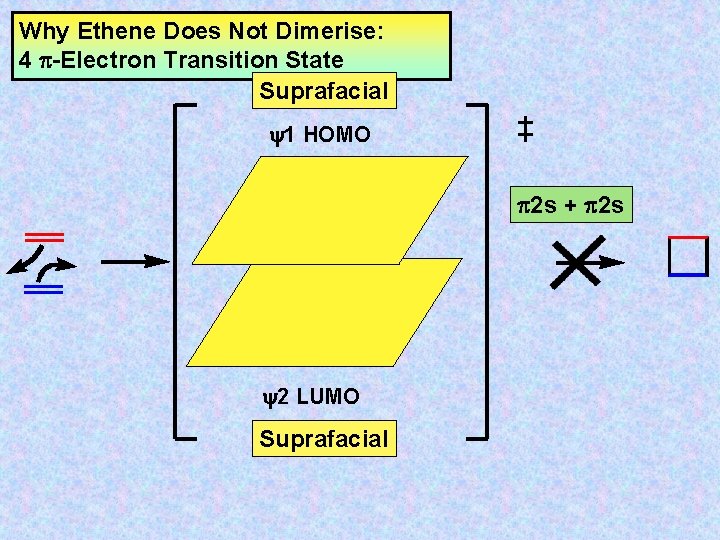
Why Ethene Does Not Dimerise: 4 -Electron Transition State Suprafacial y 1 HOMO 2 s + 2 s y 2 LUMO Suprafacial

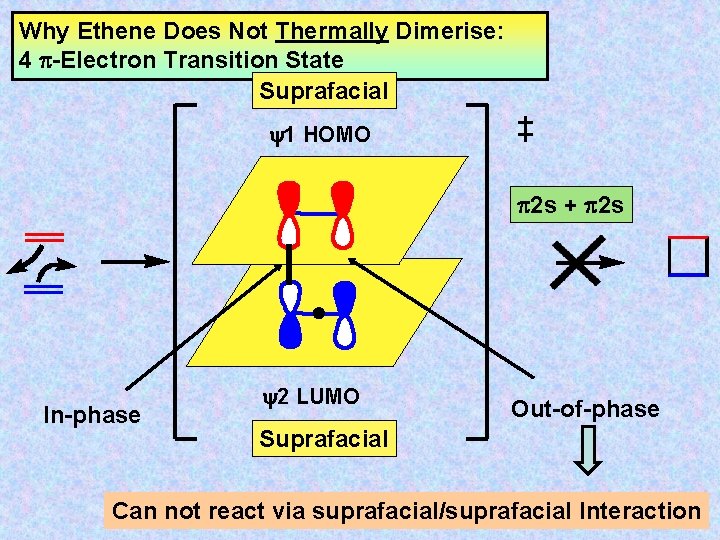
Why Ethene Does Not Thermally Dimerise: 4 -Electron Transition State Suprafacial y 1 HOMO 2 s + 2 s In-phase y 2 LUMO Out-of-phase Suprafacial Can not react via suprafacial/suprafacial Interaction

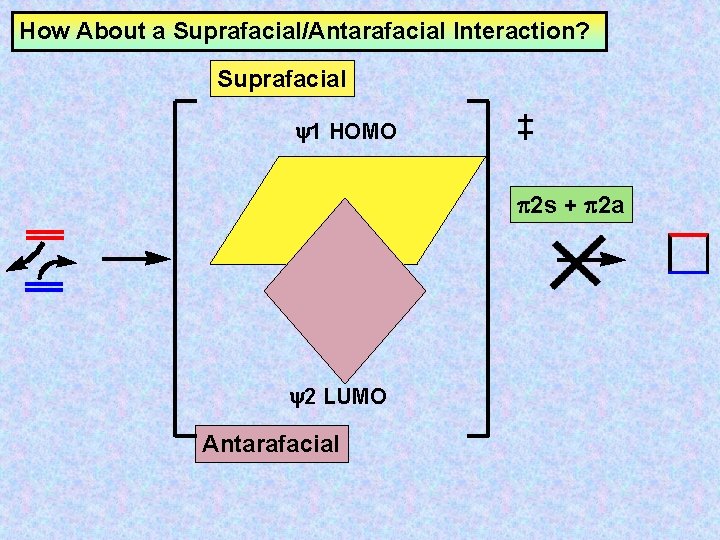
How About a Suprafacial/Antarafacial Interaction? Suprafacial y 1 HOMO 2 s + 2 a y 2 LUMO Antarafacial

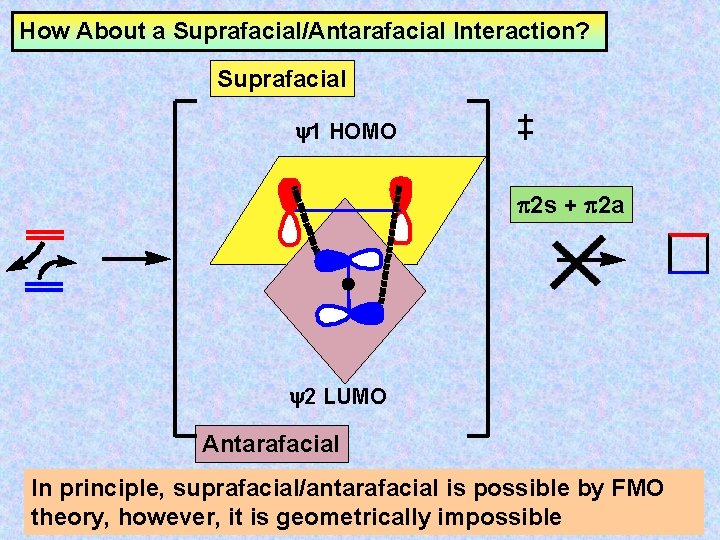
How About a Suprafacial/Antarafacial Interaction? Suprafacial y 1 HOMO 2 s + 2 a y 2 LUMO Antarafacial In principle, suprafacial/antarafacial is possible by FMO theory, however, it is geometrically impossible


The Diels-Alder Reaction: In Detail The Diels-Alder reaction is an extremely well studied cycloaddition reaction, The reason for this is that careful design of the diene component and the ene component (the dienophile) has led to a great insight into the reaction mechanism.


