SIMPOSIO EDUCACIONAL 3 AVANCES EN CNCER DE RIN



















- Slides: 59

SIMPOSIO EDUCACIONAL 3: AVANCES EN CÁNCER DE RIÑÓN Y GIST. ANTIANGIOGÉNICOS EN EL CÁNCER DE RIÑÓN AVANZADO Joaquim Bellmunt Madrid, 4 de octubre de 2007, (18: 35 a 19: 35 horas)

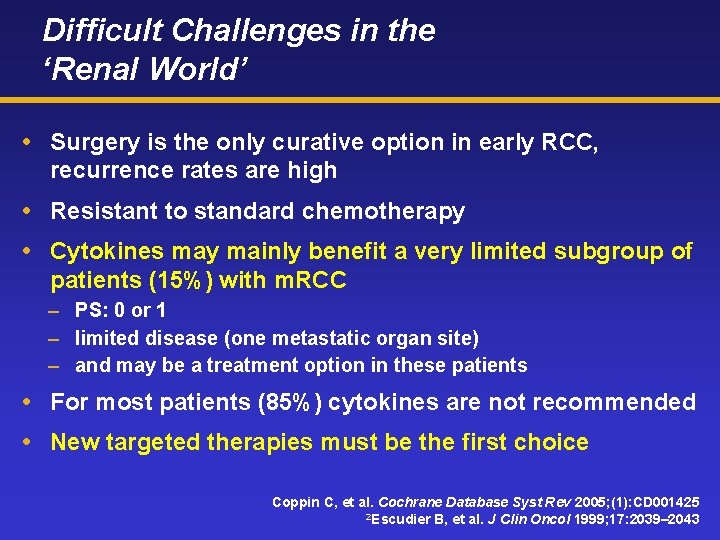
Difficult Challenges in the ‘Renal World’ Surgery is the only curative option in early RCC, recurrence rates are high Resistant to standard chemotherapy Cytokines may mainly benefit a very limited subgroup of patients (15%) with m. RCC – PS: 0 or 1 – limited disease (one metastatic organ site) – and may be a treatment option in these patients For most patients (85%) cytokines are not recommended New targeted therapies must be the first choice Coppin C, et al. Cochrane Database Syst Rev 2005; (1): CD 001425 2 Escudier B, et al. J Clin Oncol 1999; 17: 2039– 2043

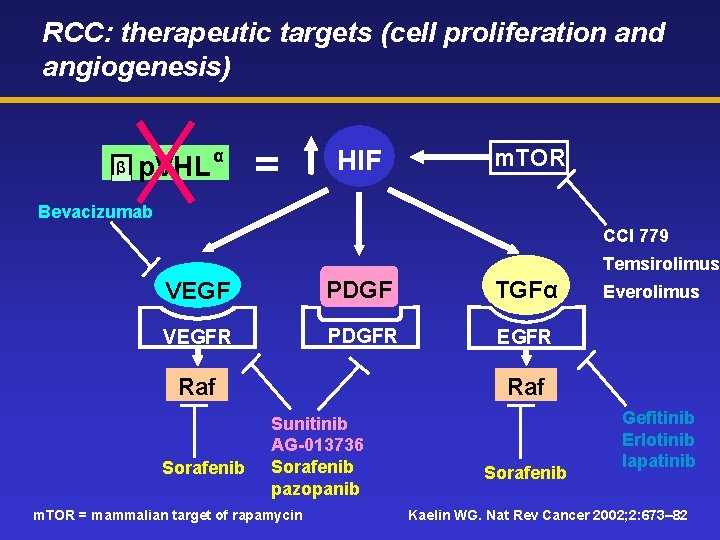
RCC: therapeutic targets (cell proliferation and angiogenesis) β p. VHL α = HIF m. TOR Bevacizumab CCI 779 Temsirolimus VEGF PDGF TGFα VEGFR PDGFR EGFR Raf Sorafenib Everolimus Raf Sunitinib AG-013736 Sorafenib pazopanib m. TOR = mammalian target of rapamycin Sorafenib Gefitinib Erlotinib lapatinib Kaelin WG. Nat Rev Cancer 2002; 2: 673– 82

