Sorafenib en el tratamiento del cncer de rin

Sorafenib en el tratamiento del cáncer de riñón

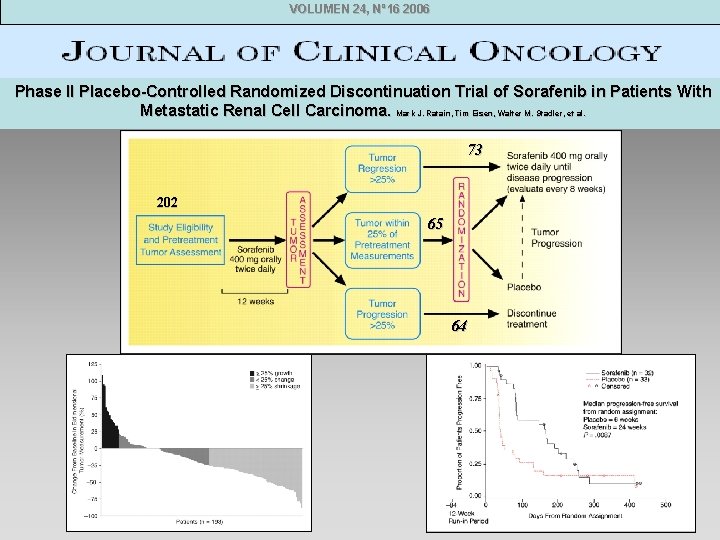
VOLUMEN 24, Nº 16 2006 Phase II Placebo-Controlled Randomized Discontinuation Trial of Sorafenib in Patients With Metastatic Renal Cell Carcinoma. Mark J. Ratain, Tim Eisen, Walter M. Stadler, et al. 73 202 65 64
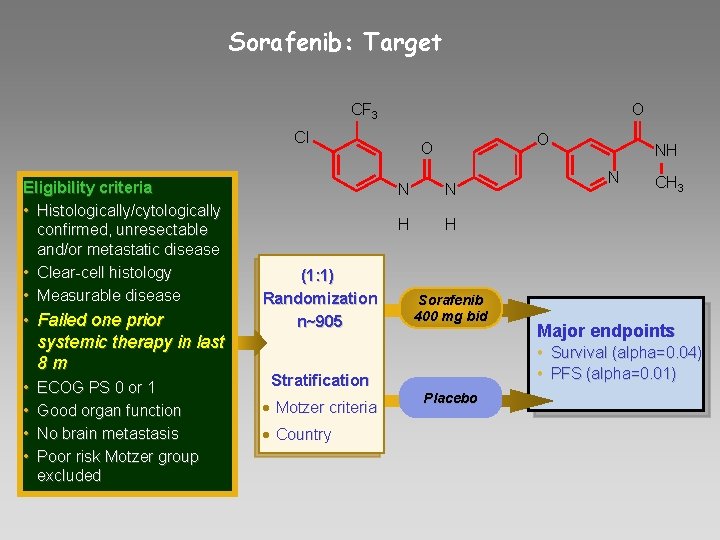
Sorafenib: Target CF 3 O Cl Eligibility criteria • Histologically/cytologically confirmed, unresectable and/or metastatic disease • Clear-cell histology • Measurable disease • Failed one prior systemic therapy in last 8 m • • ECOG PS 0 or 1 Good organ function No brain metastasis Poor risk Motzer group excluded (1: 1) Randomization n~905 O O N N H H Sorafenib 400 mg bid • Country N CH 3 Major endpoints • Survival (alpha=0. 04) • PFS (alpha=0. 01) Stratification • Motzer criteria NH Placebo
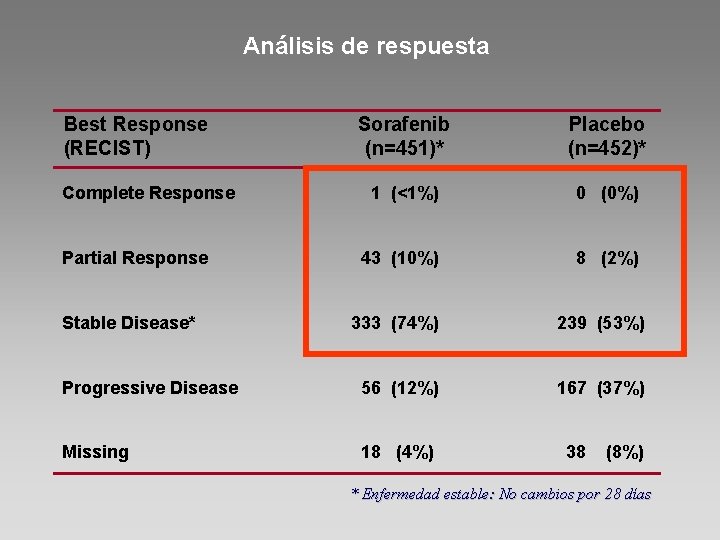
Análisis de respuesta Best Response (RECIST) Sorafenib (n=451)* Placebo (n=452)* 1 (<1%) 0 (0%) 43 (10%) 8 (2%) 333 (74%) 239 (53%) Progressive Disease 56 (12%) 167 (37%) Missing 18 (4%) Complete Response Partial Response Stable Disease* 38 (8%) * Enfermedad estable: No cambios por 28 días
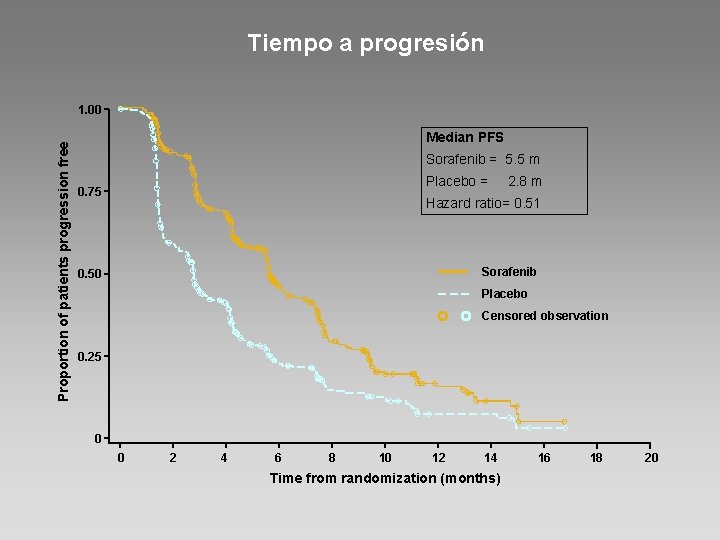
Tiempo a progresión Proportion of patients progression free 1. 00 Median PFS Sorafenib = 5. 5 m Placebo = 2. 8 m 0. 75 Hazard ratio= 0. 51 Sorafenib 0. 50 Placebo Censored observation 0. 25 0 0 2 4 6 8 10 12 14 Time from randomization (months) 16 18 20
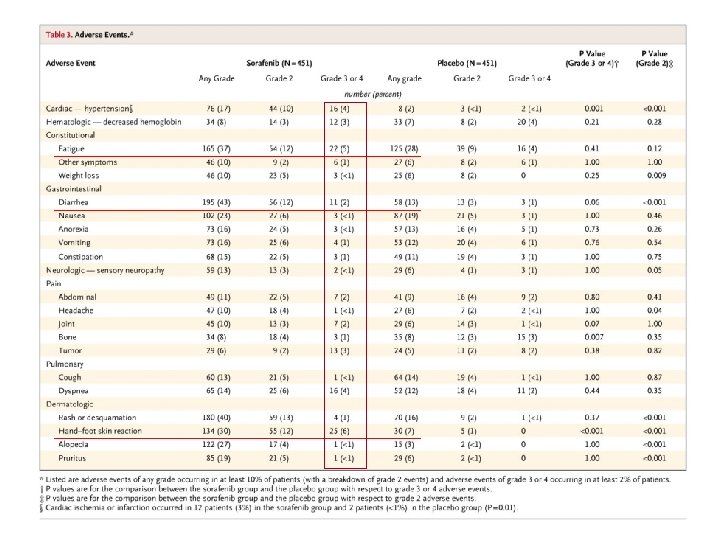
Adverse Events Escudier B et al. N Engl J Med 2007; 356: 125 -134
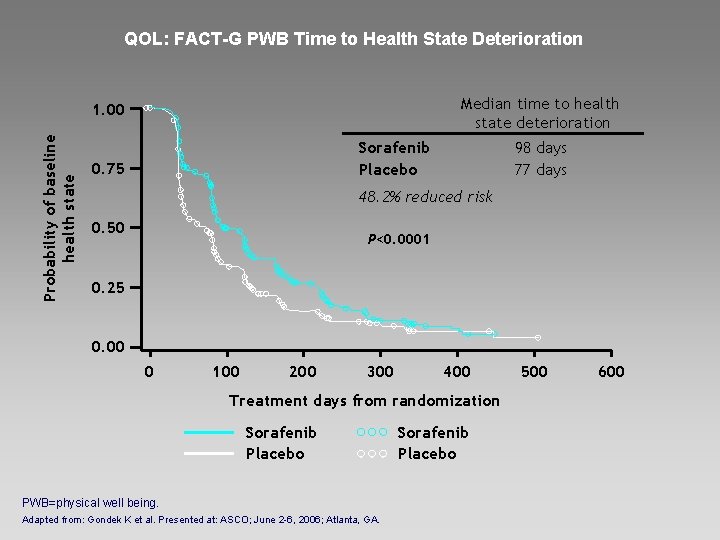
