Redefiniendo el Tratamiento del Cncer Renal Enrique Grande










- Slides: 44

Redefiniendo el Tratamiento del Cáncer Renal Enrique Grande Head of Medical Oncology Director of Clinical Research

Disclosures • • Honoraria for ad boards and/or lectures: o Pfizer, BMS, IPSEN, Roche, Eisai, Eusa Pharma, MSD, Sanofi-Genzyme, Adacap, Novartis, Pierre Fabre, Lexicon, Celgene Research Grants: o Pfizer, Astra Zeneca, MTEM/Threshold, Roche, IPSEN, Lexicon Leadership roles in medical societies: o ENETS, GETNE and GETHI Stocks or ownership interest: o None

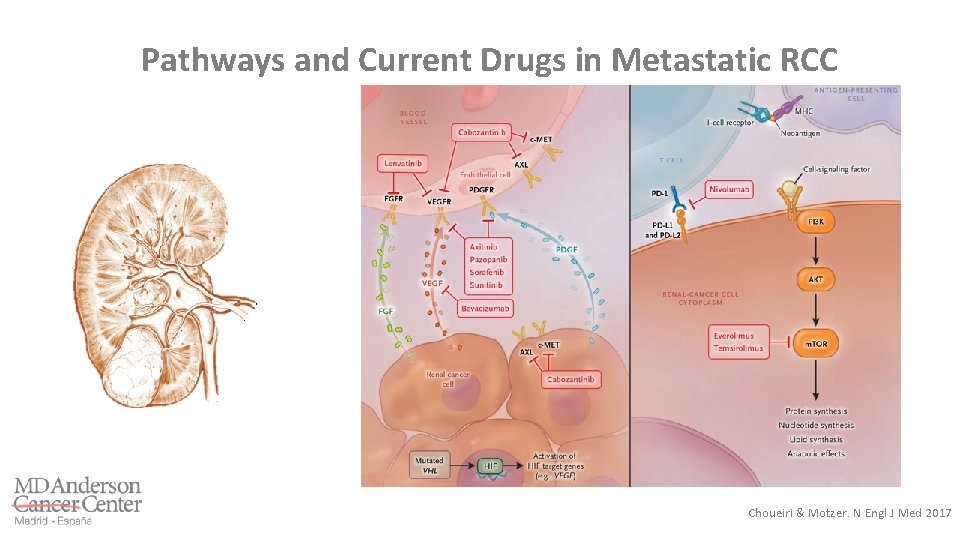
Pathways and Current Drugs in Metastatic RCC Choueiri & Motzer. N Engl J Med 2017

Curti BD. N Engl J Med 2018

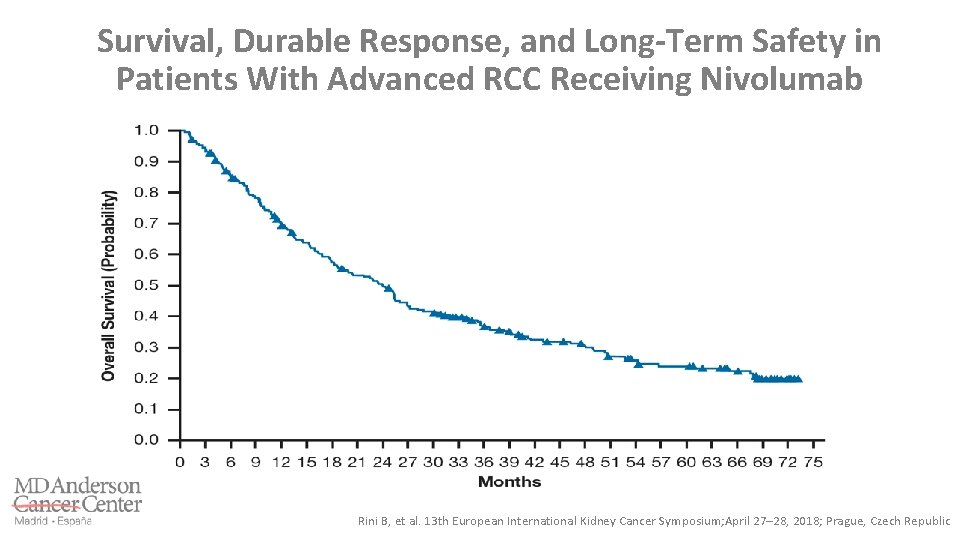
Survival, Durable Response, and Long-Term Safety in Patients With Advanced RCC Receiving Nivolumab Rini B, et al. 13 th European International Kidney Cancer Symposium; April 27– 28, 2018; Prague, Czech Republic

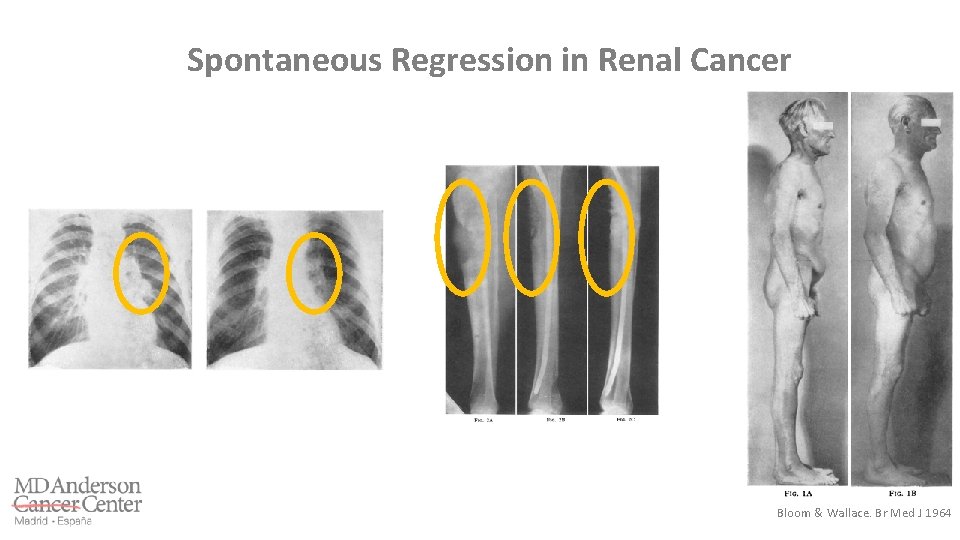
Spontaneous Regression in Renal Cancer Bloom & Wallace. Br Med J 1964

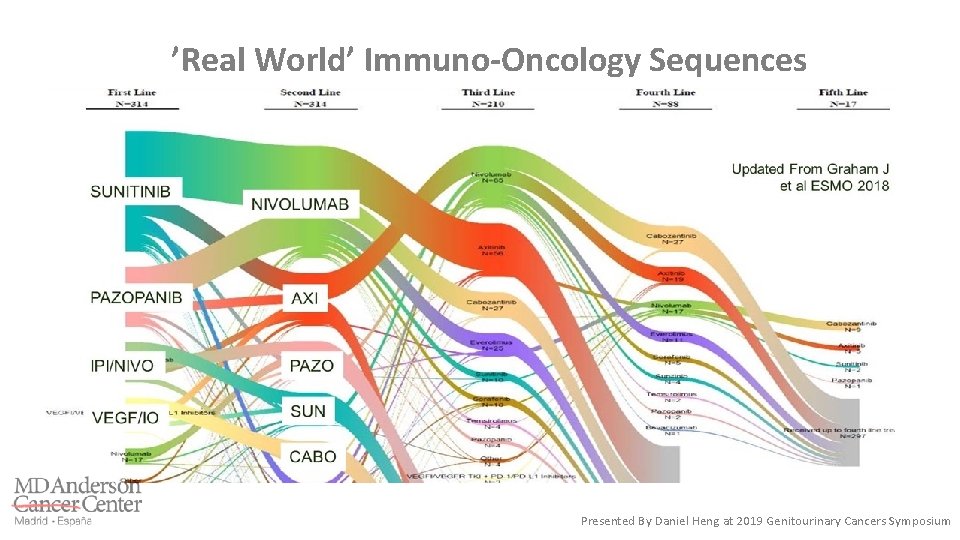
’Real World’ Immuno-Oncology Sequences Presented By Daniel Heng at 2019 Genitourinary Cancers Symposium

