The role of NICE and of the NICE





























- Slides: 45

The role of NICE, and of the NICE committee, in the evaluation of health technologies Marta Soares, Senior Research Fellow, Centre for Health Economics marta. soares@york. ac. uk Kuopio, Finland, August 2018 (some of the slides developed by Susan Griffin)

Outline ● ● ● About NICE Technology Appraisal NICE Committee Value judgements Decisions ○ Only in Research ● Impact of NICE 2

About NICE 3

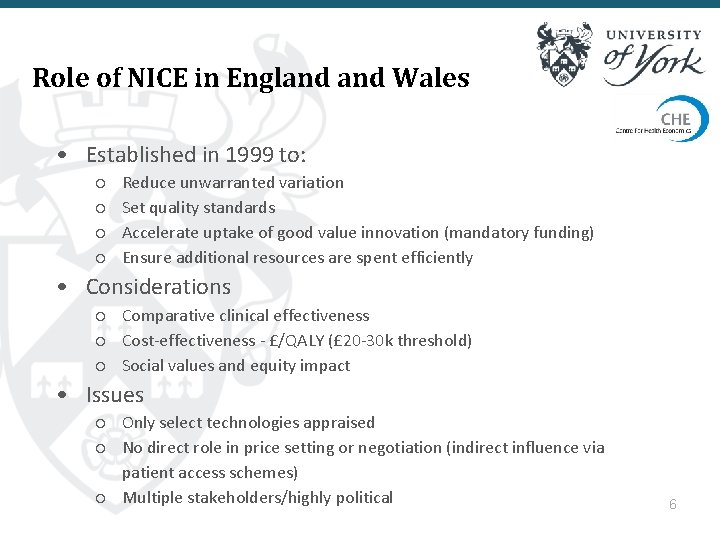
Role of NICE in England Wales • Established in 1999 to: ○ ○ Reduce unwarranted variation Set quality standards Accelerate uptake of good value innovation (mandatory funding) Ensure additional resources are spent efficiently 4

• Will NICE work? “Probably not, but it’s worth a bloody good try. ” Frank Dobson, Health Secretary, who established NICE in 1999, when asked whether he thought it would work. 5

Role of NICE in England Wales • Established in 1999 to: ○ ○ Reduce unwarranted variation Set quality standards Accelerate uptake of good value innovation (mandatory funding) Ensure additional resources are spent efficiently • Considerations ○ Comparative clinical effectiveness ○ Cost-effectiveness - £/QALY (£ 20 -30 k threshold) ○ Social values and equity impact • Issues ○ Only select technologies appraised ○ No direct role in price setting or negotiation (indirect influence via patient access schemes) ○ Multiple stakeholders/highly political 6

NICE…. what is it now ? • The National Institute for Health and Care Excellence (NICE) provides national guidance and advice to improve health and social care It does this by: • Producing evidence-based guidance and advice for health, public health • • and social care practitioners. Developing quality standards and performance metrics for those providing and commissioning health, public health and social care services; Providing a range of information services for commissioners, practitioners and managers across the spectrum of health and social care. 7

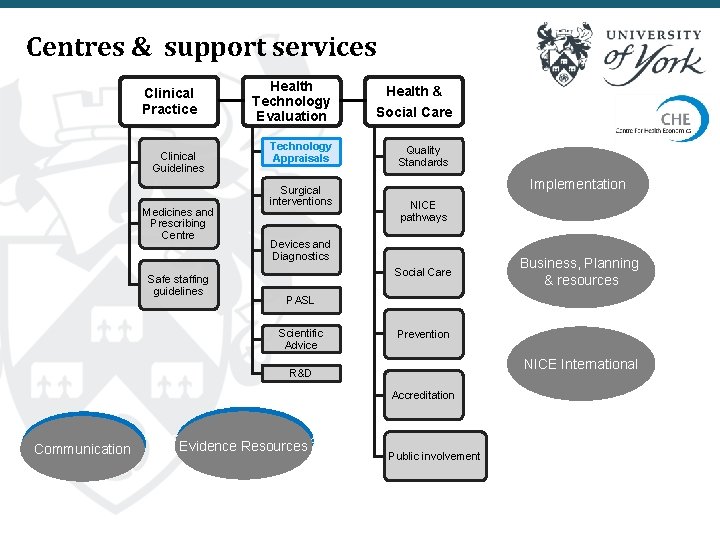
Centres & support services Clinical Practice Clinical Guidelines Medicines and Prescribing Centre Safe staffing guidelines Health Technology Evaluation Social Care Technology Appraisals Quality Standards Surgical interventions Health & Implementation NICE pathways Devices and Diagnostics Social Care PASL Scientific Advice Prevention NICE International R&D Accreditation Communication Business, Planning & resources Evidence Resources Public involvement