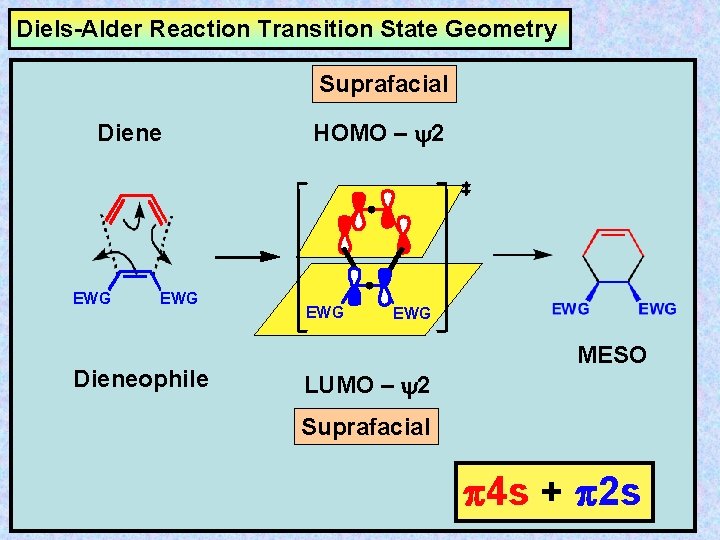
Diels-Alder Reaction Transition State Geometry Suprafacial Diene EWG Dieneophile HOMO – y 2 EWG MESO LUMO – y 2 Suprafacial 4 s + 2 s

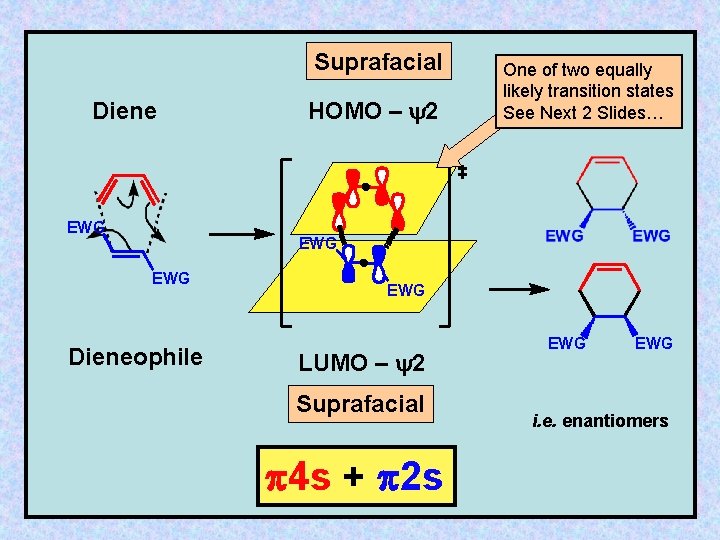
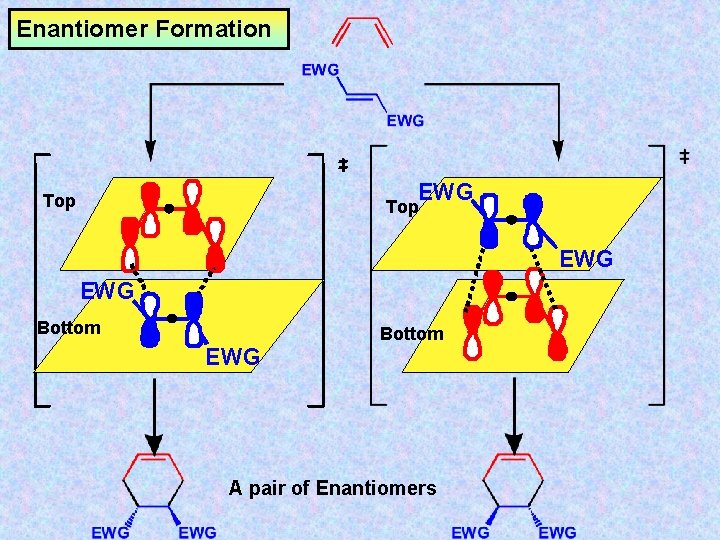
Suprafacial Diene EWG HOMO – y 2 One of two equally likely transition states See Next 2 Slides… EWG Dieneophile EWG LUMO – y 2 Suprafacial 4 s + 2 s EWG i. e. enantiomers