QOL: FACT-G PWB Time to Health State Deterioration Probability of baseline health state 1. 00 Sorafenib Placebo 0. 75 Median time to health state deterioration 98 days 77 days 48. 2% reduced risk 0. 50 P<0. 0001 0. 25 0. 00 0 100 200 300 400 Treatment days from randomization Sorafenib Placebo PWB=physical well being. Adapted from: Gondek K et al. Presented at: ASCO; June 2 -6, 2006; Atlanta, GA. Sorafenib Placebo 500 600
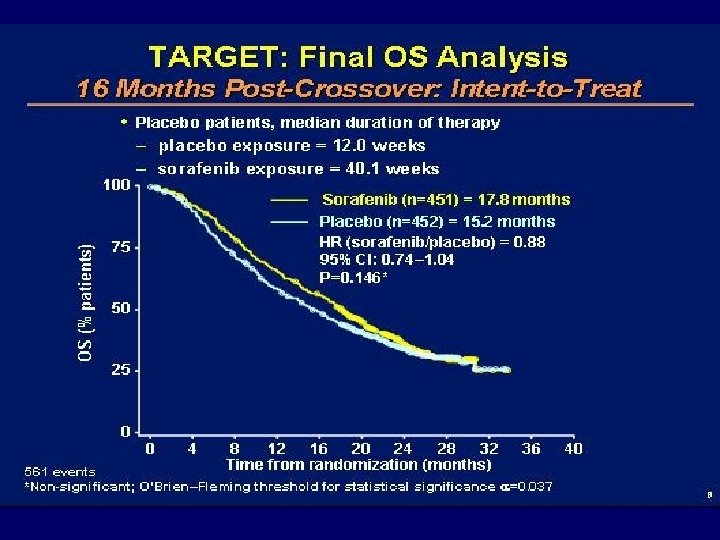
N = 903 pts

Conclusiones TARGET • Por vez primera: un fármaco no inmunoterapia prolonga tiempo a progresión en un ensayo fase III • Resultados discretos en tasa de respuesta: beneficio clínico en la mayoría de los pacientes: se trataba de un estudio de segunda línea • Toxicidad predecible y asumible • Objetivo: Resultados en primera línea
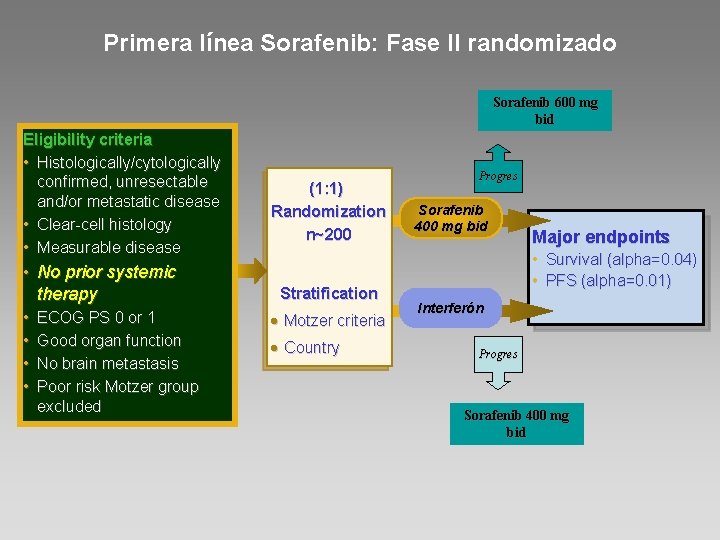
Primera línea Sorafenib: Fase II randomizado Sorafenib 600 mg bid Eligibility criteria • Histologically/cytologically confirmed, unresectable and/or metastatic disease • Clear-cell histology • Measurable disease • No prior systemic therapy • • ECOG PS 0 or 1 Good organ function No brain metastasis Poor risk Motzer group excluded (1: 1) Randomization n~200 Stratification • Motzer criteria • Country Progres Sorafenib 400 mg bid Major endpoints • Survival (alpha=0. 04) • PFS (alpha=0. 01) Interferón Progres Sorafenib 400 mg bid
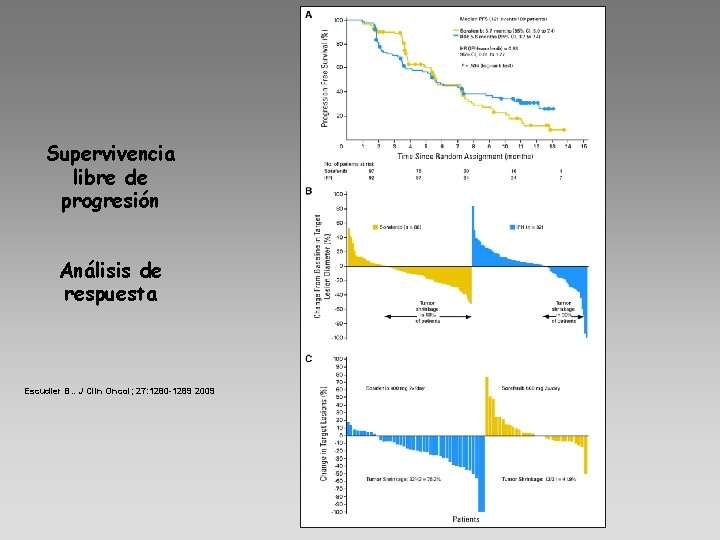
Supervivencia libre de progresión Análisis de respuesta Escudier B. . J Clin Oncol; 27: 1280 -1289 2009
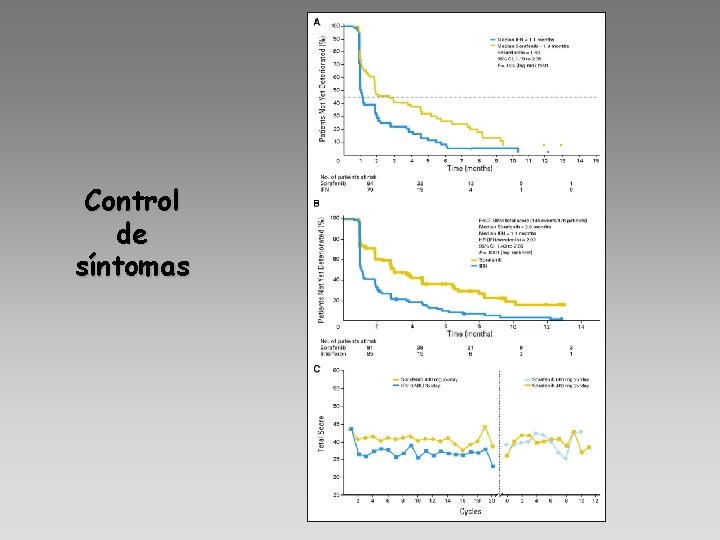
Control de síntomas