Broad range of systemic treatment options Adapted from NCCN v 2. 2019 Guidelines. https: //www. nccn. org/professionals/physician_gls/pdf/kidney. pdf

Broad range of systemic treatment options Active surveillance Adapted from NCCN v 2. 2019 Guidelines. https: //www. nccn. org/professionals/physician_gls/pdf/kidney. pdf

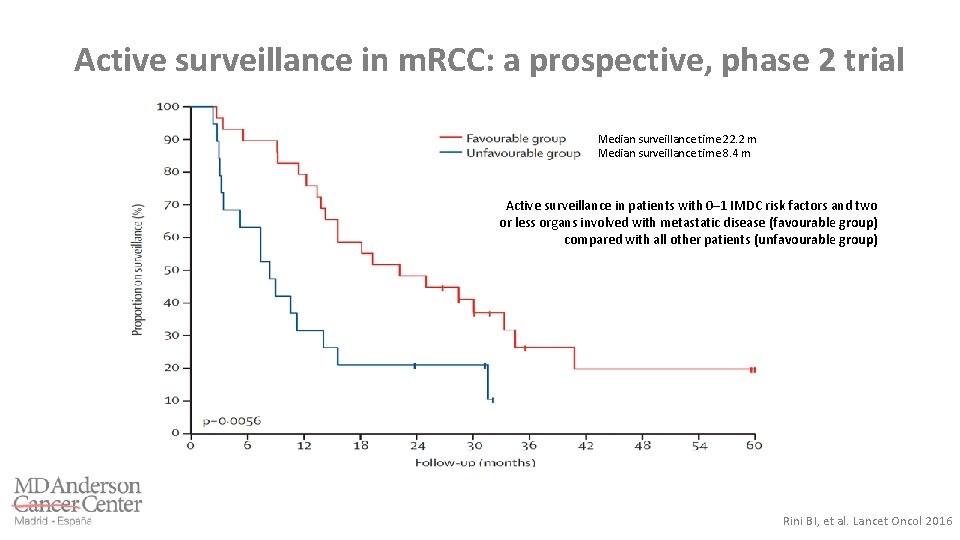
Active surveillance in m. RCC: a prospective, phase 2 trial Median surveillance time 22. 2 m Median surveillance time 8. 4 m Active surveillance in patients with 0– 1 IMDC risk factors and two or less organs involved with metastatic disease (favourable group) compared with all other patients (unfavourable group) Rini BI, et al. Lancet Oncol 2016

Broad range of systemic treatment options Active surveillance Metastasectomy Adapted from NCCN v 2. 2019 Guidelines. https: //www. nccn. org/professionals/physician_gls/pdf/kidney. pdf

Meta-analysis: Outcomes following complete metastasectomy Zaid HB, et al. J Urol 2016

Broad range of systemic treatment options Active surveillance Metastasectomy Cytoreductive Nephrectomy Adapted from NCCN v 2. 2019 Guidelines. https: //www. nccn. org/professionals/physician_gls/pdf/kidney. pdf

Role of Cytoreductive Nephrectomy in the Cytokines era Flanigan RC, et al. N Engl J Med. 2001 Mickish GHJ, et al. Lancet 2001

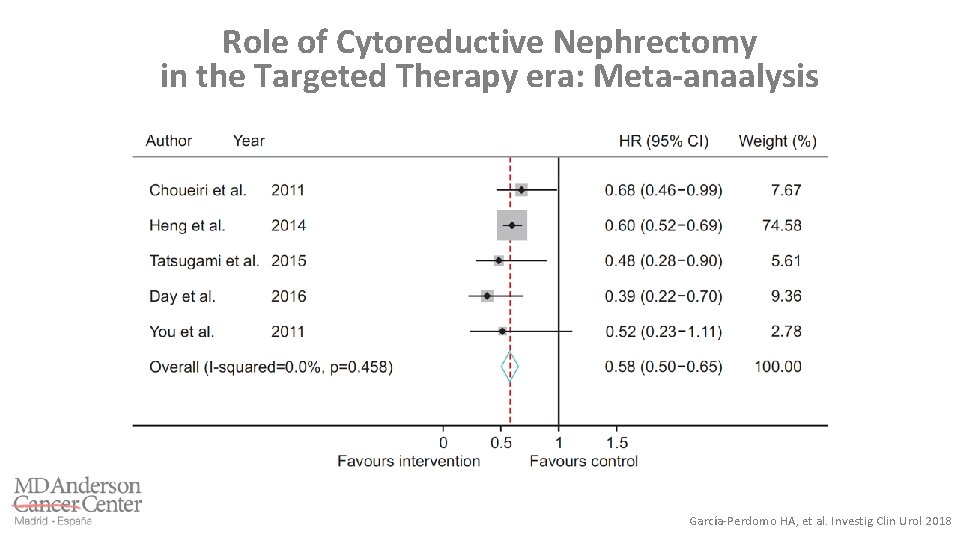
Role of Cytoreductive Nephrectomy in the Targeted Therapy era: Meta-anaalysis García-Perdomo HA, et al. Investig Clin Urol 2018

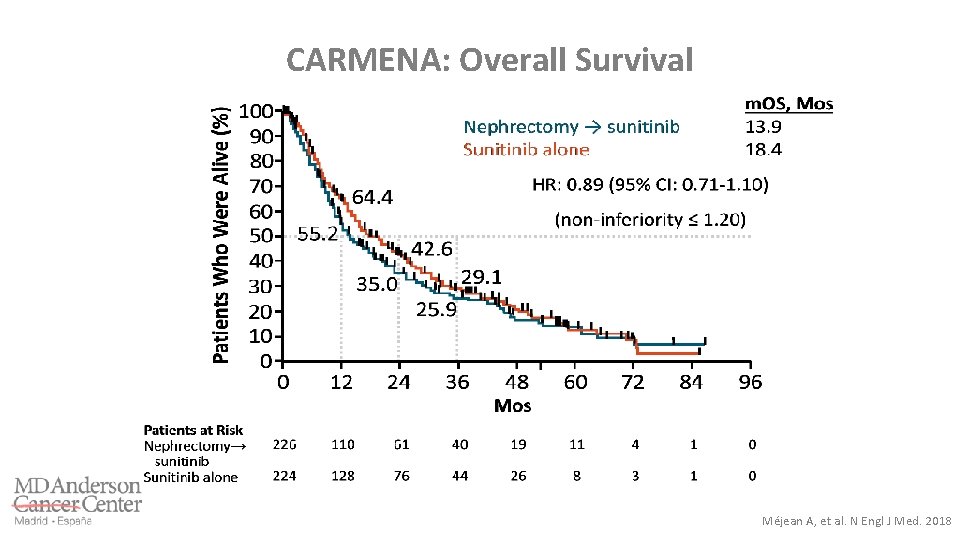
CARMENA: Overall Survival Méjean A, et al. N Engl J Med. 2018

Bex A, et al. Eur Urol 2018

Broad range of systemic treatment options Active surveillance Metastasectomy Cytoreductive Nephrectomy Systemic Treatment Adapted from NCCN v 2. 2019 Guidelines. https: //www. nccn. org/professionals/physician_gls/pdf/kidney. pdf