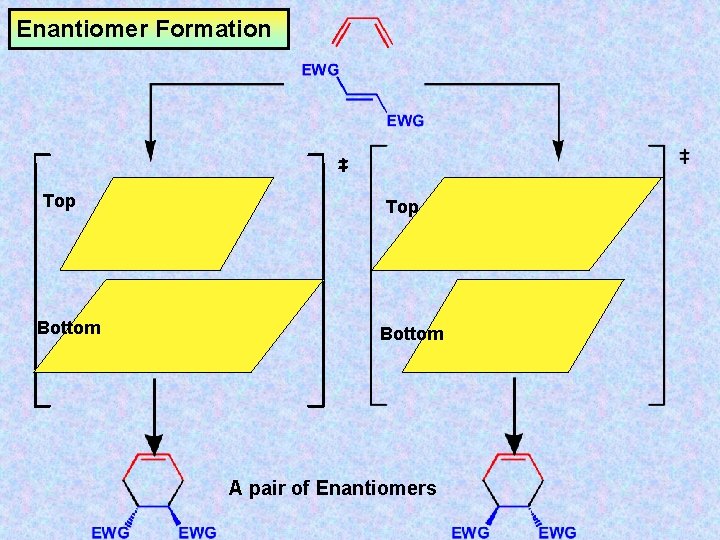
Enantiomer Formation Top Bottom A pair of Enantiomers

Enantiomer Formation EWG Top EWG Bottom EWG A pair of Enantiomers

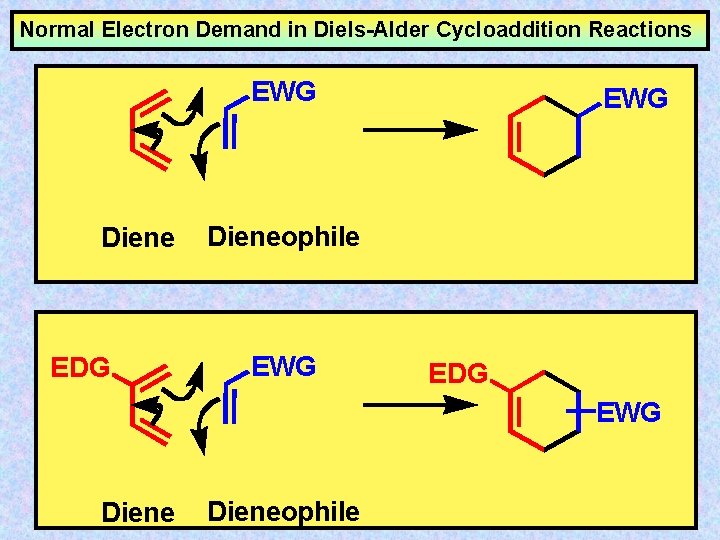
Normal Electron Demand in Diels-Alder Cycloaddition Reactions EWG Diene EDG EWG Dieneophile EWG EDG EWG Dieneophile

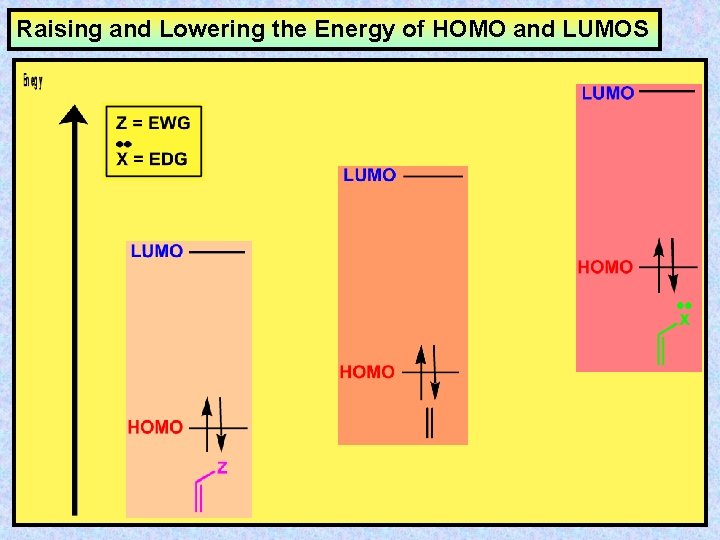
Raising and Lowering the Energy of HOMO and LUMOS

Diene HOMO/Dienophile LUMO: Normal Electron Demand


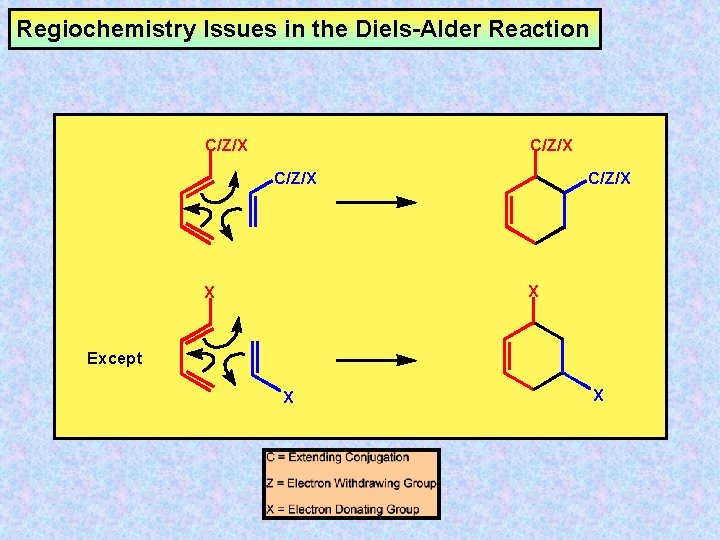
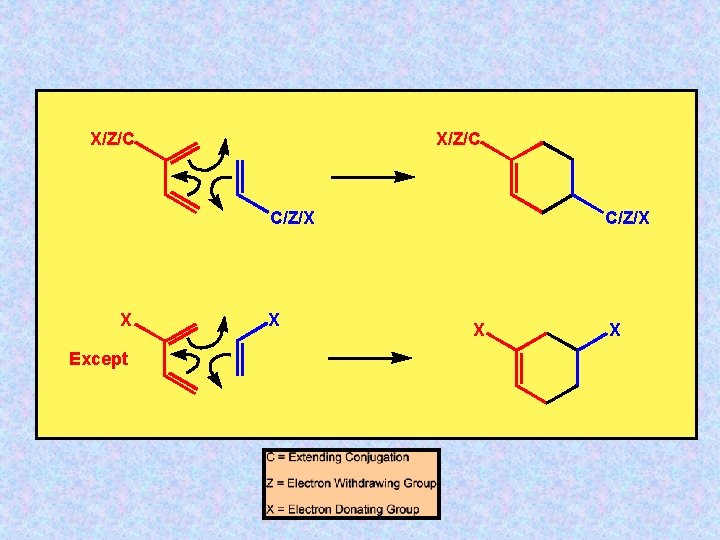
Regiochemistry Issues in the Diels-Alder Reaction C/Z/X X X Except X X