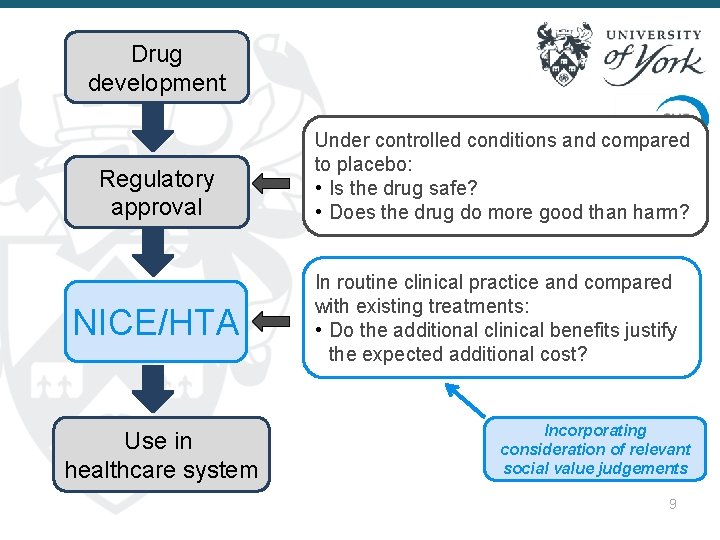
Drug development Regulatory approval NICE/HTA Use in healthcare system Under controlled conditions and compared to placebo: • Is the drug safe? • Does the drug do more good than harm? In routine clinical practice and compared with existing treatments: • Do the additional clinical benefits justify the expected additional cost? Incorporating consideration of relevant social value judgements 9

Technology Appraisals 10

NICE Technology Appraisals ● Technology appraisal one branch of NICE ● Entails assessment of clinical and cost effectiveness of health technologies ○ Medicines, surgical procedures, medical devices, diagnostic techniques, health promotion activities ● NICE decision carries legal mandate ○ Ensure all patients have access to cost-effective treatments 11

Topic selection ● Only issue guidance for selected technologies ● Topics identified by DH based on: ○ ○ ○ Health benefit across all patients for whom it is indicated Impact on NHS resources if given to all eligible patients Impact on other outcomes of interest, e. g. health inequalities Inappropriate national variation in use Institute able to add value by issuing national guidance 12

Defining the scope ● Sets out the decision problem ○ The disease area, patients, technologies covered by the appraisal and the questions it aims to answer ● Intervention and comparators ○ Must be licensed for use in UK ○ Ideally include current NHS practice ● Indication(s) ○ Scope may be narrower than license ● Identifies consultees ○ Clinical and patient groups, manufacturers and comparator manufacturers 13

Evaluation of health technologies ● Single Technology Appraisal (STA) ○ Evaluation undertaken by company ○ Critiqued by independent academic centre (Evidence Review Group) ● Multiple Technology Appraisal (MTA) ○ Evaluation by independent academic centre (Assessment Group) ○ Additional evaluations invited from manufacturers ● Fast Track Appraisal (FTA) ○ Less resource-intensive, NHS funding within 30 calendar days of guidance ○ Company base case ICER<£ 10, 000 and most plausible ICER<£ 20, 000 ○ Simple cost comparison if ≥ health benefits & ≤ cost compared to existing NICE recommended technologies for same indication ○ NICE and ERG determine eligibility with consultee input ○ NICE technical team provide report to Committee 14

Reference Case “Because the methodology of technology appraisal continues to develop, there remain areas of controversy and uncertainty, particularly in relation to the methods of cost-effectiveness analysis. However, it is important that the methods used to inform the Appraisal Committee's decision-making are consistent. For this reason, the Institute has adopted the approach of using a 'reference case' for cost-effectiveness analysis; this was chosen as most appropriate for the Appraisal Committee's purpose” http: //www. nice. org. uk/article/pmg 9/chapter/Foreword Describes preferred methods Supported by Decision Support Unit Technical Support Documents 15