Broad range of systemic treatment options Nivolumab Active surveillance Sunitinib Temsirolimus Pazopanib Tivozanib Metastasectomy Single agents Cytoreductive Nephrectomy Systemic Treatment Everolimus Sorafenib Axitinib Cabozantinib Beva + INF Lenvatinib + Eve Nivo + Ipi Atezo + Beva? Combos Avelumab + Axi? Pembro + Axi? Adapted from NCCN v 2. 2019 Guidelines. https: //www. nccn. org/professionals/physician_gls/pdf/kidney. pdf

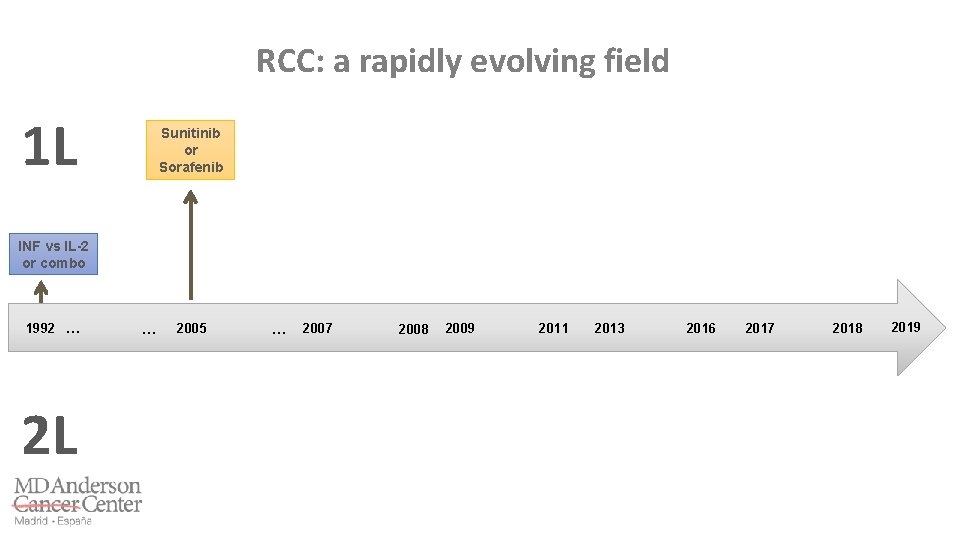
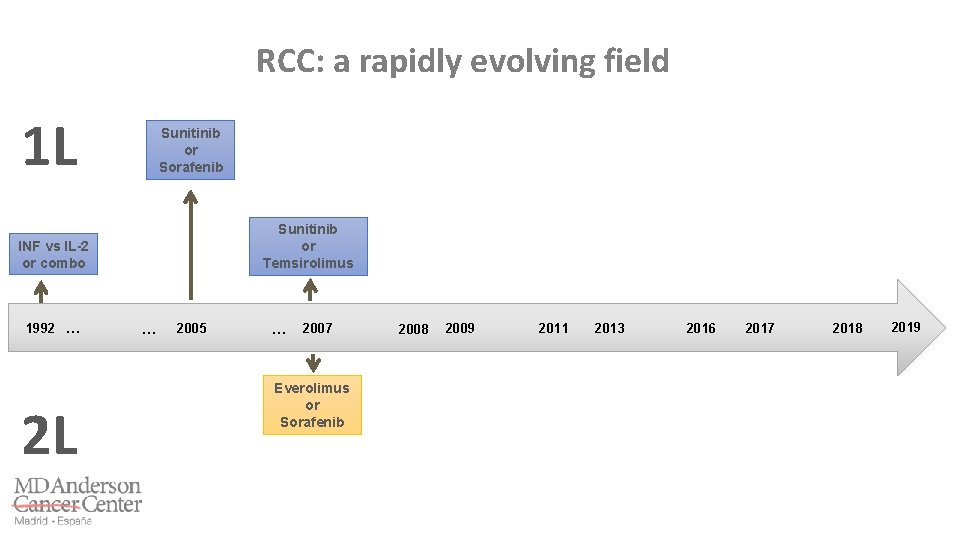
RCC: a rapidly evolving field 1 L 1992 … 2 L … 2005 … 2007 2009 2008 2011 2014 2011 2013 2016 2017 2018 2019

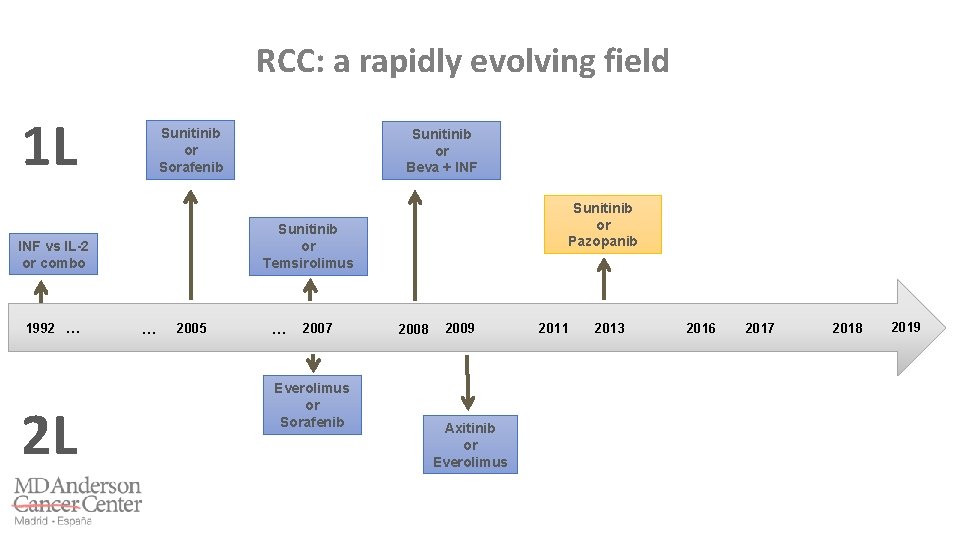
RCC: a rapidly evolving field 1 L INF vs IL-2 or combo 1992 … 2 L … 2005 … 2007 2009 2008 2011 2014 2011 2013 2016 2017 2018 2019

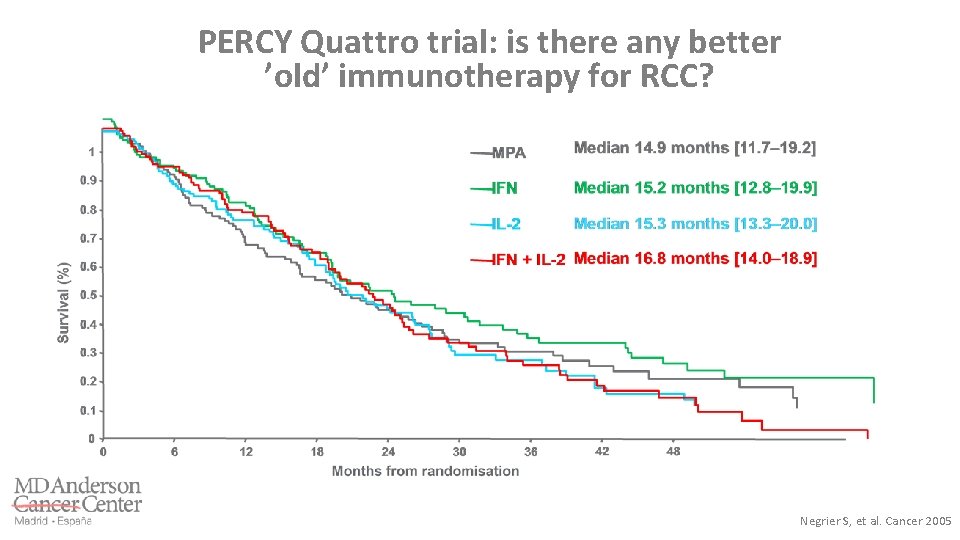
PERCY Quattro trial: is there any better ’old’ immunotherapy for RCC? Negrier S, et al. Cancer 2005

RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib INF vs IL-2 or combo 1992 … 2 L … 2005 … 2007 2009 2008 2011 2014 2011 2013 2016 2017 2018 2019

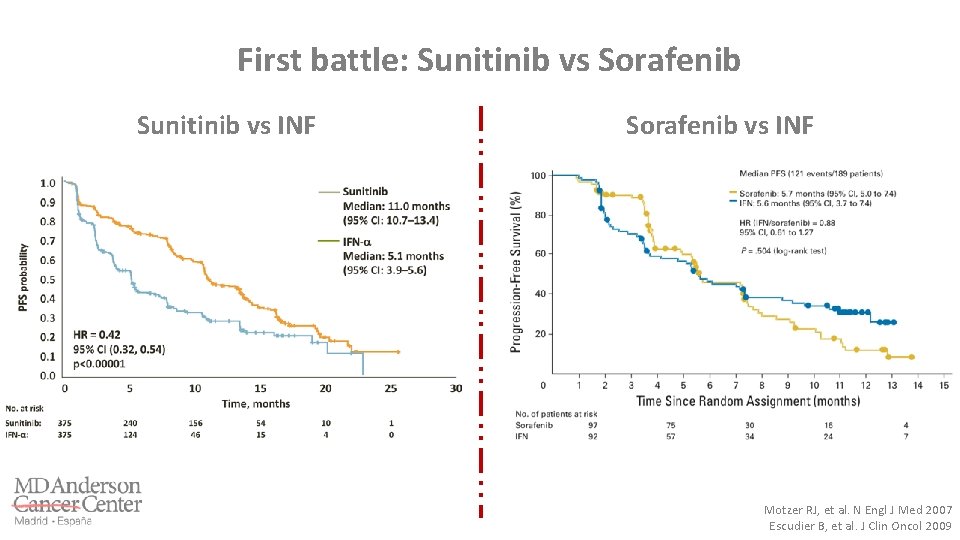
First battle: Sunitinib vs Sorafenib Sunitinib vs INF Sorafenib vs INF Motzer RJ, et al. N Engl J Med 2007 Escudier B, et al. J Clin Oncol 2009

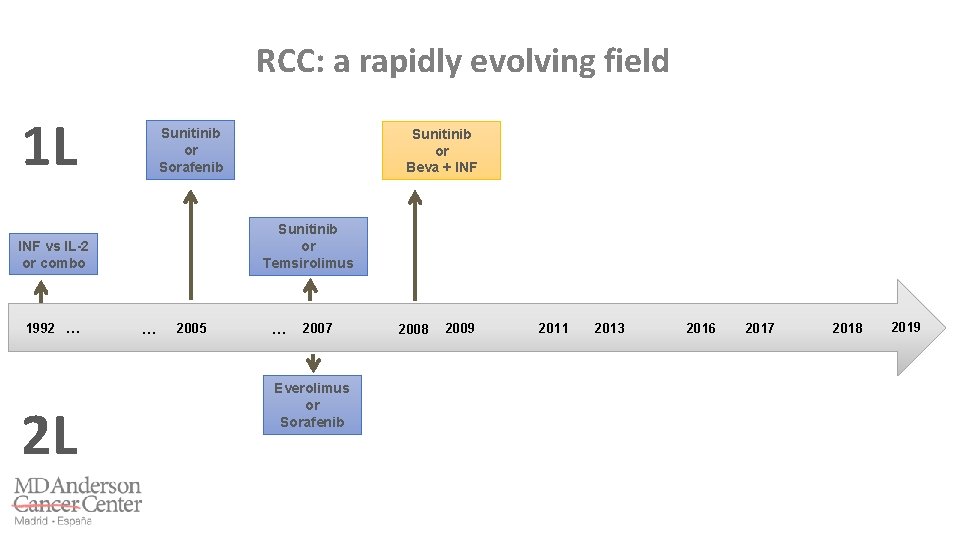
RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib Sunitinib or Temsirolimus INF vs IL-2 or combo 1992 … 2 L … 2005 … 2007 2009 2008 2011 2014 2011 2013 2016 2017 2018 2019

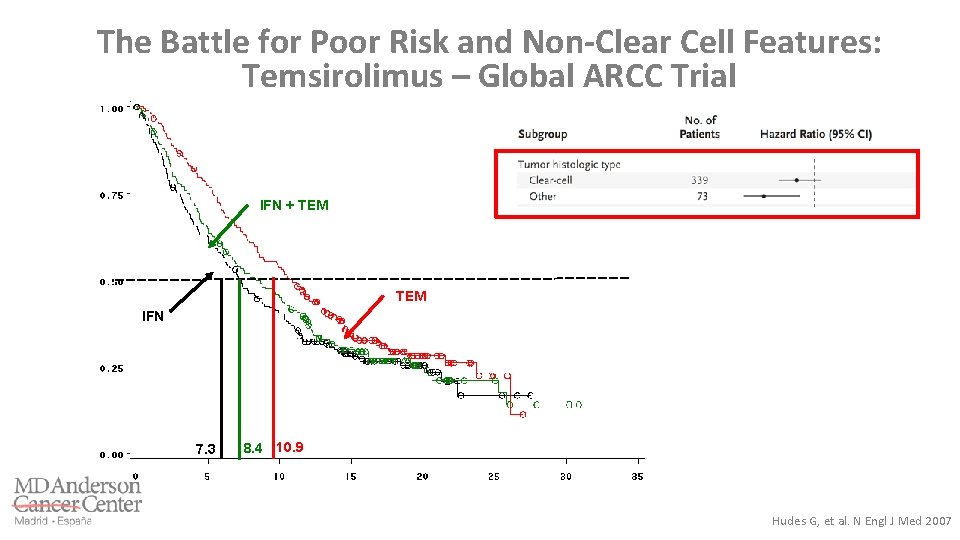
The Battle for Poor Risk and Non-Clear Cell Features: Temsirolimus – Global ARCC Trial IFN + TEM IFN 7. 3 8. 4 10. 9 Hudes G, et al. N Engl J Med 2007

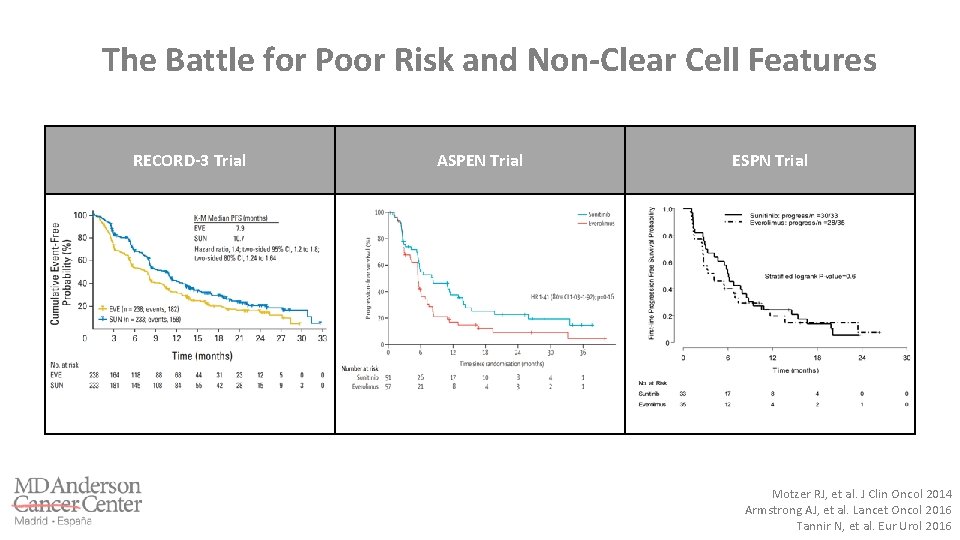
The Battle for Poor Risk and Non-Clear Cell Features RECORD-3 Trial ASPEN Trial ESPN Trial Motzer RJ, et al. J Clin Oncol 2014 Armstrong AJ, et al. Lancet Oncol 2016 Tannir N, et al. Eur Urol 2016