X/Z/C C/Z/X X Except X C/Z/X X X

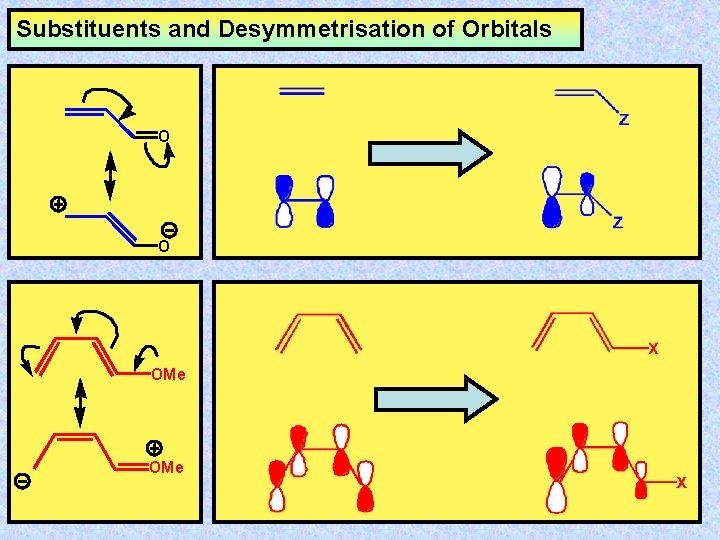
Substituents and Desymmetrisation of Orbitals O O OMe

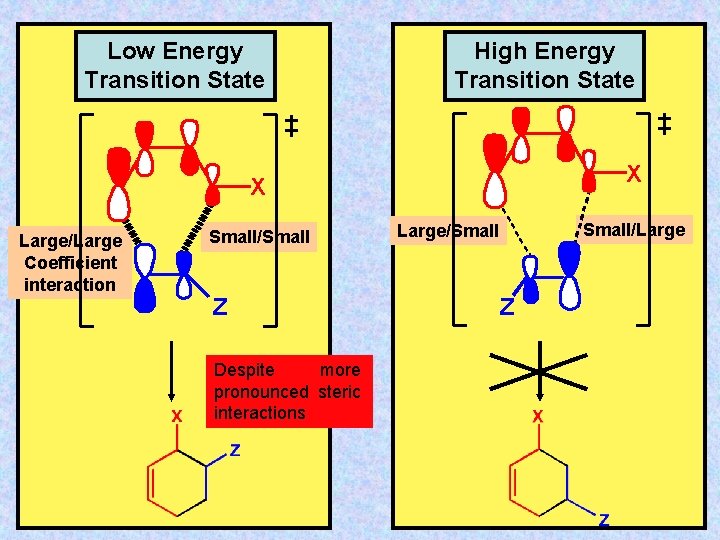
Low Energy Transition State High Energy Transition State X X Large/Large Coefficient interaction Small/Small Z Despite more pronounced steric interactions Small/Large/Small Z

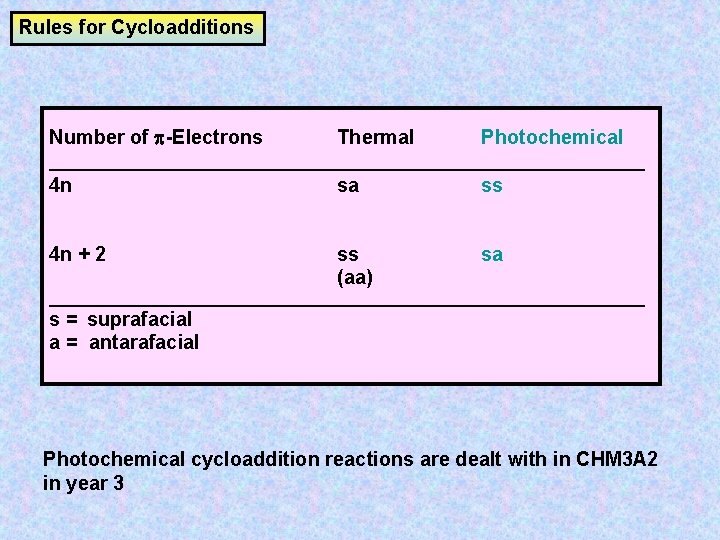
Rules for Cycloadditions Number of -Electrons Thermal Photochemical __________________________________ 4 n sa ss 4 n + 2 ss (aa) sa __________________________________ s = suprafacial a = antarafacial Photochemical cycloaddition reactions are dealt with in CHM 3 A 2 in year 3