Conclusiones • Sorafenib no fue superior a interferón en términos de supervivencia libre de progresión • Sorafenib mostró: – Mayor tasa de respuesta – Menos efectos secundarios – Mejor calidad de vida • La progresión de dosis de Sorafenib rescató algunos pacientes • Dados los resultados existentes con otras moléculas vs interferón: Sorafenib no puede considerarse un estándar de primera línea


¿Cómo intercalar Sorafenib en el tratamiento del RCC?

1. Primera línea: Perfil de Seguridad
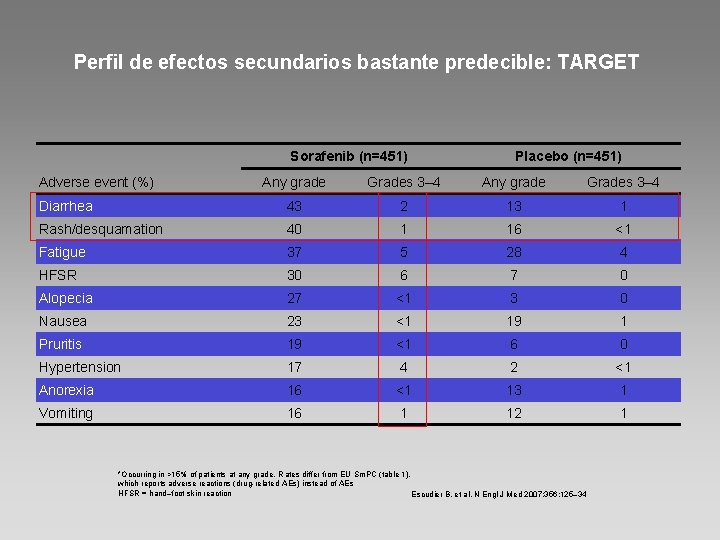
Perfil de efectos secundarios bastante predecible: TARGET Sorafenib (n=451) Adverse event (%) Placebo (n=451) Any grade Grades 3– 4 Diarrhea 43 2 13 1 Rash/desquamation 40 1 16 <1 Fatigue 37 5 28 4 HFSR 30 6 7 0 Alopecia 27 <1 3 0 Nausea 23 <1 19 1 Pruritis 19 <1 6 0 Hypertension 17 4 2 <1 Anorexia 16 <1 13 1 Vomiting 16 1 12 1 *Occurring in >15% of patients at any grade. R ates differ from EU Sm. PC (table 1), which reports adverse reactions (drug-related AEs) instead of AEs HFSR = hand–foot skin reaction Escudier B, et al. N Engl J Med 2007; 356: 125– 34
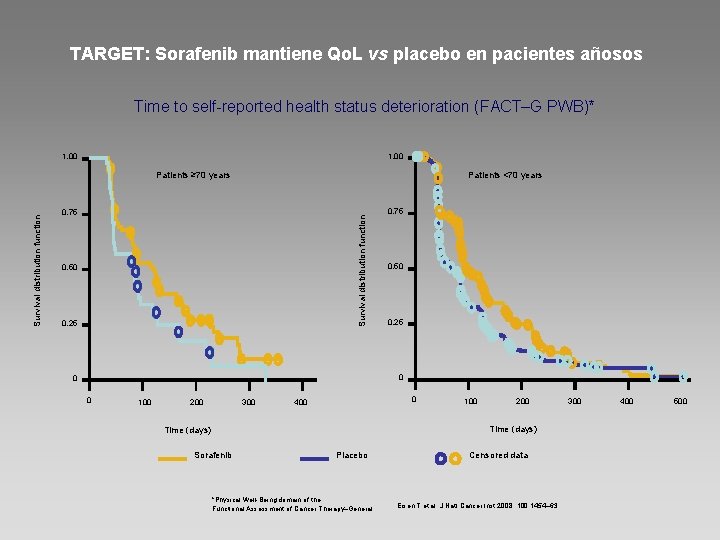
TARGET: Sorafenib mantiene Qo. L vs placebo en pacientes añosos Time to self-reported health status deterioration (FACT–G PWB)* 1. 00 Patients <70 years 0. 75 Survival distribution function Patients ≥ 70 years 0. 50 0. 25 0. 75 0. 50 0. 25 0 0 0 100 200 300 0 400 100 200 Time (days) Sorafenib Placebo *Physical Well-Being domain of the Functional Assessment of Cancer Therapy–General Censored data Eisen T et al. J Natl Cancer Inst 2008; 100: 1454– 63 300 400 500