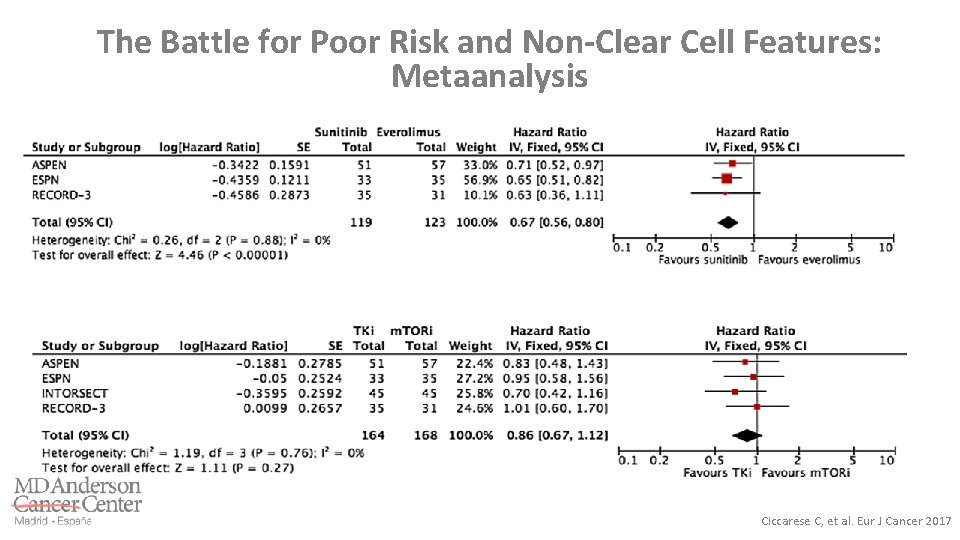
The Battle for Poor Risk and Non-Clear Cell Features: Metaanalysis Ciccarese C, et al. Eur J Cancer 2017

RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib Sunitinib or Temsirolimus INF vs IL-2 or combo 1992 … 2 L … 2005 … 2007 Everolimus or Sorafenib 2009 2008 2011 2014 2011 2013 2016 2017 2018 2019

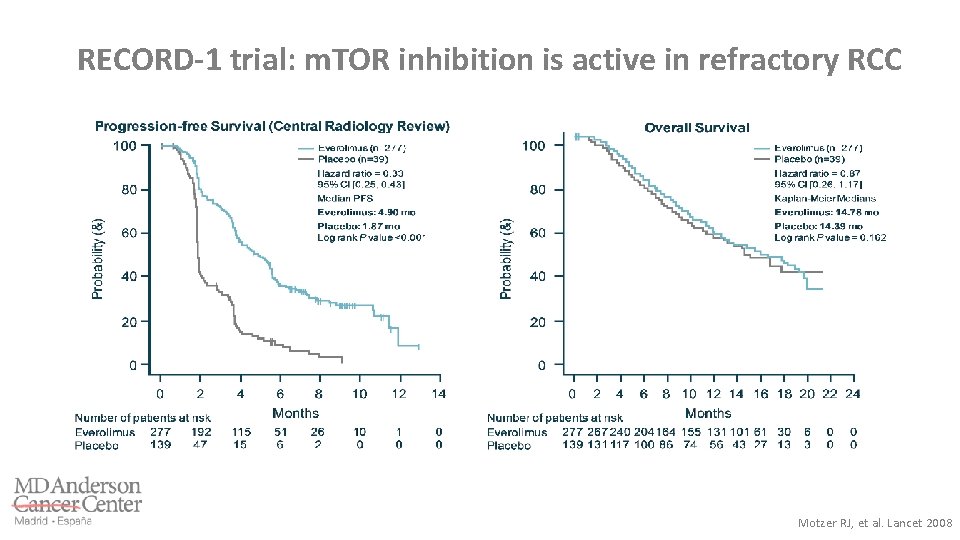
RECORD-1 trial: m. TOR inhibition is active in refractory RCC Motzer RJ, et al. Lancet 2008

RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib Sunitinib or Temsirolimus INF vs IL-2 or combo 1992 … 2 L Sunitinib or Beva + INF … 2005 … 2007 Everolimus or Sorafenib 2009 2008 2011 2014 2011 2013 2016 2017 2018 2019

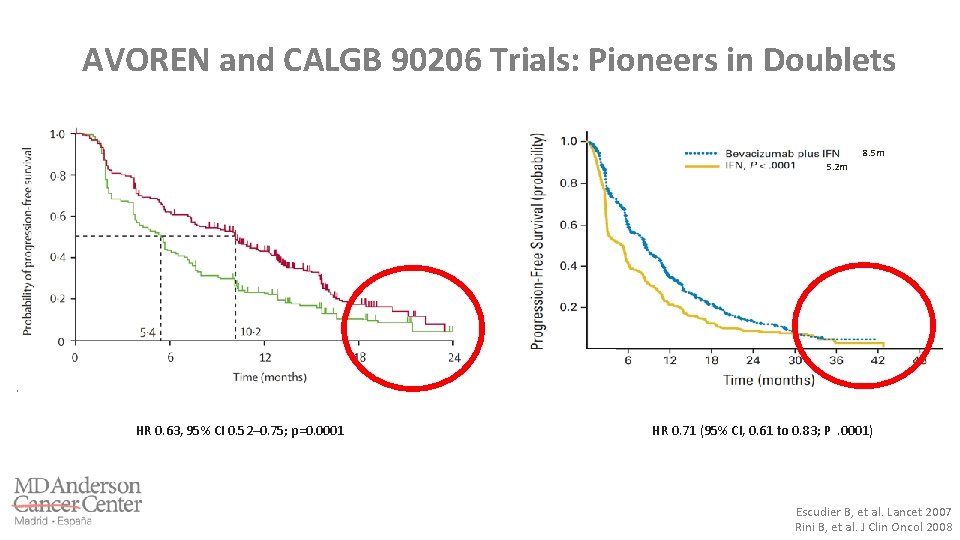
AVOREN and CALGB 90206 Trials: Pioneers in Doublets 8. 5 m 5. 2 m HR 0. 63, 95% CI 0. 52– 0. 75; p=0. 0001 HR 0. 71 (95% CI, 0. 61 to 0. 83; P. 0001) Escudier B, et al. Lancet 2007 Rini B, et al. J Clin Oncol 2008