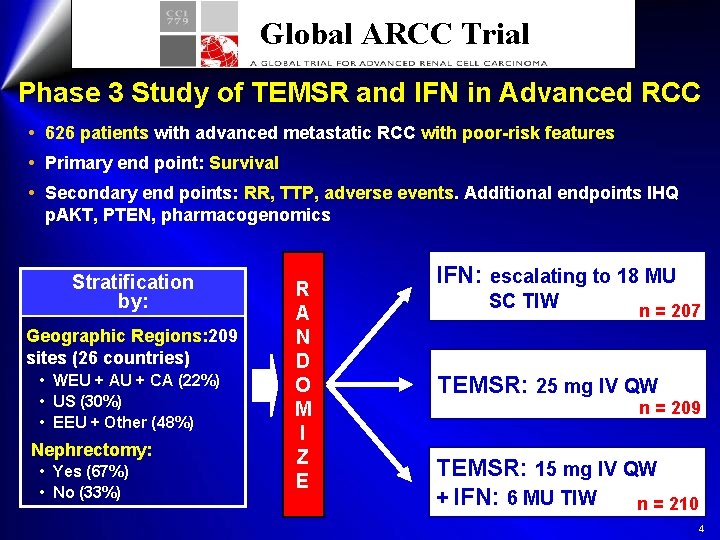
Global ARCC Trial Phase 3 Study of TEMSR and IFN in Advanced RCC 626 patients with advanced metastatic RCC with poor-risk features Primary end point: Survival Secondary end points: RR, TTP, adverse events. Additional endpoints IHQ p. AKT, PTEN, pharmacogenomics Stratification by: Geographic Regions: 209 sites (26 countries) • WEU + AU + CA (22%) • US (30%) • EEU + Other (48%) Nephrectomy: • Yes (67%) • No (33%) R A N D O M I Z E IFN: escalating to 18 MU SC TIW n = 207 TEMSR: 25 mg IV QW n = 209 TEMSR: 15 mg IV QW + IFN: 6 MU TIW n = 210 4

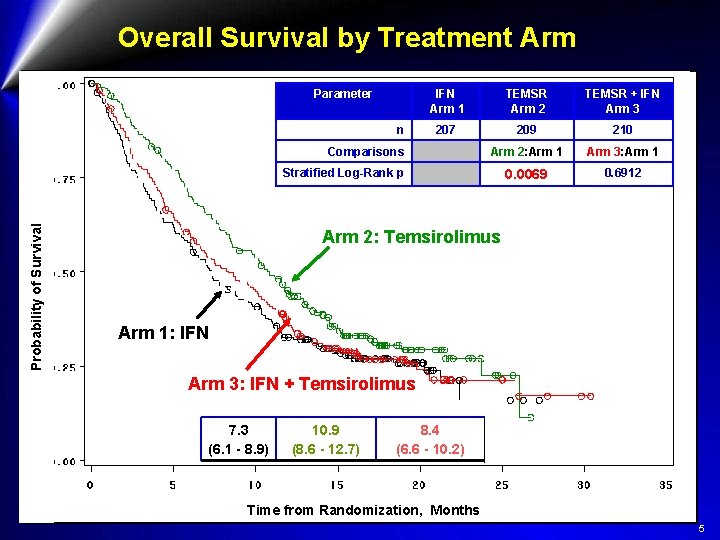
Overall Survival by Treatment Arm Parameter n IFN Arm 1 TEMSR Arm 2 TEMSR + IFN Arm 3 207 209 210 Arm 2: Arm 1 Arm 3: Arm 1 0. 0069 0. 6912 Comparisons Probability of Survival Stratified Log-Rank p Arm 2: Temsirolimus Arm 1: IFN Arm 3: IFN + Temsirolimus 7. 3 (6. 1 - 8. 9) 10. 9 (8. 6 - 12. 7) 8. 4 (6. 6 - 10. 2) Time from Randomization, Months 5

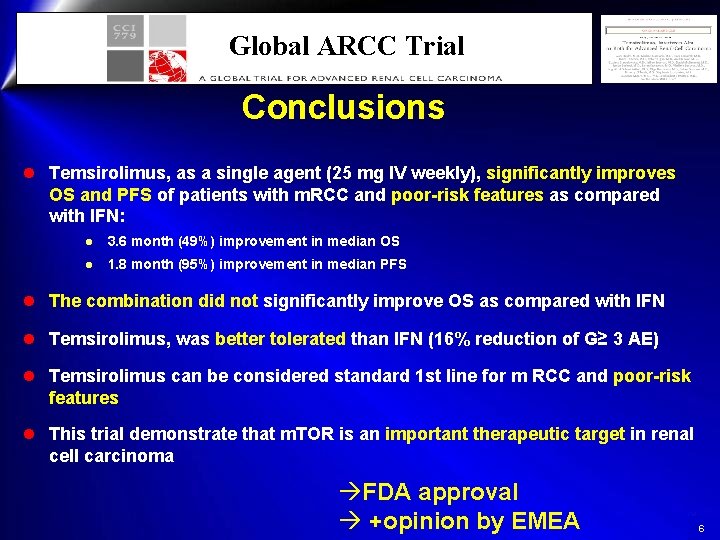
Global ARCC Trial Conclusions l Temsirolimus, as a single agent (25 mg IV weekly), significantly improves OS and PFS of patients with m. RCC and poor-risk features as compared with IFN: l 3. 6 month (49%) improvement in median OS l 1. 8 month (95%) improvement in median PFS l The combination did not significantly improve OS as compared with IFN l Temsirolimus, was better tolerated than IFN (16% reduction of G≥ 3 AE) l Temsirolimus can be considered standard 1 st line for m RCC and poor-risk features l This trial demonstrate that m. TOR is an important therapeutic target in renal cell carcinoma àFDA approval à +opinion by EMEA 6