Sorafenib parece tener la menor incidencia reportada de efectos adversos grado III-IV “Overall… a cumulative count of all grade 3– 4 side effects for each agent shows that sorafenib causes the fewest grade 3– 4 side effects” “Sorafenib, sunitinib, and temsirolimus represent effective treatment options for patients with metastatic RCC, but the drugs are not devoid of toxicity” “Head-to-head trials with the same patient inclusion criteria are required to enable valid comparisons of safety profiles of targeted therapies” Bhojani N et al. Eur Urol 2008; 53: 917– 30
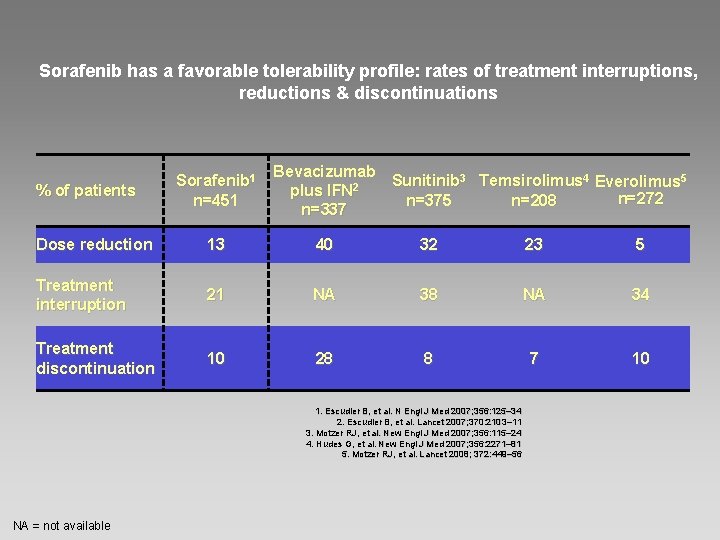
Sorafenib has a favorable tolerability profile: rates of treatment interruptions, reductions & discontinuations % of patients Sorafenib 1 n=451 Bevacizumab Sunitinib 3 Temsirolimus 4 Everolimus 5 plus IFN 2 n=272 n=375 n=208 n=337 Dose reduction 13 40 32 23 5 Treatment interruption 21 NA 38 NA 34 Treatment discontinuation 10 28 8 7 10 1. Escudier B, et al. N Engl J Med 2007; 356: 125– 34 2. Escudier B, et al. Lancet 2007; 370: 2103– 11 3. Motzer RJ, et al. New Engl J Med 2007; 356: 115– 24 4. Hudes G, et al. New Engl J Med 2007; 356: 2271– 81 5. Motzer RJ, et al. Lancet 2008; 372: 449– 56 NA = not available
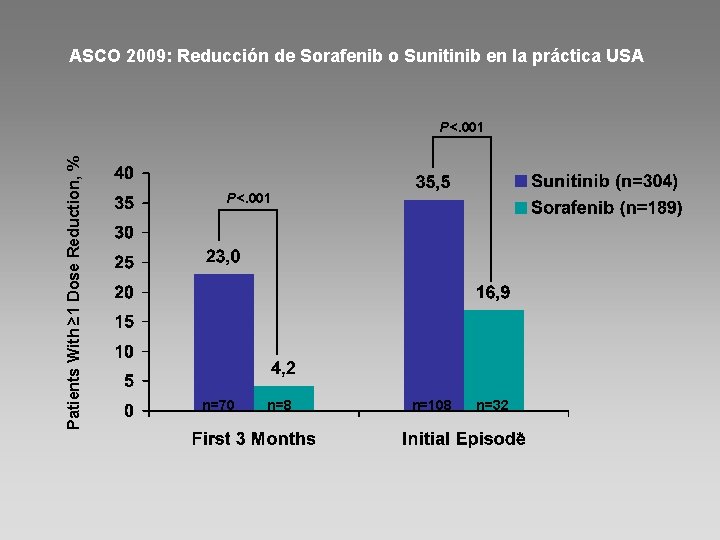
ASCO 2009: Reducción de Sorafenib o Sunitinib en la práctica USA Patients With ≥ 1 Dose Reduction, % P<. 001 n=70 n=8 n=108 n=32 *

2. Segundas o ulteriores líneas: Secuenciación

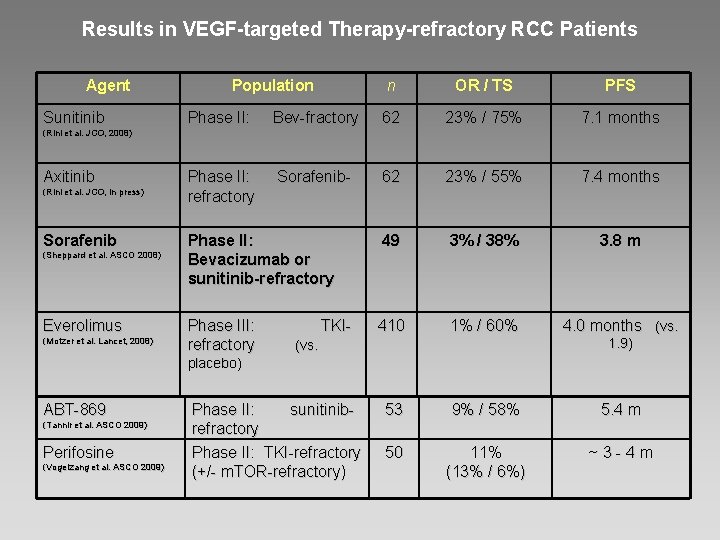
Results in VEGF-targeted Therapy-refractory RCC Patients Agent Sunitinib Population n OR / TS PFS Phase II: Bev-fractory 62 23% / 75% 7. 1 months Phase II: Sorafenibrefractory 62 23% / 55% 7. 4 months Phase II: Bevacizumab or sunitinib-refractory 49 3% / 38% 3. 8 m Phase III: TKIrefractory (vs. 410 1% / 60% 4. 