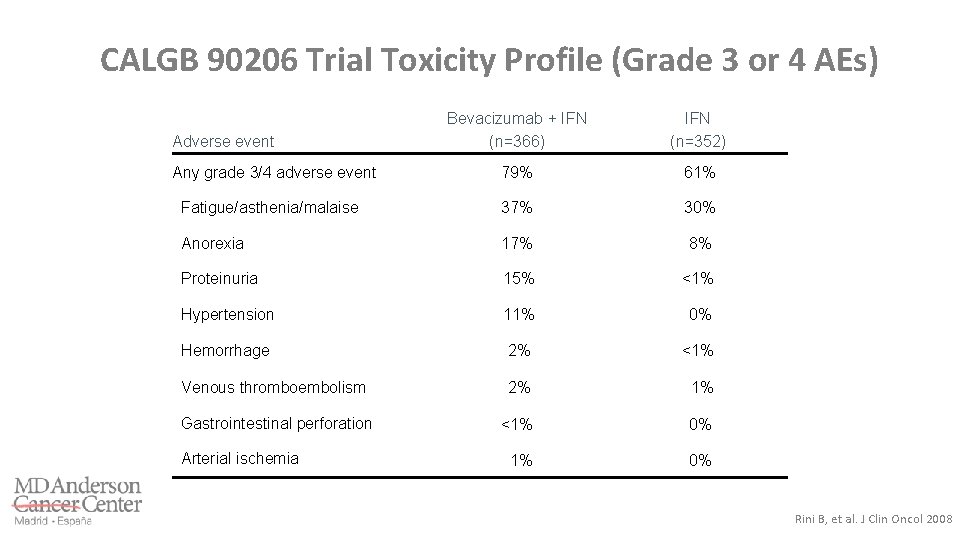
CALGB 90206 Trial Toxicity Profile (Grade 3 or 4 AEs) Bevacizumab + IFN (n=366) IFN (n=352) Any grade 3/4 adverse event 79% 61% Fatigue/asthenia/malaise 37% 30% Anorexia 17% 8% Proteinuria 15% <1% Hypertension 11% 0% Hemorrhage 2% <1% Venous thromboembolism 2% 1% Gastrointestinal perforation <1% 0% Adverse event Arterial ischemia Rini B, et al. J Clin Oncol 2008

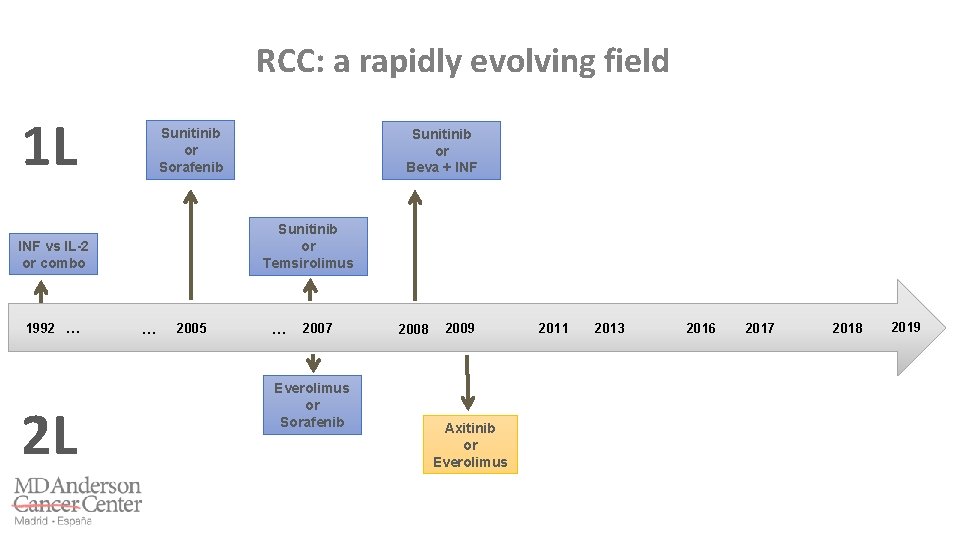
RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib Sunitinib or Temsirolimus INF vs IL-2 or combo 1992 … 2 L Sunitinib or Beva + INF … 2005 … 2007 Everolimus or Sorafenib 2009 2008 2011 Axitinib or Everolimus 2014 2011 2013 2016 2017 2018 2019

AXIS trial: Axitinib can overcome the resistance to sunitinib Rini BI, et al. Lancet 2011

RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib 2 L Sunitinib or Pazopanib Sunitinib or Temsirolimus INF vs IL-2 or combo 1992 … Sunitinib or Beva + INF … 2005 … 2007 Everolimus or Sorafenib 2009 2008 2011 Axitinib or Everolimus 2014 2011 2013 2016 2017 2018 2019

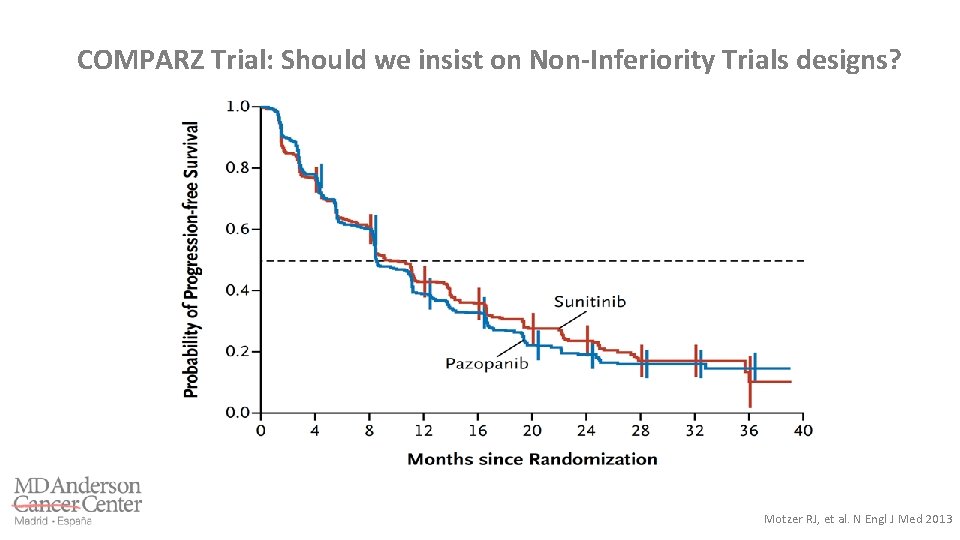
COMPARZ Trial: Should we insist on Non-Inferiority Trials designs? Motzer RJ, et al. N Engl J Med 2013

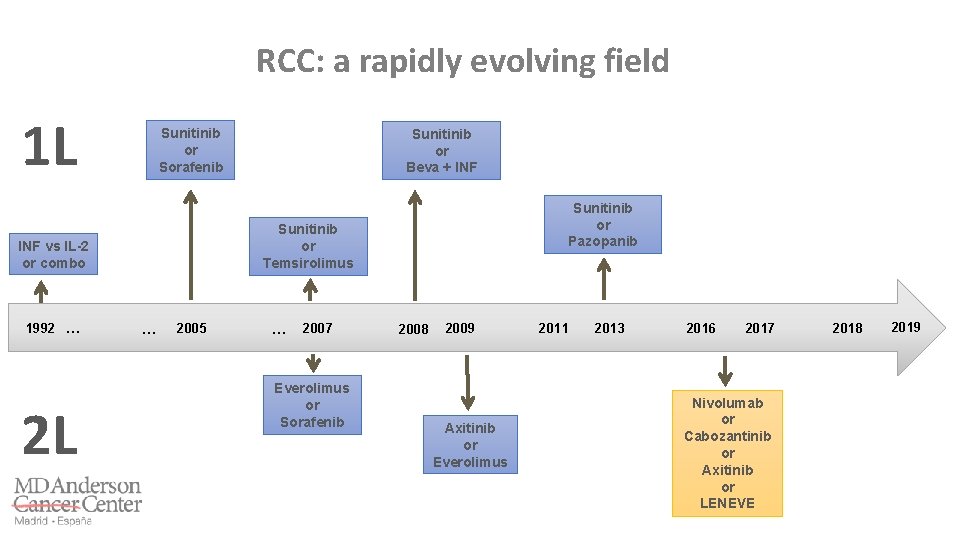
RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib 2 L Sunitinib or Pazopanib Sunitinib or Temsirolimus INF vs IL-2 or combo 1992 … Sunitinib or Beva + INF … 2005 … 2007 Everolimus or Sorafenib 2009 2008 2011 Axitinib or Everolimus 2014 2011 2013 2016 2017 Nivolumab or Cabozantinib or Axitinib or LENEVE 2018 2019