Antiangiogenic therapy in RCC Drugs and clinical trials based on VHL biology

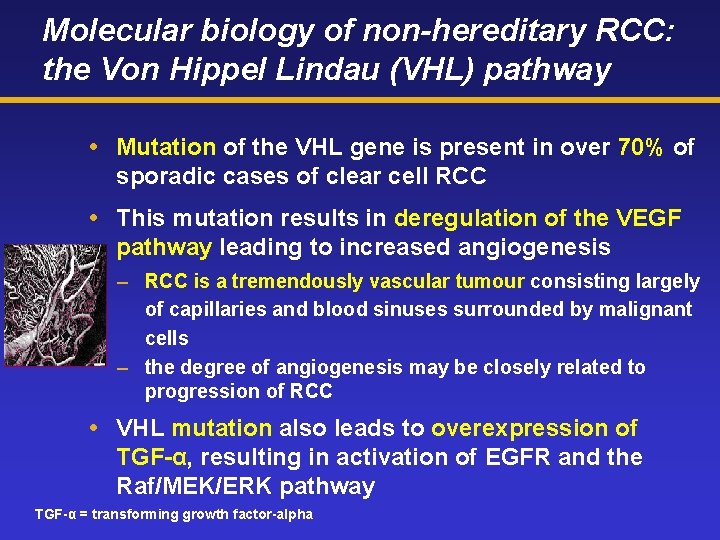
Molecular biology of non-hereditary RCC: the Von Hippel Lindau (VHL) pathway Mutation of the VHL gene is present in over 70% of sporadic cases of clear cell RCC This mutation results in deregulation of the VEGF pathway leading to increased angiogenesis – RCC is a tremendously vascular tumour consisting largely of capillaries and blood sinuses surrounded by malignant cells – the degree of angiogenesis may be closely related to progression of RCC VHL mutation also leads to overexpression of TGF-α, resulting in activation of EGFR and the Raf/MEK/ERK pathway TGF-α = transforming growth factor-alpha

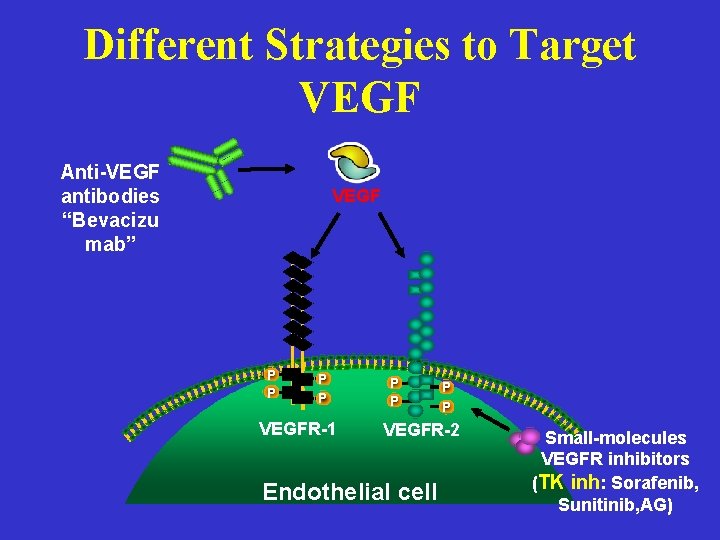
Different Strategies to Target VEGF Anti-VEGF antibodies “Bevacizu mab” VEGF P P VEGFR-1 P P VEGFR-2 Endothelial cell Small-molecules VEGFR inhibitors (TK inh: Sorafenib, Sunitinib, AG)