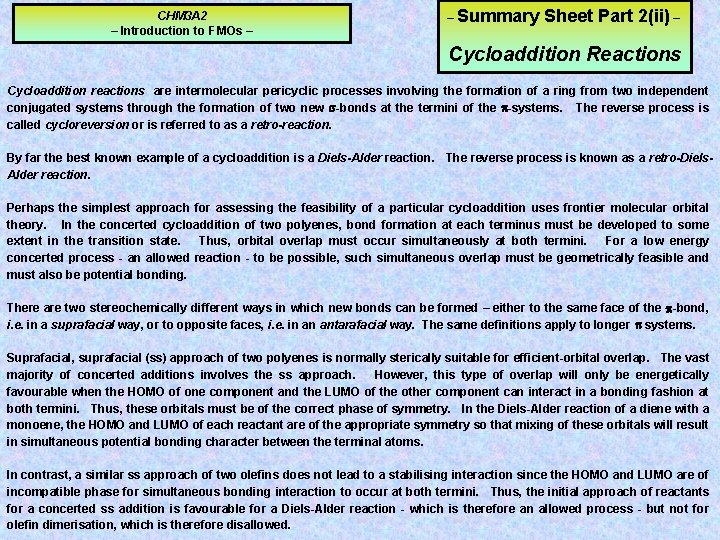
CHM 3 A 2 – Introduction to FMOs – – Summary Sheet Part 2(ii) – Cycloaddition Reactions Cycloaddition reactions are intermolecular pericyclic processes involving the formation of a ring from two independent conjugated systems through the formation of two new -bonds at the termini of the -systems. The reverse process is called cycloreversion or is referred to as a retro-reaction. By far the best known example of a cycloaddition is a Diels-Alder reaction. The reverse process is known as a retro-Diels. Alder reaction. Perhaps the simplest approach for assessing the feasibility of a particular cycloaddition uses frontier molecular orbital theory. In the concerted cycloaddition of two polyenes, bond formation at each terminus must be developed to some extent in the transition state. Thus, orbital overlap must occur simultaneously at both termini. For a low energy concerted process - an allowed reaction - to be possible, such simultaneous overlap must be geometrically feasible and must also be potential bonding. There are two stereochemically different ways in which new bonds can be formed – either to the same face of the -bond, i. e. in a suprafacial way, or to opposite faces, i. e. in an antarafacial way. The same definitions apply to longer systems. Suprafacial, suprafacial (ss) approach of two polyenes is normally sterically suitable for efficient-orbital overlap. The vast majority of concerted additions involves the ss approach. However, this type of overlap will only be energetically favourable when the HOMO of one component and the LUMO of the other component can interact in a bonding fashion at both termini. Thus, these orbitals must be of the correct phase of symmetry. In the Diels-Alder reaction of a diene with a monoene, the HOMO and LUMO of each reactant are of the appropriate symmetry so that mixing of these orbitals will result in simultaneous potential bonding character between the terminal atoms. In contrast, a similar ss approach of two olefins does not lead to a stabilising interaction since the HOMO and LUMO are of incompatible phase for simultaneous bonding interaction to occur at both termini. Thus, the initial approach of reactants for a concerted ss addition is favourable for a Diels-Alder reaction - which is therefore an allowed process - but not for olefin dimerisation, which is therefore disallowed.

Exercise 1: 4 n+2 Cycloadditions Explain the difference in the rates of reaction of the two reaction shown right.

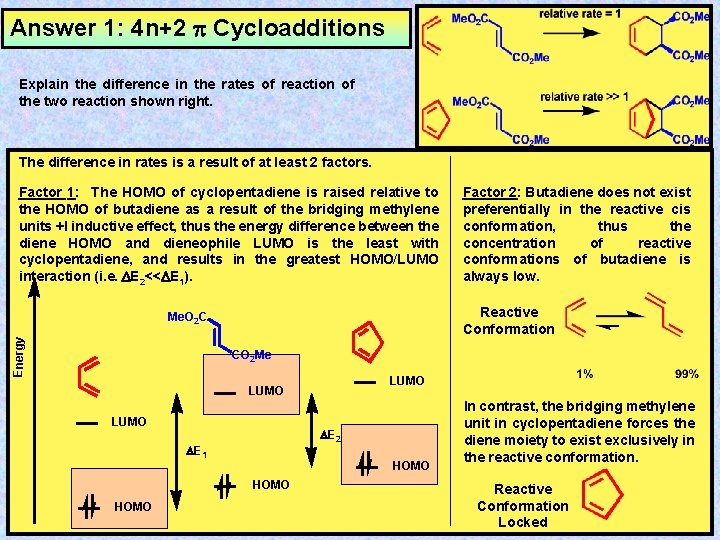
Answer 1: 4 n+2 Cycloadditions Explain the difference in the rates of reaction of the two reaction shown right. The difference in rates is a result of at least 2 factors. Factor 1: The HOMO of cyclopentadiene is raised relative to the HOMO of butadiene as a result of the bridging methylene units +I inductive effect, thus the energy difference between the diene HOMO and dieneophile LUMO is the least with cyclopentadiene, and results in the greatest HOMO/LUMO interaction (i. e. DE 2<<DE 1). Reactive Conformation Me. O 2 C Energy Factor 2: Butadiene does not exist preferentially in the reactive cis conformation, thus the concentration of reactive conformations of butadiene is always low. CO 2 Me LUMO DE 2 DE 1 HOMO In contrast, the bridging methylene unit in cyclopentadiene forces the diene moiety to exist exclusively in the reactive conformation. Reactive Conformation Locked