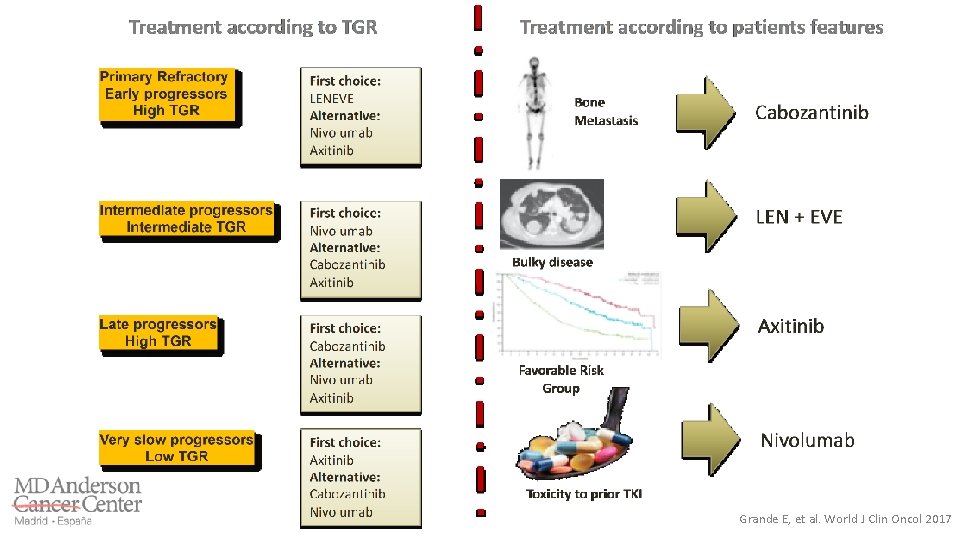
Grande E, et al. World J Clin Oncol 2017

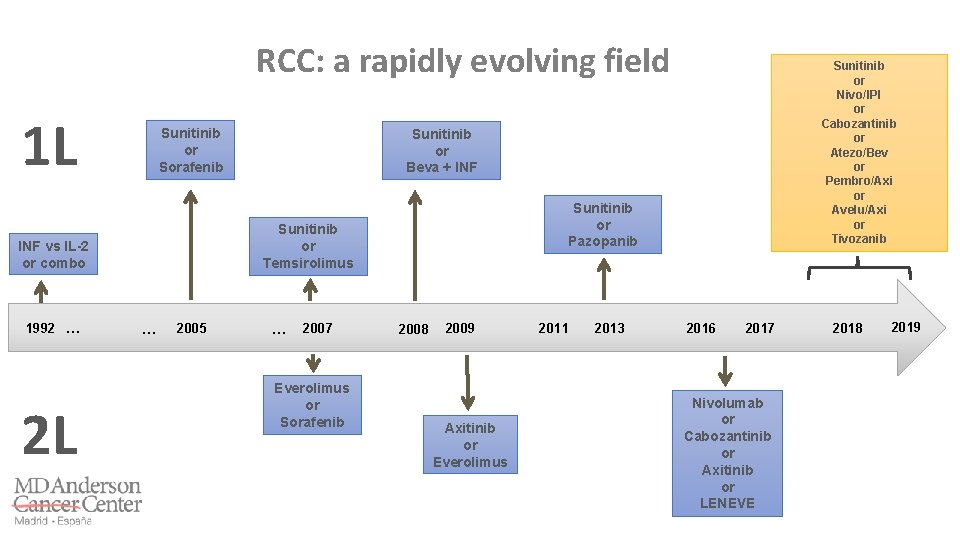
RCC: a rapidly evolving field 1 L Sunitinib or Sorafenib 1992 … 2 L Sunitinib or Beva + INF Sunitinib or Pazopanib Sunitinib or Temsirolimus INF vs IL-2 or combo … 2005 Sunitinib or Nivo/IPI or Cabozantinib or Atezo/Bev or Pembro/Axi or Avelu/Axi or Tivozanib … 2007 Everolimus or Sorafenib 2009 2008 2011 Axitinib or Everolimus 2014 2011 2013 2016 2017 Nivolumab or Cabozantinib or Axitinib or LENEVE 2018 2019

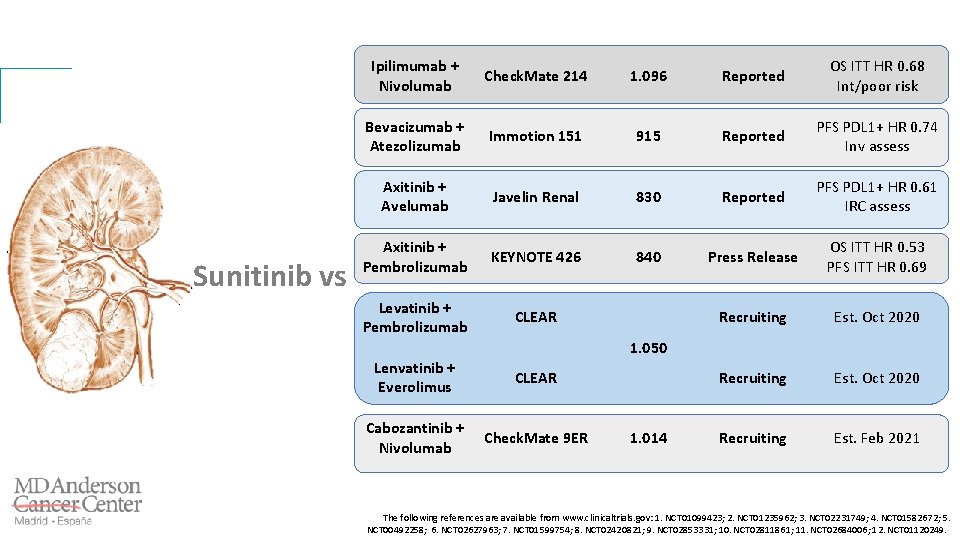
Sunitinib vs Ipilimumab + Nivolumab Check. Mate 214 1. 096 Reported OS ITT HR 0. 68 Int/poor risk Bevacizumab + Atezolizumab Immotion 151 915 Reported PFS PDL 1+ HR 0. 74 Inv assess Axitinib + Avelumab Javelin Renal 830 Reported PFS PDL 1+ HR 0. 61 IRC assess Axitinib + Pembrolizumab KEYNOTE 426 840 Press Release OS ITT HR 0. 53 PFS ITT HR 0. 69 Levatinib + Pembrolizumab CLEAR Recruiting Est. Oct 2020 Recruiting Est. Feb 2021 1. 050 Lenvatinib + Everolimus CLEAR Cabozantinib + Nivolumab Check. Mate 9 ER 1. 014 The following references are available from www. clinicaltrials. gov: 1. NCT 01099423; 2. NCT 01235962; 3. NCT 02231749; 4. NCT 01582672; 5. NCT 00492258; 6. NCT 02627963; 7. NCT 01599754; 8. NCT 02420821; 9. NCT 02853331; 10. NCT 02811861; 11. NCT 02684006; 12. NCT 01120249.