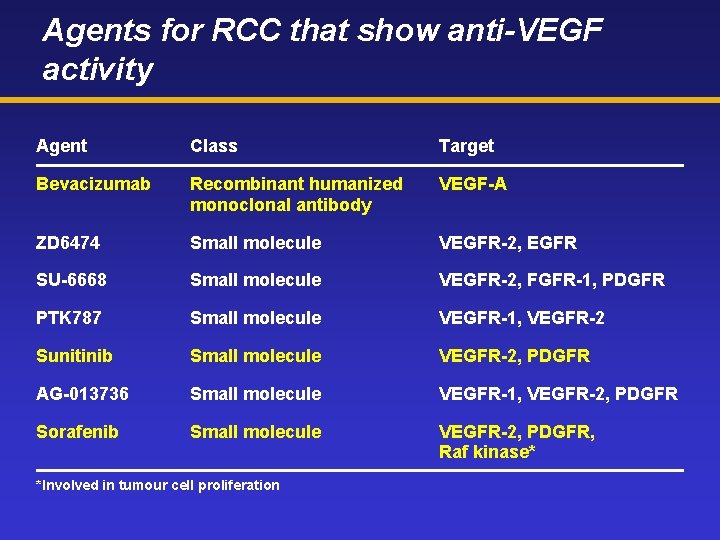
Agents for RCC that show anti-VEGF activity Agent Class Target Bevacizumab Recombinant humanized monoclonal antibody VEGF-A ZD 6474 Small molecule VEGFR-2, EGFR SU-6668 Small molecule VEGFR-2, FGFR-1, PDGFR PTK 787 Small molecule VEGFR-1, VEGFR-2 Sunitinib Small molecule VEGFR-2, PDGFR AG-013736 Small molecule VEGFR-1, VEGFR-2, PDGFR Sorafenib Small molecule VEGFR-2, PDGFR, Raf kinase* *Involved in tumour cell proliferation

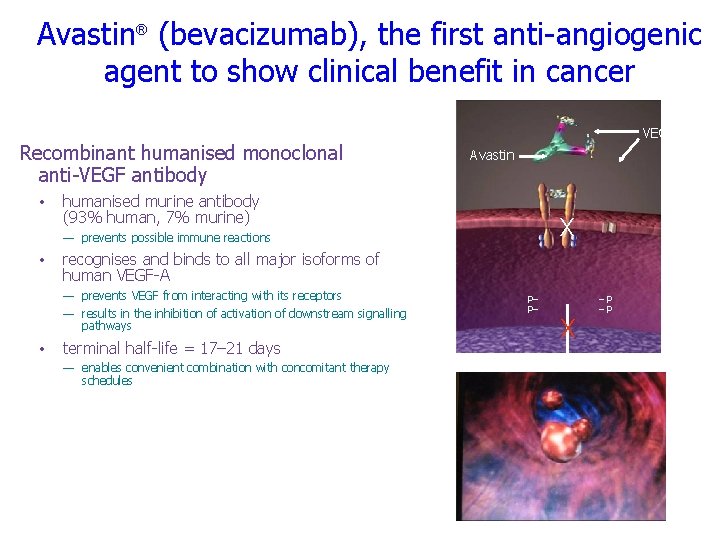
Avastin® (bevacizumab), the first anti-angiogenic agent to show clinical benefit in cancer Recombinant humanised monoclonal anti-VEGF antibody • VEGF Avastin humanised murine antibody (93% human, 7% murine) X — prevents possible immune reactions • recognises and binds to all major isoforms of human VEGF-A — prevents VEGF from interacting with its receptors — results in the inhibition of activation of downstream signalling pathways • terminal half-life = 17– 21 days — enables convenient combination with concomitant therapy schedules P– P– X –P –P Growth Proliferation Migration Survival

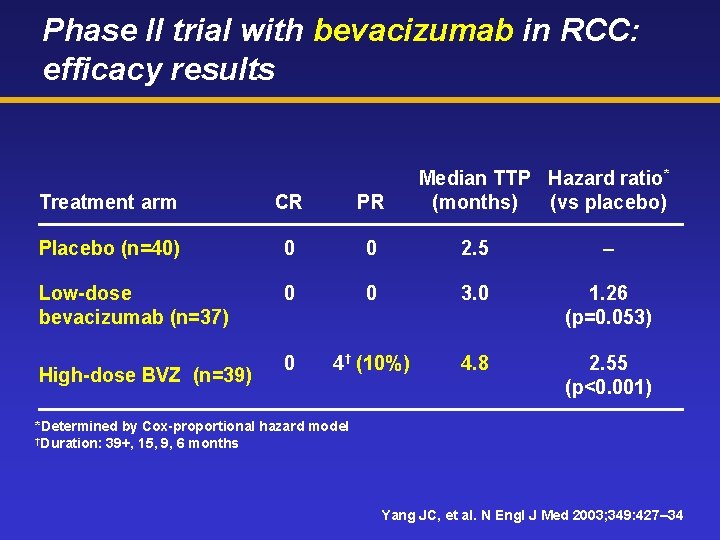
Phase II trial with bevacizumab in RCC: efficacy results Median TTP Hazard ratio* (months) (vs placebo) Treatment arm CR PR Placebo (n=40) 0 0 2. 5 – Low-dose bevacizumab (n=37) 0 0 3. 0 1. 26 (p=0. 053) 0 4† (10%) 4. 8 2. 55 (p<0. 001) High-dose BVZ (n=39) *Determined by Cox-proportional hazard model †Duration: 39+, 15, 9, 6 months Yang JC, et al. N Engl J Med 2003; 349: 427– 34