Reference Case (Cost-Effectiveness) ● ● Use of the QALY central Health service perspective for costs Reference case is prescriptive and generic No intention to limit methods development of innovative techniques ● Reference case ≠ standardisation 16

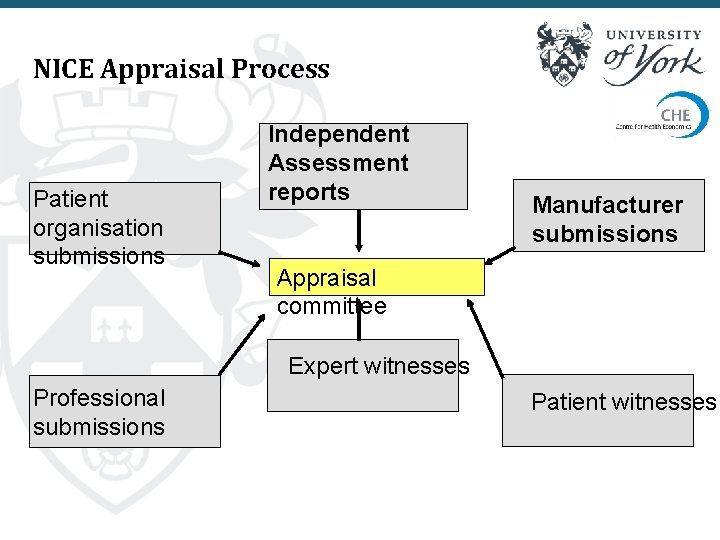
NICE Appraisal Process Patient organisation submissions Independent Assessment reports Manufacturer submissions Appraisal committee Expert witnesses Professional submissions Patient witnesses

Appraisal committee 18

Technology Appraisal Committee ● Independent advisory committee drawn from: ○ Academia, NHS, patient and carer organisations, manufacturers ○ Reflect spread of interests and expertise ● Tasks ○ Consider evidence ○ Come to conclusions about cost effectiveness ○ Come to conclusions about impact on other outcomes of interest (e. g. health inequalities) ○ Follow NICE’s principles on appropriate threshold (end of life, uncertainty) ○ Issue guidance 19

Committees in action ● Four TAC, meet once per month ● Discuss up to six topics in one day ○ Most appear over at least two meetings ● Documents sent out in advance ○ Seen early by NICE, chair, vice chair, lead team ○ Prepare pre-meeting briefing (PMB) ○ Documents include final scope, PMB, submissions from company(s), submission from independent academic centre, consultee comments 20

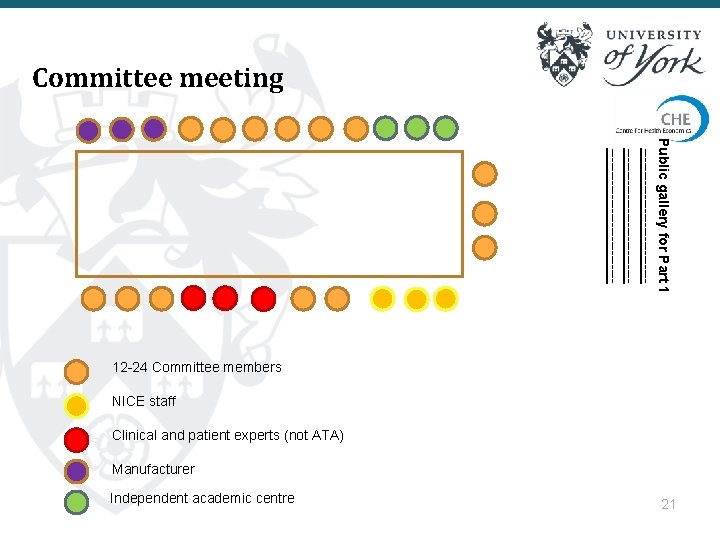
Committee meeting Public gallery for Part 1 ------------------------------------------- 12 -24 Committee members NICE staff Clinical and patient experts (not ATA) Manufacturer Independent academic centre 21