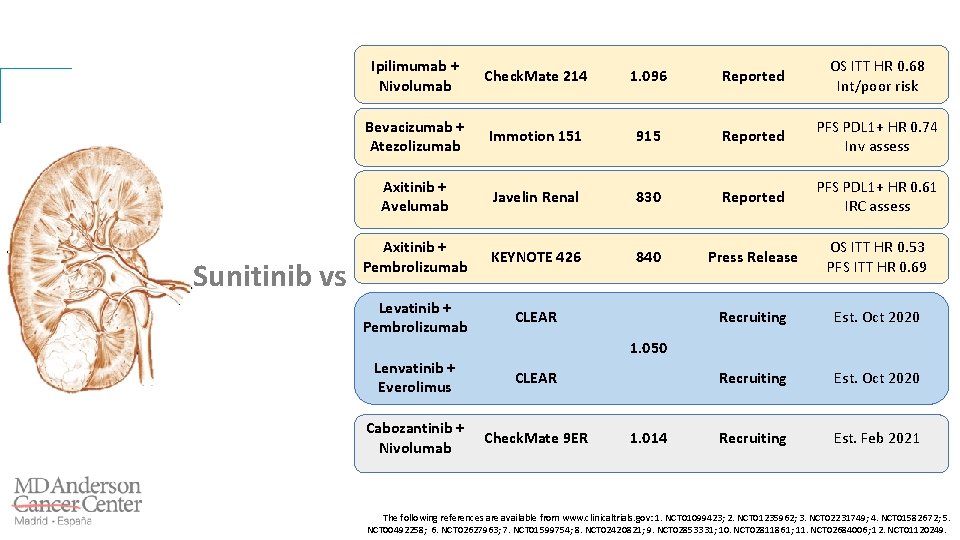
Sunitinib vs Ipilimumab + Nivolumab Check. Mate 214 1. 096 Reported OS ITT HR 0. 68 Int/poor risk Bevacizumab + Atezolizumab Immotion 151 915 Reported PFS PDL 1+ HR 0. 74 Inv assess Axitinib + Avelumab Javelin Renal 830 Reported PFS PDL 1+ HR 0. 61 IRC assess Axitinib + Pembrolizumab KEYNOTE 426 840 Press Release OS ITT HR 0. 53 PFS ITT HR 0. 69 Levatinib + Pembrolizumab CLEAR Recruiting Est. Oct 2020 Recruiting Est. Feb 2021 1. 050 Lenvatinib + Everolimus CLEAR Cabozantinib + Nivolumab Check. Mate 9 ER 1. 014 The following references are available from www. clinicaltrials. gov: 1. NCT 01099423; 2. NCT 01235962; 3. NCT 02231749; 4. NCT 01582672; 5. NCT 00492258; 6. NCT 02627963; 7. NCT 01599754; 8. NCT 02420821; 9. NCT 02853331; 10. NCT 02811861; 11. NCT 02684006; 12. NCT 01120249.

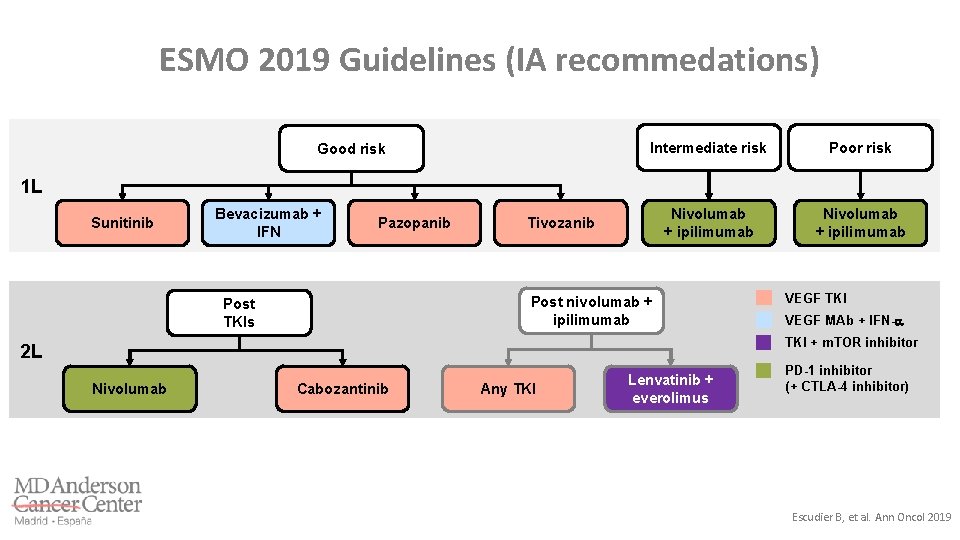
ESMO 2019 Guidelines (IA recommedations) Good risk Intermediate risk Poor risk Nivolumab + ipilimumab 1 L Sunitinib Bevacizumab + IFN Pazopanib Tivozanib Post nivolumab + ipilimumab Post TKIs VEGF TKI VEGF MAb + IFN- TKI + m. TOR inhibitor 2 L Nivolumab Cabozantinib Any TKI Lenvatinib + everolimus PD-1 inhibitor (+ CTLA-4 inhibitor) Escudier B, et al. Ann Oncol 2019

egrande@mdanderson. es @drenriquegrande
 Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Teoria do nefron intacto
Teoria do nefron intacto Cncer
Cncer Cncer
Cncer Vasa recta vs peritubular capillaries
Vasa recta vs peritubular capillaries Grande muy grande
Grande muy grande Enrique del barco
Enrique del barco Al final del tubo
Al final del tubo Ligamento periodontal
Ligamento periodontal Enrique feoli md
Enrique feoli md Enrique helmut meyer mercado
Enrique helmut meyer mercado Enrique garcia godoy
Enrique garcia godoy Enrique velazco
Enrique velazco Enrique cortés reyes
Enrique cortés reyes Casa hogar beato padre enrique rebuschini
Casa hogar beato padre enrique rebuschini Enrique santos discépolo cambalache letras
Enrique santos discépolo cambalache letras Dr enrique saguil
Dr enrique saguil Enrique zapata reyes
Enrique zapata reyes Luca enriques
Luca enriques Jose enrique villa rivera
Jose enrique villa rivera Mario enrique guzman vega
Mario enrique guzman vega Hoy rezo por ti
Hoy rezo por ti Enrique zapata reyes
Enrique zapata reyes Liceo scientifico enriques
Liceo scientifico enriques Enrique orbegozo
Enrique orbegozo Juan enrique garrido figueroa
Juan enrique garrido figueroa Enrique higareda almaraz
Enrique higareda almaraz Enrique zapata
Enrique zapata Enrique rosero puerto
Enrique rosero puerto Jose vicente y mariano angulo
Jose vicente y mariano angulo Luis enrique arango muñoz
Luis enrique arango muñoz Cuales son los recursos humanos de un club deportivo
Cuales son los recursos humanos de un club deportivo Enrique lastra
Enrique lastra Enrique penalosa quotes
Enrique penalosa quotes Dr enrique wulff
Dr enrique wulff Dialéctica hegeliana
Dialéctica hegeliana Enrique guzmn
Enrique guzmn Etnometodología
Etnometodología Order pasado participio
Order pasado participio Haleine oenolique
Haleine oenolique Enrique godoy duran
Enrique godoy duran Enrique cabrero mendoza
Enrique cabrero mendoza Auditoria administrativa benjamin franklin
Auditoria administrativa benjamin franklin Calidad según crosby
Calidad según crosby Enrique guevara ortiz
Enrique guevara ortiz