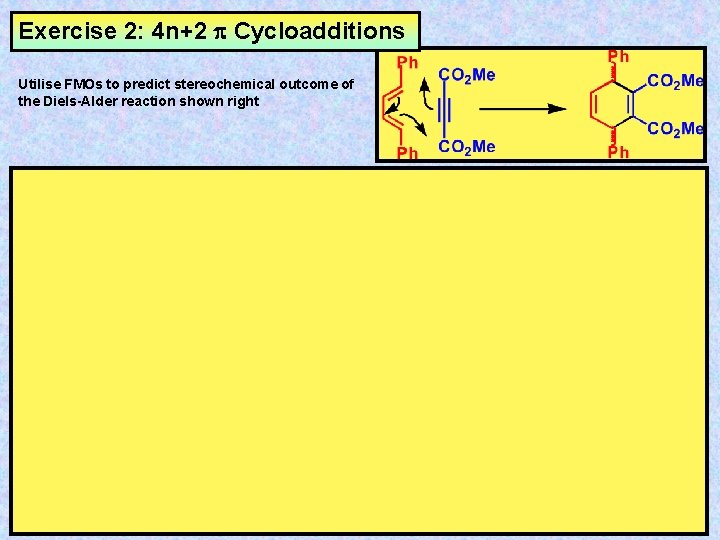
Exercise 2: 4 n+2 Cycloadditions Utilise FMOs to predict stereochemical outcome of the Diels-Alder reaction shown right

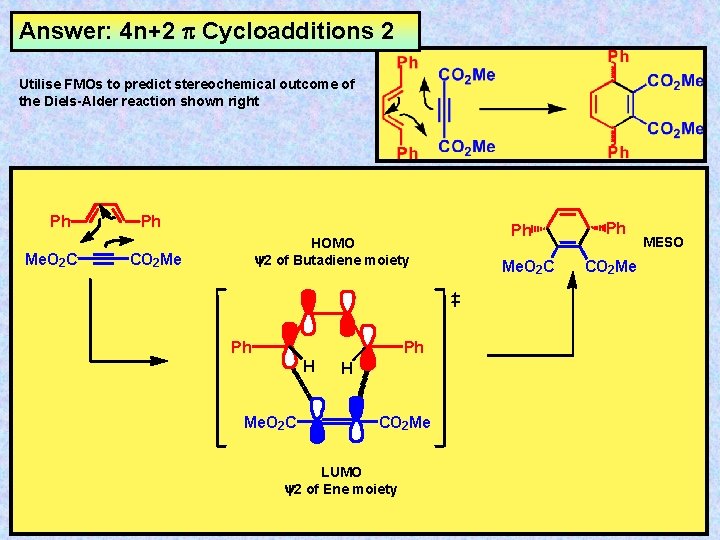
Answer: 4 n+2 Cycloadditions 2 Utilise FMOs to predict stereochemical outcome of the Diels-Alder reaction shown right Ph Me. O 2 C Ph HOMO y 2 of Butadiene moiety CO 2 Me Ph Ph H Me. O 2 C H CO 2 Me LUMO y 2 of Ene moiety Ph Me. O 2 C Ph CO 2 Me MESO

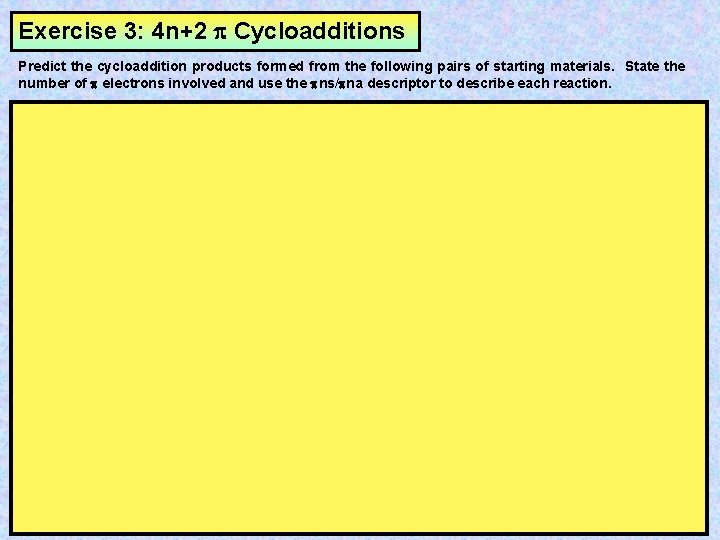
Exercise 3: 4 n+2 Cycloadditions Predict the cycloaddition products formed from the following pairs of starting materials. State the number of electrons involved and use the ns/ na descriptor to describe each reaction.