0 months (vs. (Rini et al. JCO , 2008) (Rini et al. JCO, 2008) Axitinib (Rini et al. JCO , in press) (Rini et al. JCO, in press) Sorafenib (Sheppard et al. ASCO 2008) Everolimus (Motzer et al. Lancet, 2008) 1. 9) placebo) ABT-869 (Tannir et al. ASCO 2009) Perifosine (Vogelzang et al. ASCO 2009) Phase II: sunitinibrefractory Phase II: TKI-refractory (+/- m. TOR-refractory) 53 9% / 58% 5. 4 m 50 11% (13% / 6%) ~ 3 - 4 m
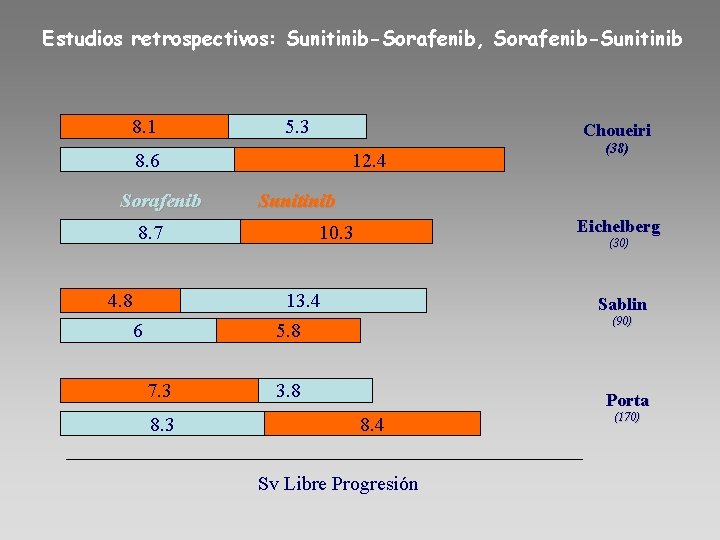
Estudios retrospectivos: Sunitinib-Sorafenib, Sorafenib-Sunitinib 8. 1 5. 3 Choueiri 8. 6 Sorafenib 12. 4 Sunitinib 8. 7 4. 8 Eichelberg 10. 3 (30) 13. 4 5. 8 6 7. 3 8. 3 (38) Sablin (90) 3. 8 Porta 8. 4 Sv Libre Progresión (170)
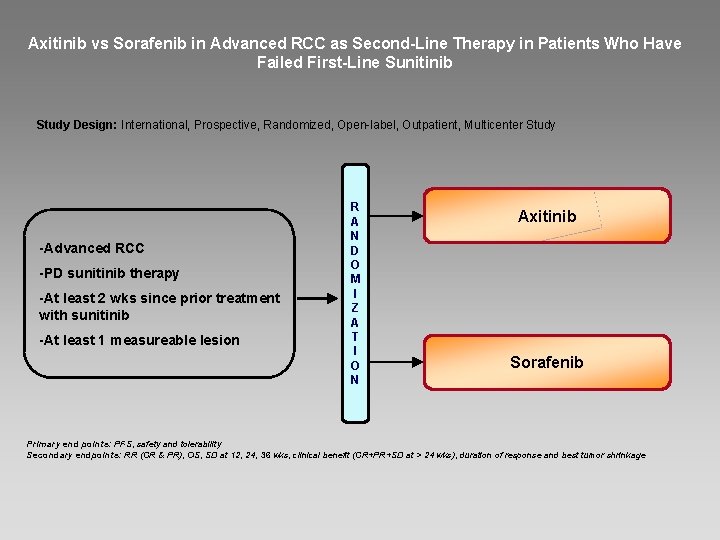
Axitinib vs Sorafenib in Advanced RCC as Second-Line Therapy in Patients Who Have Failed First-Line Sunitinib Study Design: International, Prospective, Randomized, Open-label, Outpatient, Multicenter Study -Advanced RCC -PD sunitinib therapy -At least 2 wks since prior treatment with sunitinib -At least 1 measureable lesion R A N D O M I Z A T I O N Axitinib Sorafenib Primary end points: PFS, safety and tolerability Secondary endpoints: RR (CR & PR), OS, SD at 12, 24, 36 wks, clinical benefit (CR+PR+SD at > 24 wks), duration of response and best tumor shrinkage
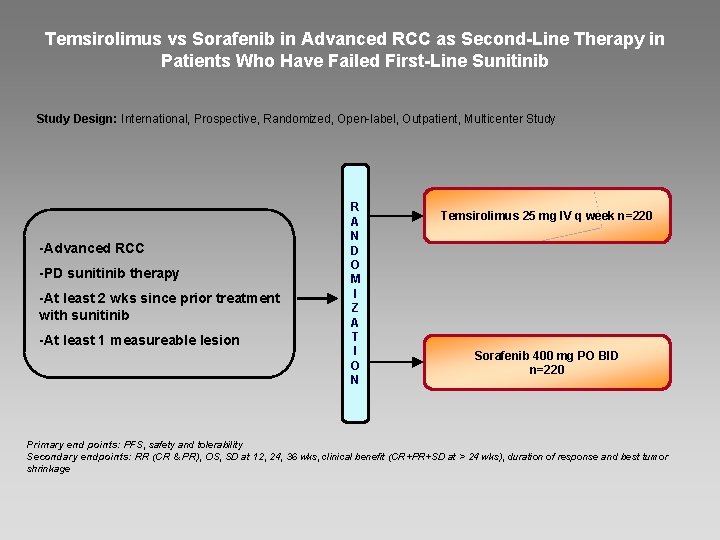
Temsirolimus vs Sorafenib in Advanced RCC as Second-Line Therapy in Patients Who Have Failed First-Line Sunitinib Study Design: International, Prospective, Randomized, Open-label, Outpatient, Multicenter Study -Advanced RCC -PD sunitinib therapy -At least 2 wks since prior treatment with sunitinib -At least 1 measureable lesion R A N D O M I Z A T I O N Temsirolimus 25 mg IV q week n=220 Sorafenib 400 mg PO BID n=220 Primary end points: PFS, safety and tolerability Secondary endpoints: RR (CR & PR), OS, SD at 12, 24, 36 wks, clinical benefit (CR+PR+SD at > 24 wks), duration of response and best tumor shrinkage