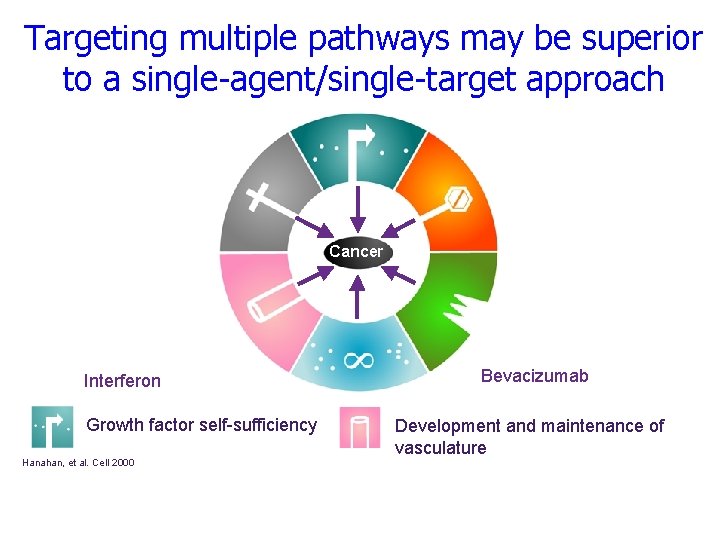
Targeting multiple pathways may be superior to a single-agent/single-target approach Cancer Interferon Growth factor self-sufficiency Hanahan, et al. Cell 2000 Bevacizumab Development and maintenance of vasculature

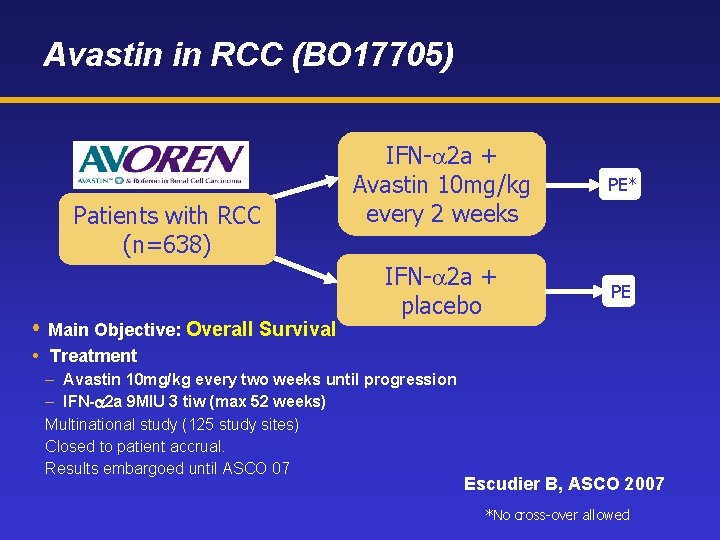
Avastin in RCC (BO 17705) Patients with RCC (n=638) Main Objective: Overall Survival IFN-a 2 a + Avastin 10 mg/kg every 2 weeks PE* IFN-a 2 a + placebo PE Treatment – Avastin 10 mg/kg every two weeks until progression – IFN- 2 a 9 MIU 3 tiw (max 52 weeks) Multinational study (125 study sites) Closed to patient accrual. Results embargoed until ASCO 07 Escudier B, ASCO 2007 *No cross-over allowed

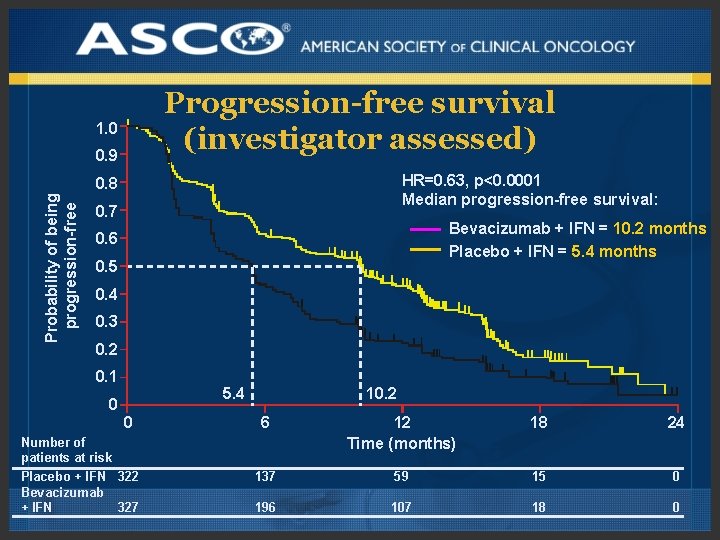
Progression-free survival (investigator assessed) 1. 0 0. 9 HR=0. 63, p<0. 0001 Median progression-free survival: Probability of being progression-free 0. 8 0. 7 Bevacizumab + IFN = 10. 2 months Placebo + IFN = 5. 4 months 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 5. 4 0 0 Number of patients at risk Placebo + IFN 322 Bevacizumab + IFN 327 10. 2 6 12 Time (months) 18 24 137 59 15 0 196 107 18 0