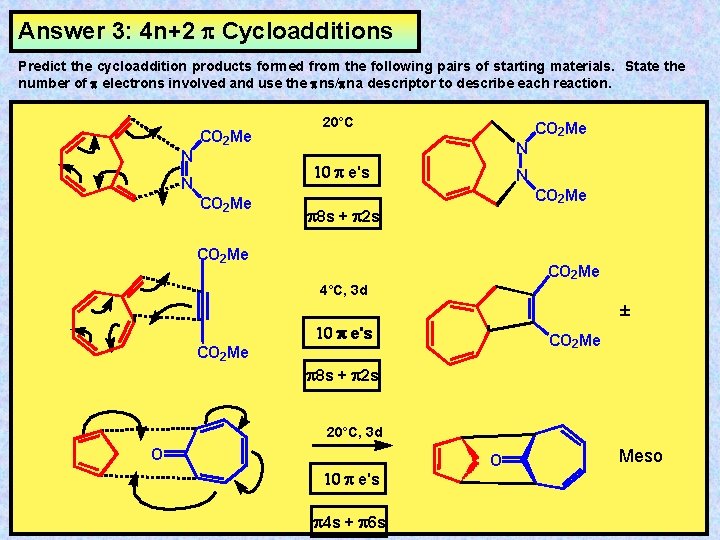
Answer 3: 4 n+2 Cycloadditions Predict the cycloaddition products formed from the following pairs of starting materials. State the number of electrons involved and use the ns/ na descriptor to describe each reaction. CO 2 Me N 20°C CO 2 Me N 10 e's N CO 2 Me 8 s + 2 s CO 2 Me 4°C, 3 d ± 10 e's CO 2 Me 8 s + 2 s 20°C, 3 d O 10 e's 4 s + 6 s O Meso

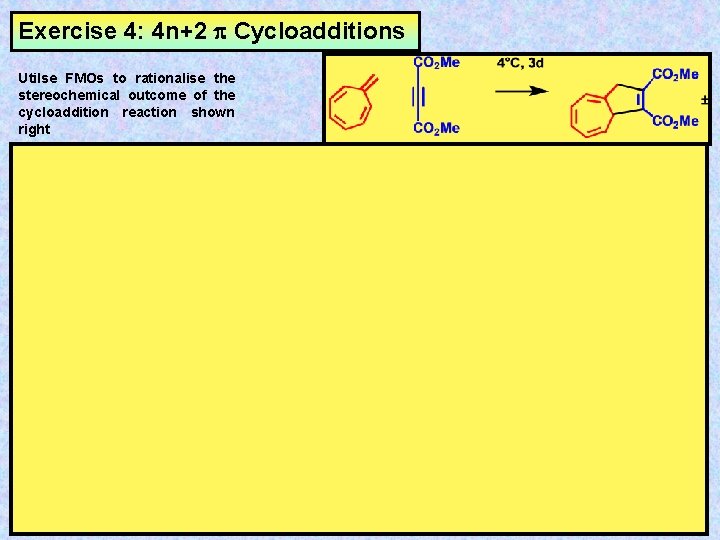
Exercise 4: 4 n+2 Cycloadditions Utilse FMOs to rationalise the stereochemical outcome of the cycloaddition reaction shown right

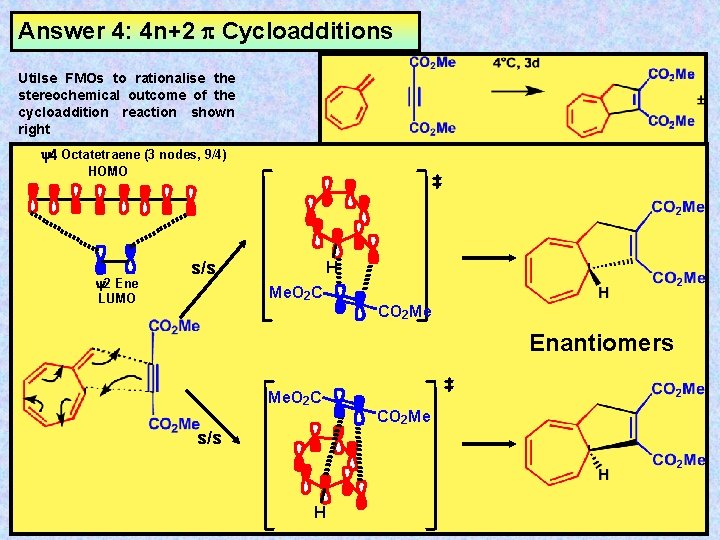
Answer 4: 4 n+2 Cycloadditions Utilse FMOs to rationalise the stereochemical outcome of the cycloaddition reaction shown right y 4 Octatetraene (3 nodes, 9/4) HOMO y 2 Ene LUMO s/s H Me. O 2 C CO 2 Me Enantiomers Me. O 2 C s/s H CO 2 Me

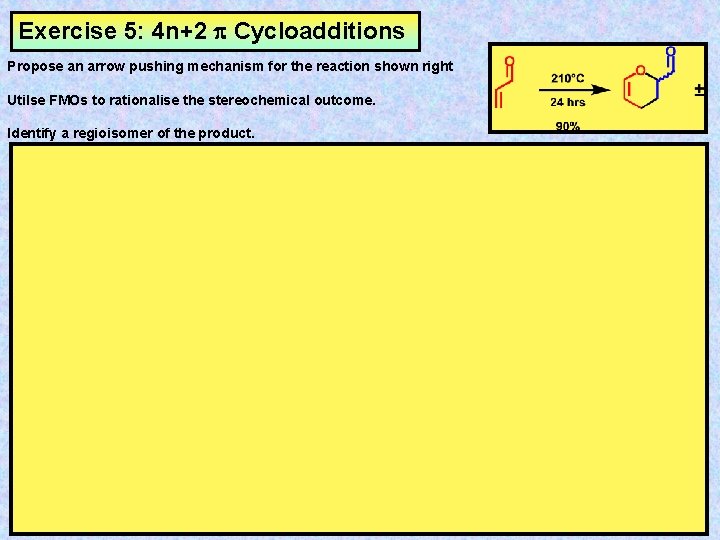
Exercise 5: 4 n+2 Cycloadditions Propose an arrow pushing mechanism for the reaction shown right Utilse FMOs to rationalise the stereochemical outcome. Identify a regioisomer of the product.