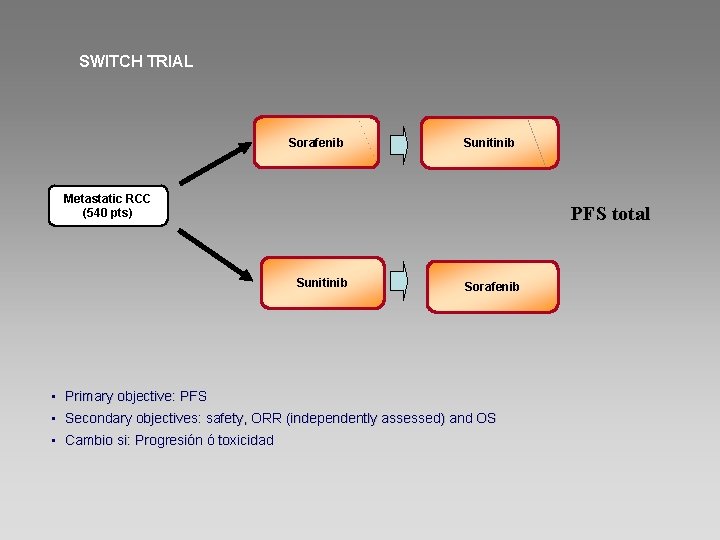
SWITCH TRIAL Sorafenib Sunitinib Metastatic RCC (540 pts) PFS total Sunitinib Sorafenib • Primary objective: PFS • Secondary objectives: safety, ORR (independently assessed) and OS • Cambio si: Progresión ó toxicidad
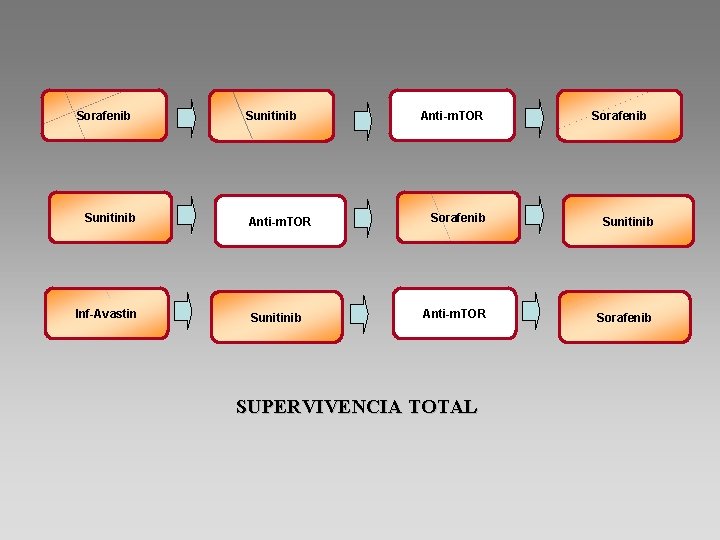
Sorafenib Sunitinib Inf-Avastin Sunitinib Anti-m. TOR Sorafenib SUPERVIVENCIA TOTAL

3. Combinación
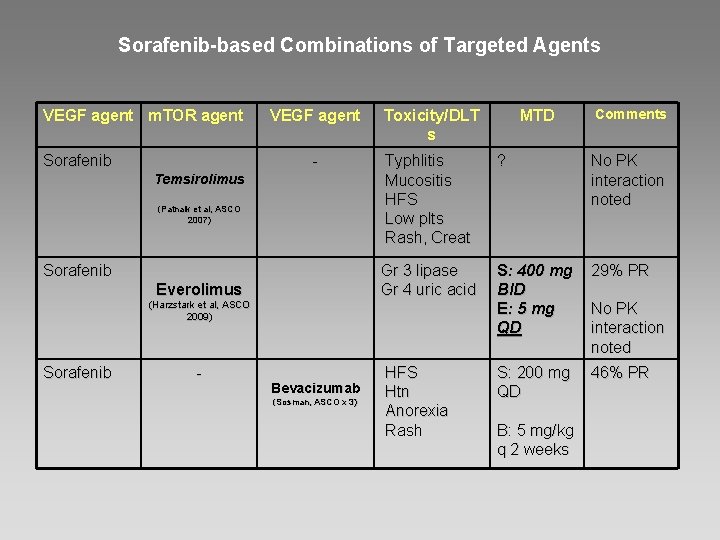
Sorafenib-based Combinations of Targeted Agents VEGF agent m. TOR agent Sorafenib VEGF agent - Temsirolimus (Patnaik et al, ASCO 2007) Sorafenib Everolimus Toxicity/DLT s - Bevacizumab (Sosman, ASCO x 3) Comments Typhlitis Mucositis HFS Low plts Rash, Creat ? No PK interaction noted Gr 3 lipase Gr 4 uric acid S: 400 mg BID E: 5 mg QD 29% PR S: 200 mg QD 46% PR (Harzstark et al, ASCO 2009) Sorafenib MTD HFS Htn Anorexia Rash B: 5 mg/kg q 2 weeks No PK interaction noted
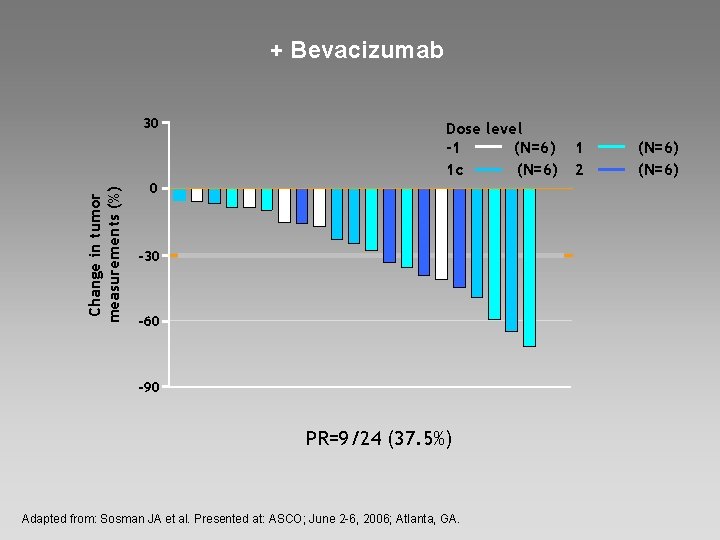
+ Bevacizumab Change in tumor measurements (%) 30 Dose level -1 (N=6) 1 c (N=6) 0 -30 -60 -90 PR=9/24 (37. 5%) Adapted from: Sosman JA et al. Presented at: ASCO; June 2 -6, 2006; Atlanta, GA. 1 2 (N=6)
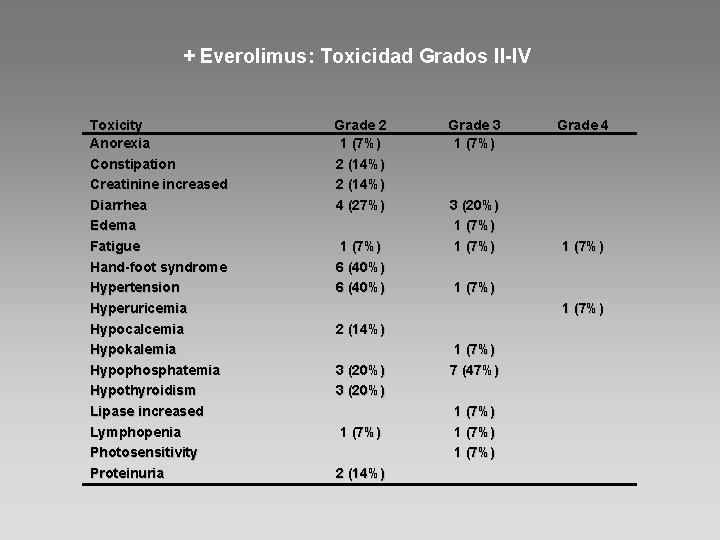
+ Everolimus: Toxicidad Grados II-IV Toxicity Anorexia Constipation Creatinine increased Diarrhea Edema Fatigue Hand-foot syndrome Hypertension Hyperuricemia Hypocalcemia Hypokalemia Hypophosphatemia Hypothyroidism Lipase increased Lymphopenia Photosensitivity Proteinuria Grade 2 1 (7%) 2 (14%) 4 (27%) 1 (7%) 6 (40%) Grade 3 1 (7%) 3 (20%) 1 (7%) Grade 4 1 (7%) 2 (14%) 3 (20%) 1 (7%) 2 (14%) 1 (7%) 7 (47%) 1 (7%)