Evidence considered ● Prior to the meeting ○ Submissions from company(s) and independent academic group ○ Consultees comments ● At the meeting ○ Opportunity to put questions to experts, manufacturers and independent academic centre ○ Clinical and patient expert testimony 22

Role of Committee ● Consider generalisability to NHS in England Wales ● Come to conclusions about most appropriate value judgements, analytical choices, assumptions ● Request further evidence ○ Lead team to ERG/AG in advance of meeting ○ Questions during meeting ○ Specify further analyses during committee meetings ● Form guidance 23

Value judgements subjective statement of opinion rather than a fact that can be tested by looking at the available evidence. Normative statements are subjective statements – i. e. they carry value judgments 24

Value judgements ● When conducting economic evaluation we are required to make a series of value judgements ● Scope sets out decision problem but may not define population for CEA ● NICE Reference Case (methods guides) sets out preferred approach but is not exhaustive ● Further, evaluation may depart from scope and/or methods guide 25

Who should make value judgements? ● The results can be sensitive to these ○ What evidence is ‘relevant’ ○ How does evidence relate to setting (generalisability) ○ How should we combine the evidence using decision model (structural uncertainty) ○ How should we analyse the evidence (model uncertainty) ● Committee must form a view about most appropriate approach and on what impact on the ICER will be if approach inadequate 26

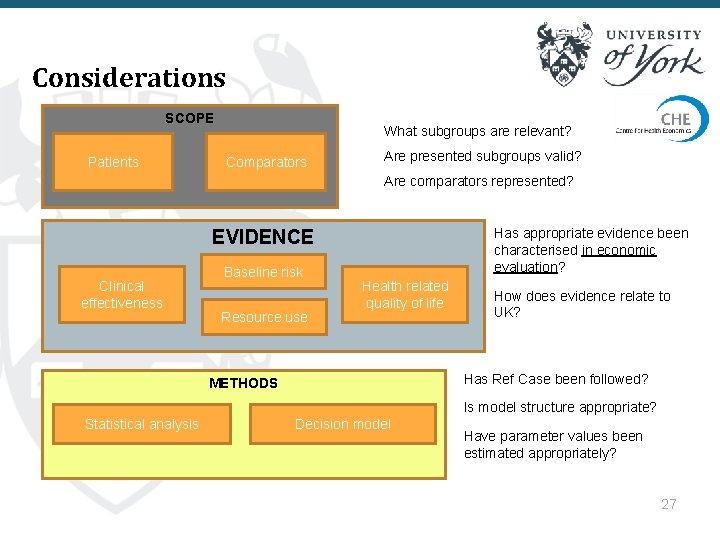
Considerations SCOPE Patients What subgroups are relevant? Comparators Are presented subgroups valid? Are comparators represented? Has appropriate evidence been characterised in economic evaluation? EVIDENCE Clinical effectiveness Baseline risk Resource use Health related quality of life How does evidence relate to UK? Has Ref Case been followed? METHODS Is model structure appropriate? Statistical analysis Decision model Have parameter values been estimated appropriately? 27

Value judgements - scope ● Why might evaluation depart from scope? ○ Clinical trials for interventions include only subset of population ○ Company targets high risk subgroup ○ Comparators not used in certain patients ○ Characterisation of comparator ■ ■ E. g. best supportive care, current practice, watchful waiting Dose/schedule differs from that used in practice 28

Value judgements - evidence ● ● ● Clinical trials conducted outside UK Clinical trial does not match license Which trials to include Other evidence from different settings Evidence out of date 29

Value judgement - methods ● Departure from Reference Case should only occur if unavailable ● Reference Case cannot cover all options ● Often no ‘right’ answer ○ E. g. Survival analysis for extrapolation ○ Scientific approach but no statistical test ● Structure of decision model 30

Decisions 31

Conclusions ● What is most plausible ICER? ○ Across whole population ○ In subgroup(s) ● Is current evidence sufficient? ● What is appropriate threshold? ○ Range £ 20 -£ 30, 000 ○ Do End of Life criteria apply? ■ ■ Survival extension > 3 months for life expectancy < 24 months Threshold up to £ 50 k 32