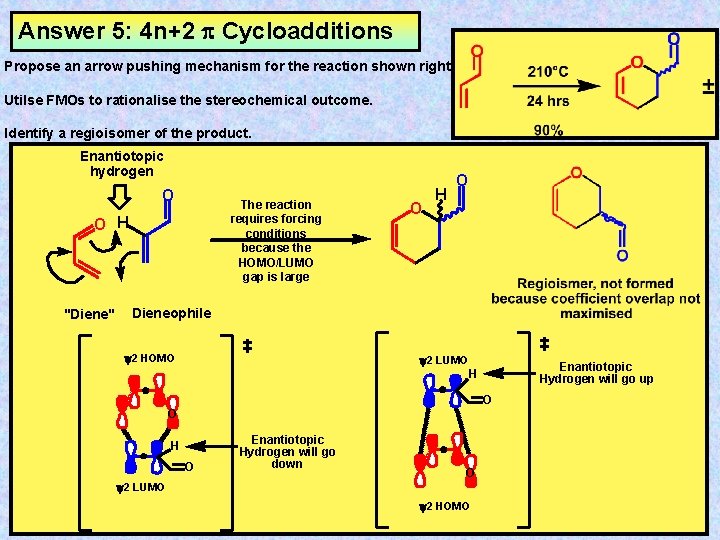
Answer 5: 4 n+2 Cycloadditions Propose an arrow pushing mechanism for the reaction shown right Utilse FMOs to rationalise the stereochemical outcome. Identify a regioisomer of the product. Enantiotopic hydrogen O The reaction requires forcing conditions because the HOMO/LUMO gap is large O H "Diene" O H O Dieneophile y 2 HOMO y 2 LUMO Enantiotopic Hydrogen will go up H O O H O Enantiotopic Hydrogen will go down O y 2 LUMO y 2 HOMO

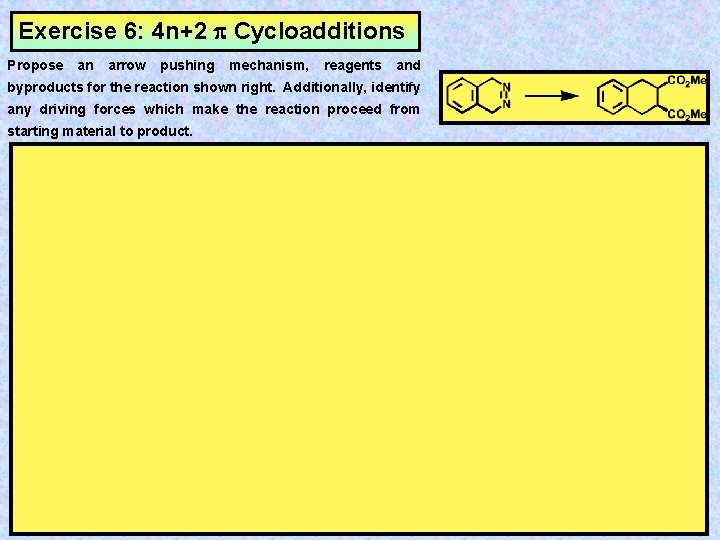
Exercise 6: 4 n+2 Cycloadditions Propose an arrow pushing mechanism, reagents and byproducts for the reaction shown right. Additionally, identify any driving forces which make the reaction proceed from starting material to product.

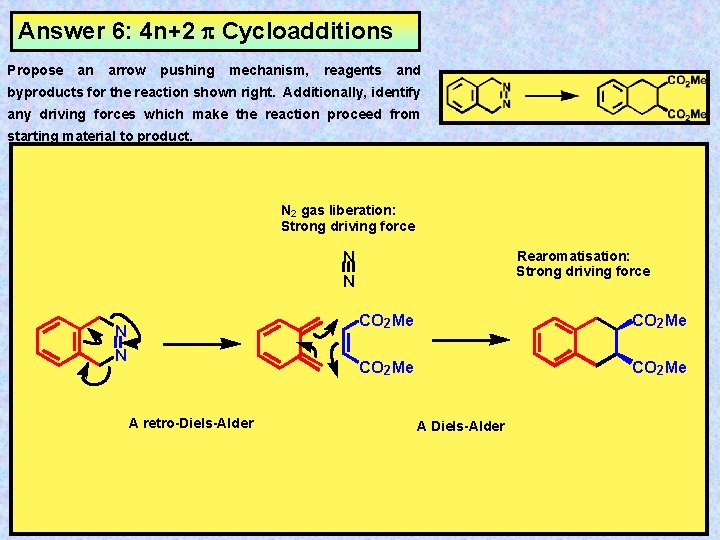
Answer 6: 4 n+2 Cycloadditions Propose an arrow pushing mechanism, reagents and byproducts for the reaction shown right. Additionally, identify any driving forces which make the reaction proceed from starting material to product. N 2 gas liberation: Strong driving force N N A retro-Diels-Alder Rearomatisation: Strong driving force CO 2 Me A Diels-Alder