Tumor response (investigator assessed) Response 13 2 11 IFN + Bevacizumab (n=306) } Overall response rate (%)* Complete response Partial response IFN + placebo (n=289) 31 1 30 p<0. 0001 Median duration of response (months) Median duration of stable disease (months) *Patients with measurable disease only 11 13 7 10

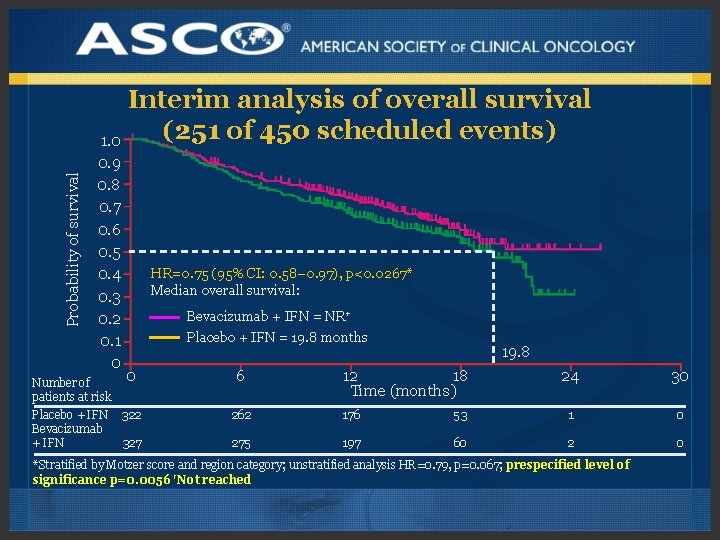
Probability of survival Interim analysis of overall survival (251 of 450 scheduled events) 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 0 Number of patients at risk Placebo + IFN 322 Bevacizumab + IFN 327 HR=0. 75 (95% CI: 0. 58– 0. 97), p<0. 0267* Median overall survival: Bevacizumab + IFN = NR† Placebo + IFN = 19. 8 months 6 19. 8 12 18 Time (months) 24 30 262 176 53 1 0 275 197 60 2 0 *Stratified by Motzer score and region category; unstratified analysis HR=0. 79, p=0. 067; prespecified level of significance p=0. 0056 †Not reached

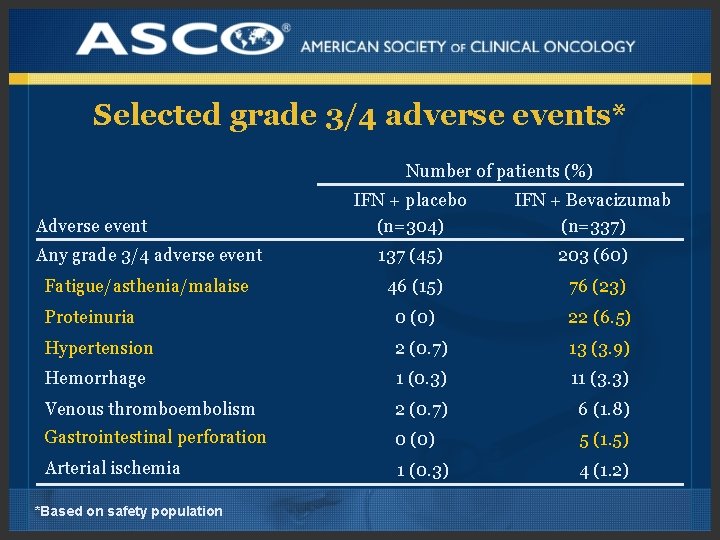
Selected grade 3/4 adverse events* Number of patients (%) IFN + placebo (n=304) IFN + Bevacizumab (n=337) Any grade 3/4 adverse event 137 (45) 203 (60) Fatigue/asthenia/malaise 46 (15) 76 (23) Proteinuria 0 (0) 22 (6. 5) Hypertension 2 (0. 7) 13 (3. 9) Hemorrhage 1 (0. 3) 11 (3. 3) Venous thromboembolism 2 (0. 7) 6 (1. 8) Gastrointestinal perforation 0 (0) 5 (1. 5) Arterial ischemia 1 (0. 3) 4 (1. 2) Adverse event *Based on safety population

Bevacizumab ± IFN Phase III: Conclusions –ASCO 2007 • In this placebo-controlled study, the addition of bevacizumab to IFN results in clinically important and statistically significant improvement in progression-free survival and tumor response • Trend in favor of improved survival exists • The treatments were well tolerated and no new toxicities emerged outside of those known with IFN and bevacizumab Adapted from Escudier B et al. Presented at ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