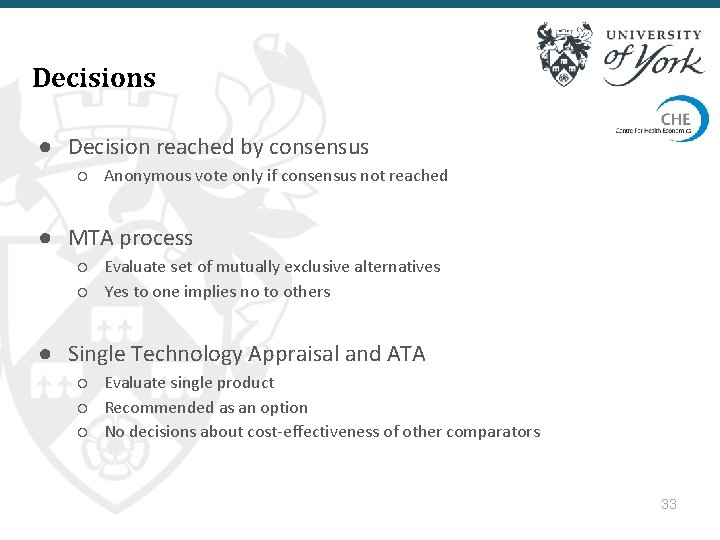
Decisions ● Decision reached by consensus ○ Anonymous vote only if consensus not reached ● MTA process ○ Evaluate set of mutually exclusive alternatives ○ Yes to one implies no to others ● Single Technology Appraisal and ATA ○ Evaluate single product ○ Recommended as an option ○ No decisions about cost-effectiveness of other comparators 33

Types of decisions ● Recommend ○ In line with marketing authorisation ○ NHS legally obliged to fund and resource within 3 months as standard ● Optimise ○ Recommended only in subgroup of licensed population ● Only in research ● Recommended for use within CDF ● Not recommended 34

Only in Research ● Requires judgement that ○ Current evidence insufficient ○ Reasonable prospect of technology being CE ○ On balance benefits outweigh cost of research ● Complex considerations at end of assessment of cost effectiveness ● Committee not routinely provided with supporting evidence ○ Value of information ○ Feasibility and costs of further research 35

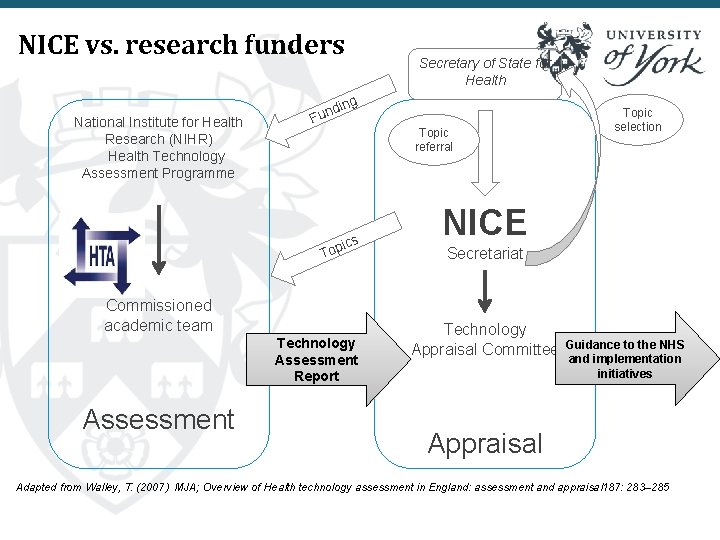
NICE vs. research funders National Institute for Health Research (NIHR) Health Technology Assessment Programme ing d n Fu ics Top Commissioned academic team Technology Assessment Report Assessment Secretary of State for Health Topic referral Topic selection NICE Secretariat Technology Appraisal Committee Guidance to the NHS and implementation initiatives Appraisal Adapted from Walley, T. (2007) MJA; Overview of Health technology assessment in England: assessment and appraisal 187: 283– 285

Cancer Drugs Fund ● Requires judgement that ○ Current evidence insufficient ○ Reasonable prospect of technology being CE ● Managed Access Scheme agreed between company and NHS England ○ Data Collection Arrangement to detail evidence required ○ CDF Commercial Agreement to determine price ● Drug becomes available immediately in CDF while further evidence is collected ○ Further data collection not necessarily via CDF ○ Review in approximately 2 years 37