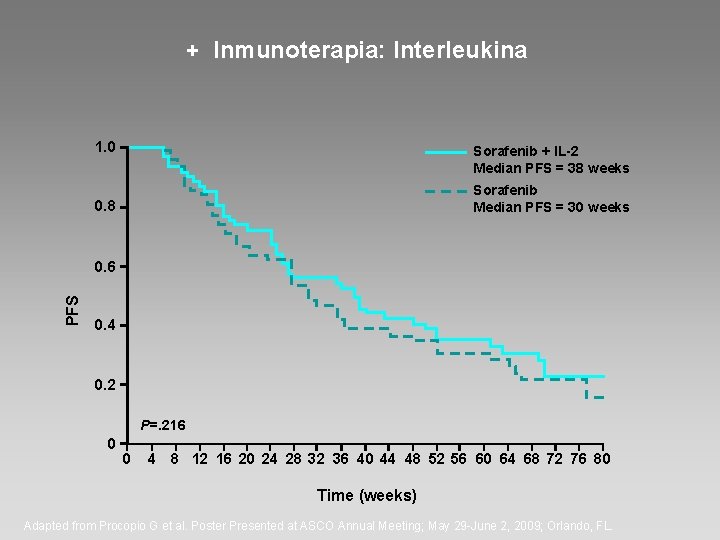
+ Inmunoterapia: Interleukina 1. 0 Sorafenib + IL-2 Median PFS = 38 weeks Sorafenib Median PFS = 30 weeks 0. 8 PFS 0. 6 0. 4 0. 2 P=. 216 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 Time (weeks) Adapted from Procopio G et al. Poster Presented at ASCO Annual Meeting; May 29 -June 2, 2009; Orlando, FL.
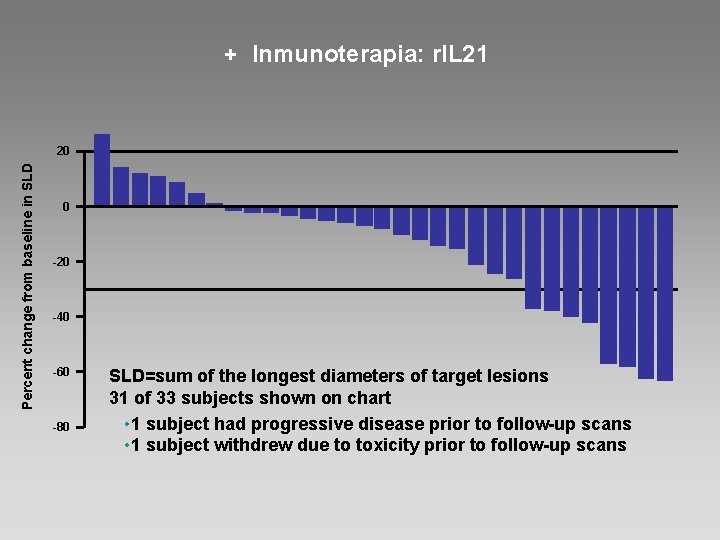
+ Inmunoterapia: r. IL 21 Percent change from baseline in SLD 20 0 -20 -40 -60 -80 SLD=sum of the longest diameters of target lesions 31 of 33 subjects shown on chart • 1 subject had progressive disease prior to follow-up scans • 1 subject withdrew due to toxicity prior to follow-up scans
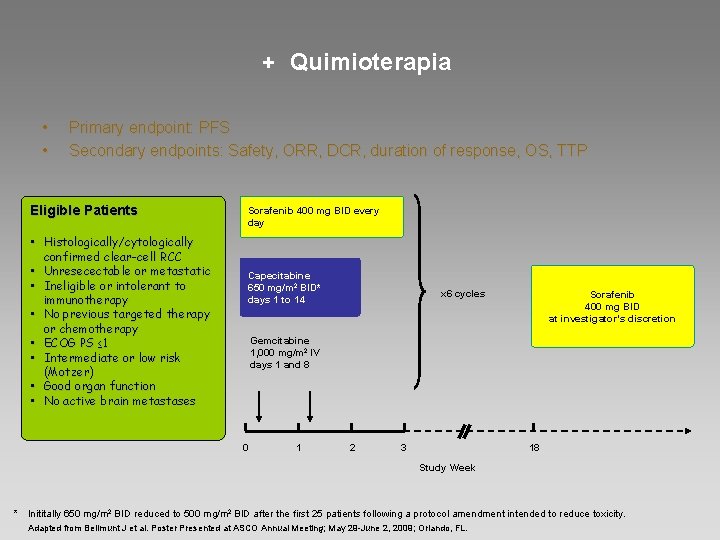
+ Quimioterapia • • Primary endpoint: PFS Secondary endpoints: Safety, ORR, DCR, duration of response, OS, TTP Eligible Patients • Histologically/cytologically confirmed clear-cell RCC • Unresecectable or metastatic • Ineligible or intolerant to immunotherapy • No previous targeted therapy or chemotherapy • ECOG PS ≤ 1 • Intermediate or low risk (Motzer) • Good organ function • No active brain metastases Sorafenib 400 mg BID every day Capecitabine 650 mg/m 2 BID* days 1 to 14 x 6 cycles Sorafenib 400 mg BID at investigator’s discretion Gemcitabine 1, 000 mg/m 2 IV days 1 and 8 0 1 2 3 18 Study Week * Inititally 650 mg/m 2 BID reduced to 500 mg/m 2 BID after the first 25 patients following a protocol amendment intended to reduce toxicity. Adapted from Bellmunt J et al. Poster Presented at ASCO Annual Meeting; May 29 -June 2, 2009; Orlando, FL.