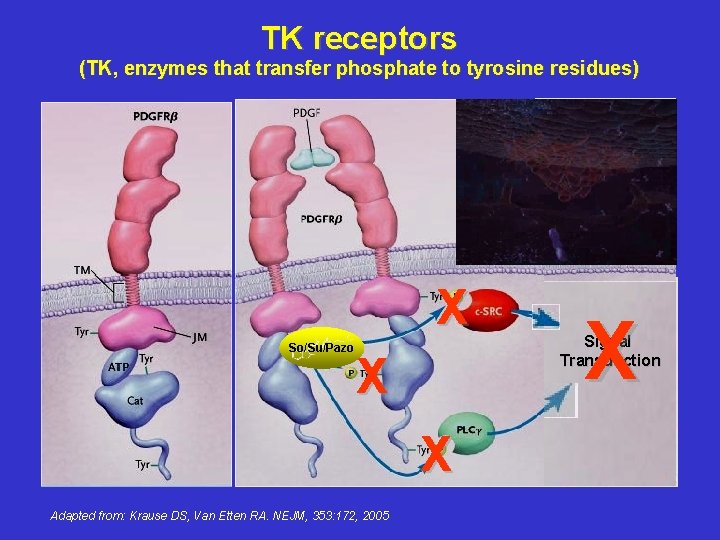
TK receptors (TK, enzymes that transfer phosphate to tyrosine residues) X So/Su/Pazo X X Adapted from: Krause DS, Van Etten RA. NEJM, 353: 172, 2005 X Signal Transduction

Sunitinib in RCC Sunitinib is an orally bioavailable small molecule angiogenesis inhibitor Sunitinib inhibits the tyrosine kinase activity of VEGFR, PDGFR and c-KIT Two single-arm, multicentre, phase II trials in advanced RCC have been completed – sunitinib was administered in cycles of 50 mg orally, daily for 4 weeks, followed by 2 weeks’ rest

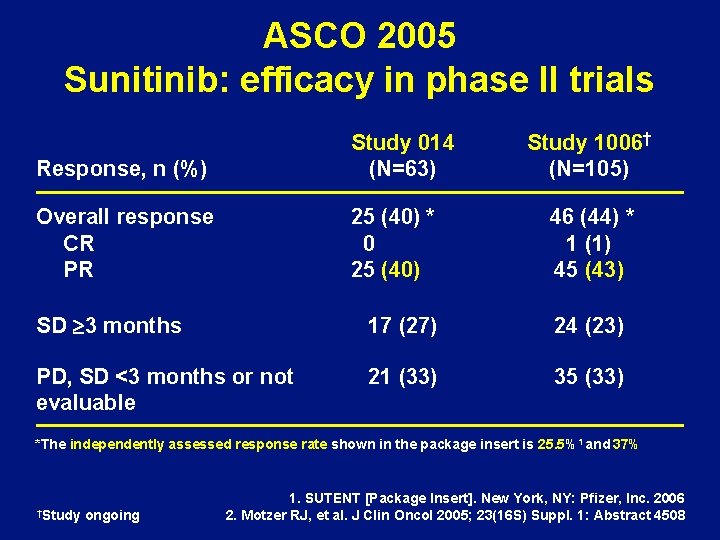
ASCO 2005 Sunitinib: efficacy in phase II trials Response, n (%) Study 014 (N=63) Overall response CR PR 25 (40) * 0 25 (40) Study 1006† (N=105) 46 (44) * 1 (1) 45 (43) SD ³ 3 months 17 (27) 24 (23) PD, SD <3 months or not evaluable 21 (33) 35 (33) *The independently assessed response rate shown in the package insert is 25. 5%1 and 37% †Study ongoing 1. SUTENT [Package Insert]. New York, NY: Pfizer, Inc. 2006 2. Motzer RJ, et al. J Clin Oncol 2005; 23(16 S) Suppl. 1: Abstract 4508