Consultation ● YES – Recommended and within license requires no consultation ● In all other cases Appraisal Consultation Document issued for consultation ● Consider responses and further analyses and/or evidence at second meeting ● Ultimately issue guidance ● Opportunity for appeal ○ Failed to act fairly or exceeded powers ○ Decision is perverse in light of evidence submitted 38

Impact of NICE 39

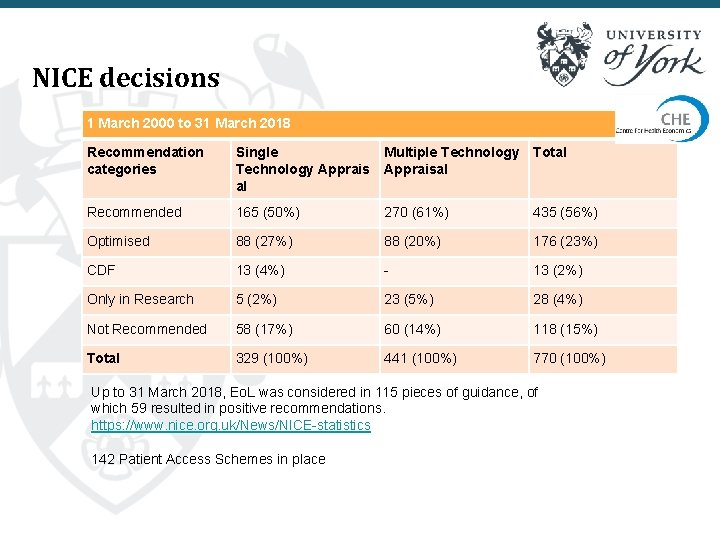
NICE decisions 1 March 2000 to 31 March 2018 Recommendation categories Single Technology Apprais al Multiple Technology Total Appraisal Recommended 165 (50%) 270 (61%) 435 (56%) Optimised 88 (27%) 88 (20%) 176 (23%) CDF 13 (4%) - 13 (2%) Only in Research 5 (2%) 23 (5%) 28 (4%) Not Recommended 58 (17%) 60 (14%) 118 (15%) Total 329 (100%) 441 (100%) 770 (100%) Up to 31 March 2018, Eo. L was considered in 115 pieces of guidance, of which 59 resulted in positive recommendations. https: //www. nice. org. uk/News/NICE-statistics 142 Patient Access Schemes in place

Impact of NICE ● Independent body ● Rationing made explicit and public ○ Hitting the headlines ■ “Betrayal of 20, 000 cancer patients: Rationing body rejects ten drugs (allowed in Europe) that could have extended lives” ● Opportunity for public to take part ○ Consultation ○ View Committee meeting 41

Impact of NICE on health ● In principle approve technology only if generate more health than displaced ● Evidence suggests this is not the case and ICERs>£ 30 k not rejected ○ Dakin et al. Health Economics 2014; ● Further, empirical threshold may be lower than £ 20 -£ 30, 000 range ○ Claxton et al. Health Economics 2015; 24(4): 1 -7 42

43

Some references and websites ● BBC Documentary ‘The Price of Life’ https: //vimeo. com/4796083 ● https: //www. nice. org. uk/ ● http: //www. nicedsu. org. uk/technical-support● ● documents%281985314%29. htm Claxton K, et al. Informing a decision framework for when NICE should recommend the use of health technologies only in the context of an appropriately designed programme of evidence development. Health Technol Assess 2012; 16(46) Collins M, Latimer N. NICE’s end of life decision making scheme: impact on population health. BMJ 2013; 346: f 1363 Dakin H, et al. 2014. The influence of cost-effectiveness and other factors on NICE decisions. DOI: 10. 1002/hec. 3086', Health Economics Claxton K, et al. Causes for concern: is nice failing to uphold its responsibilities to all nhs patients? Health Econ 2015; 24: 1 -7 44

The role of NICE, and of the NICE committee, in the evaluation of health technologies Marta Soares, Senior Research Fellow, Centre for Health Economics marta. soares@york. ac. uk Kuopio, Finland, August 2018
 Role of nice
Role of nice Azure worker role
Azure worker role Interaktionistisches rollenmodell
Interaktionistisches rollenmodell Statuses and their related roles determine
Statuses and their related roles determine Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi
Hát lên người ơi Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Các số nguyên tố là gì
Các số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi





































