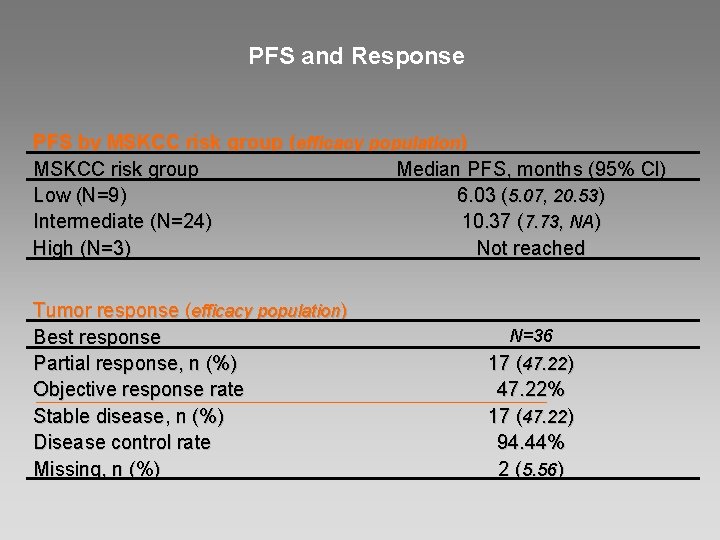
PFS and Response PFS by MSKCC risk group (efficacy population) MSKCC risk group Median PFS, months (95% Cl) Low (N=9) 6. 03 (5. 07, 20. 53) Intermediate (N=24) 10. 37 (7. 73, NA) High (N=3) Not reached Tumor response (efficacy population) Best response Partial response, n (%) Objective response rate Stable disease, n (%) Disease control rate Missing, n (%) N=36 17 (47. 22) 47. 22% 17 (47. 22) 94. 44% 2 (5. 56)

4. Adyuvancia
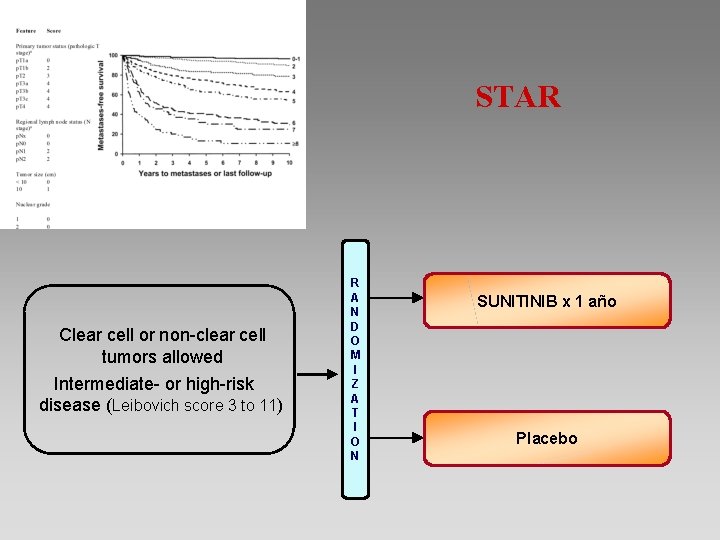
STAR Clear cell or non-clear cell tumors allowed Intermediate- or high-risk disease (Leibovich score 3 to 11) R A N D O M I Z A T I O N SUNITINIB x 1 año Placebo
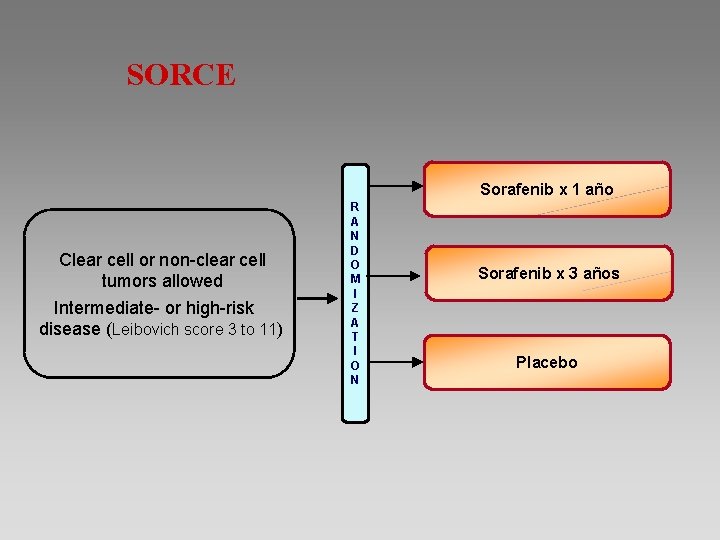
SORCE Sorafenib x 1 año Clear cell or non-clear cell tumors allowed Intermediate- or high-risk disease (Leibovich score 3 to 11) R A N D O M I Z A T I O N Sorafenib x 3 años Placebo
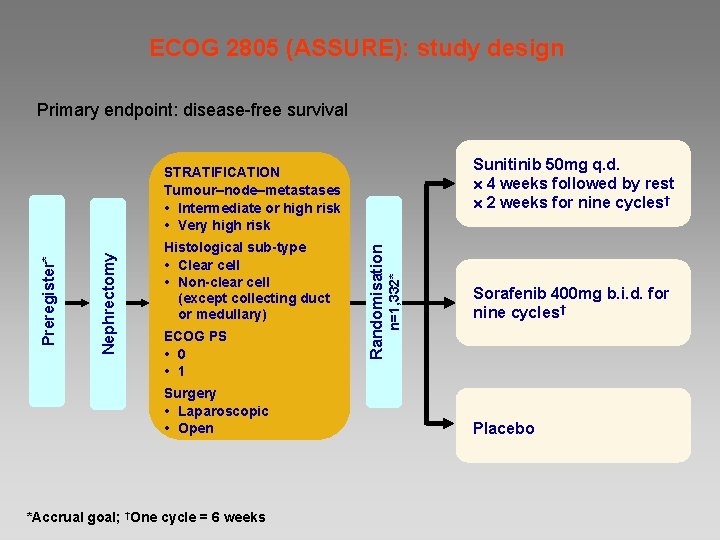
ECOG 2805 (ASSURE): study design Primary endpoint: disease-free survival Sunitinib 50 mg q. d. 4 weeks followed by rest 2 weeks for nine cycles† ECOG PS 0 1 Surgery Laparoscopic Open *Accrual goal; †One cycle = 6 weeks n=1, 332* Histological sub-type Clear cell Non-clear cell (except collecting duct or medullary) Randomisation Nephrectomy Preregister* STRATIFICATION Tumour–node–metastases Intermediate or high risk Very high risk Sorafenib 400 mg b. i. d. for nine cycles† Placebo

Gracias
- Slides: 43