Cochrane and NICE Guidelines A view from NICE














- Slides: 19

Cochrane and NICE Guidelines: A view from NICE Dr Nichole Taske Centre for Clinical Practice, NICE

To cover 1. An outline of the NICE guideline development process; 2. How to find out about guidelines in development; 3. How to get involved/register interest in a particular guideline.

1. The NICE guideline development process

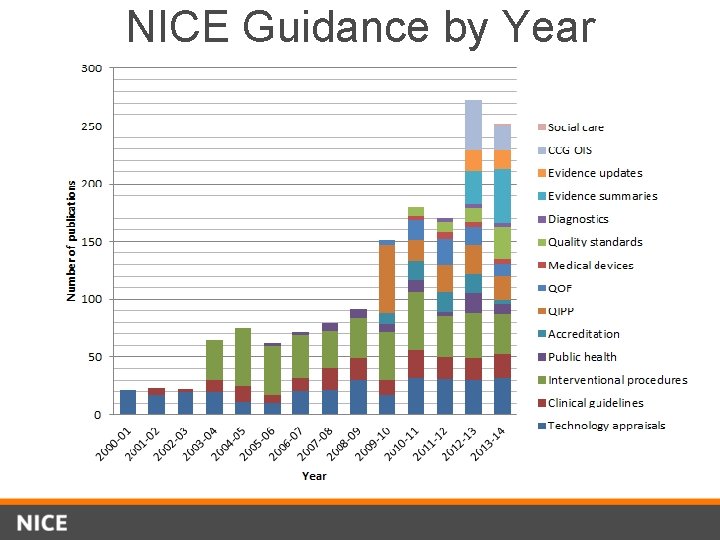
NICE clinical guideline programme • The largest publicly-funded national guidelines programme in the world • 233 guidelines published since 2002 (172 new topics, 47 updates and 14 standing committee updates) • Involving over 1000 people (most on a voluntary basis) • Includes areas of public health, social care and service delivery.

NICE Guidance by Year

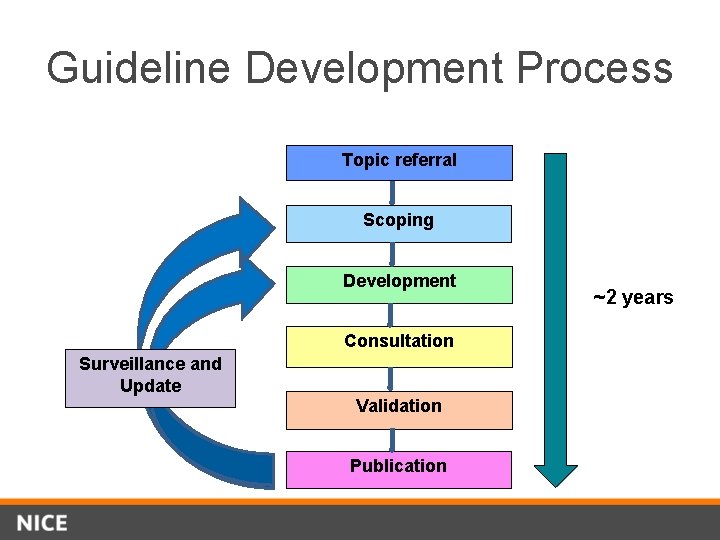
Guideline Development Process Topic referral Scoping Development Consultation Surveillance and Update Validation Publication ~2 years

2. How to find out about guidelines in development

Guidelines in development-1

Guidelines in development-2

Guidelines in development-2 a

Guidelines in development-2 b Milestone dates for all Clinical Guidelines currently in development To register your organisation as a stakeholder for any clinical guidelines currently in development (i. e listed below) please follow this link: http: //www. nice. org. uk/get-involved/stakeholder-registration Please note: This table is updated regularly and dates may change. All changes since the last update are shown in red. Development dates are listed as 'TBC' until the development time has been formally agreed with the guideline developer. Date of last update: 17 th Feb 2016 To request to attend any upcoming stakeholder workshops, and for all other queries regarding any of the guidelines listed below, please contact: guidelines@nice. org. uk To view all published clincal guidelines please follow this link: http: //www. nice. org. uk/Guidance/published? type=Guidelines Guideline GDG member recruitment opens / Scope consultation starts GDG Chair (and Stakeholder early member) Developer* recruitment opens recruitment closes workshop GDG member recruitment closes / Scope consultation closes 1 Motor Neurone Disease NCGC 2 Neonatal jaundice (update SC) NCC WCH 3 Safe use of controlled drugs MPC 4 Preoperative tests (update) NCGC 08 -Oct-13 22 -Oct-13 14 -Feb-14 5 Chest pain of recent onset (stable) (update) CGUT tbc tbc 6 Psychosis and schizophrenia in children and young people (SC update) CGUT tbc tbc 7 Haematological cancers, service guidance tbc tbc NCC C Draft guideline consultation opens Draft guideline consultation closes Publication Notes 15 -Jul-13 13 -Aug-13 30 -Oct-13 15 -Nov-13 13 -Dec-13 01 -Sep-15 13 -Oct-15 24 -Feb-16 tbc tbc tbc 22 -Jul-15 20 -Aug-15 05 -Mar-16 13 -Nov-15 11 -Dec-15 31 -Mar-16 03 -Mar-14 31 -Mar-14 12 -Oct-15 23 -Nov-15 06 -Apr-16 tbc 22 -Dec-15 11 -May-16 tbc 01 -Feb-16 25 -Feb-16 11 -May-16 30 -Apr-15 30 -Nov-15 14 -Jan-16 25 -May-16

2. How to get involved/register interest in a guideline: - Stakeholder; - Committee member; - Guideline development; - Guideline surveillance

Stakeholder registration

The scoping process § The scoping process comprises: – A pre-consultation stakeholder workshop – A 4 week consultation period with stakeholders See Chapter 2 of ‘Developing NICE Guidelines: The Manual’ http: //www. nice. org. uk/article/pmg 20/chapter/2 -the-scope

NICE Guideline Committees Ø Clinicians Ø Researchers Ø Service managers Ø Health economists Ø Patients/carers

Guideline development • Ensure reviews relevant to the NHS are up -to-date • Work alongside guideline developers to provide updated Cochrane reviews; – Challenges: • Aligning timelines; • Ensuring review questions (PICO) are compatible • Stakeholder consultation on draft guideline

Updating our guidelines Keeping up to date with new evidence Maintaining the catalogue of 233 published guidelines: • Surveillance reviews • Ability to update discrete areas of guideline

Surveillance process • Guidelines are reviewed every 2 years post-publication; • Process encompasses: – Literature review; – Topic-specific strategies (CRG liaison) • e. g. Dementia; NE; Eczema – Intelligence gathering – Stakeholder consultation (‘no to update’ decisions at 4 & 8 years only);

Thank you. Nichole. Taske@nice. org. uk