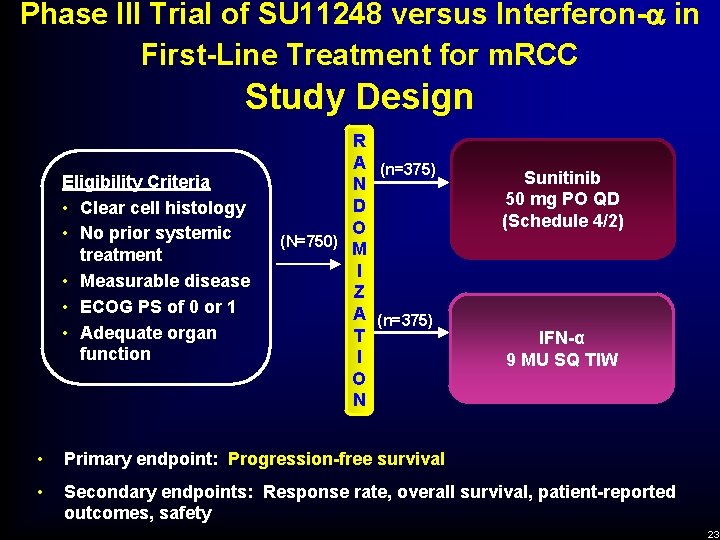
Phase III Trial of SU 11248 versus Interferon- in First-Line Treatment for m. RCC Study Design Eligibility Criteria • Clear cell histology • No prior systemic treatment • Measurable disease • ECOG PS of 0 or 1 • Adequate organ function R A (n=375) N D O (N=750) M I Z A (n=375) T I O N Sunitinib 50 mg PO QD (Schedule 4/2) IFN-α 9 MU SQ TIW • Primary endpoint: Progression-free survival • Secondary endpoints: Response rate, overall survival, patient-reported outcomes, safety 23

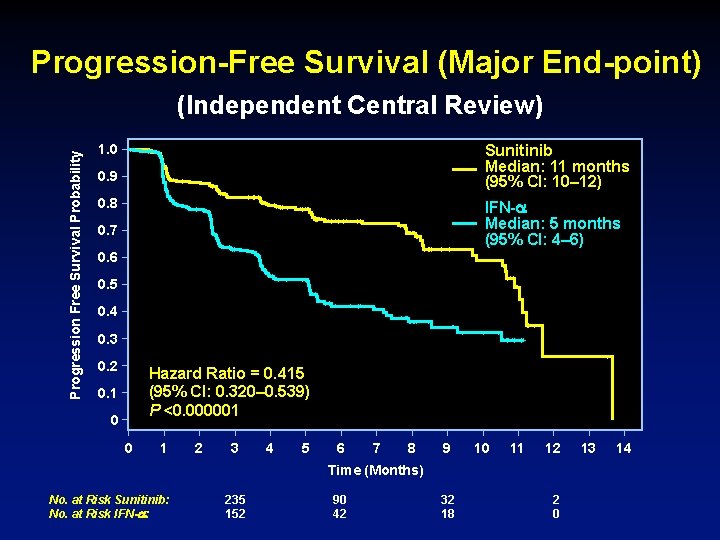
Progression-Free Survival (Major End-point) Progression Free Survival Probability (Independent Central Review) Sunitinib Median: 11 months (95% CI: 10– 12) 1. 0 0. 9 0. 8 IFN- Median: 5 months (95% CI: 4– 6) 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Hazard Ratio = 0. 415 (95% CI: 0. 320– 0. 539) P <0. 000001 0. 1 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Time (Months) No. at Risk Sunitinib: No. at Risk IFN- : 235 152 90 42 32 18 2 0 13 14

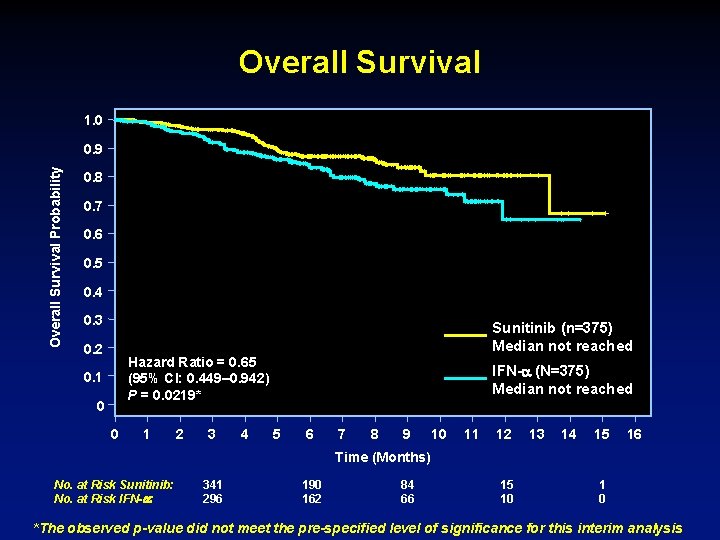
Overall Survival 1. 0 Overall Survival Probability 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Sunitinib (n=375) Median not reached Hazard Ratio = 0. 65 (95% CI: 0. 449– 0. 942) P = 0. 0219* 0. 1 0 0 1 2 3 4 IFN- (N=375) Median not reached 5 6 7 8 9 10 11 12 13 14 15 16 Time (Months) No. at Risk Sunitinib: No. at Risk IFN- : 341 296 190 162 84 66 15 10 1 0 *The observed p-value did not meet the pre-specified level of significance for this interim analysis

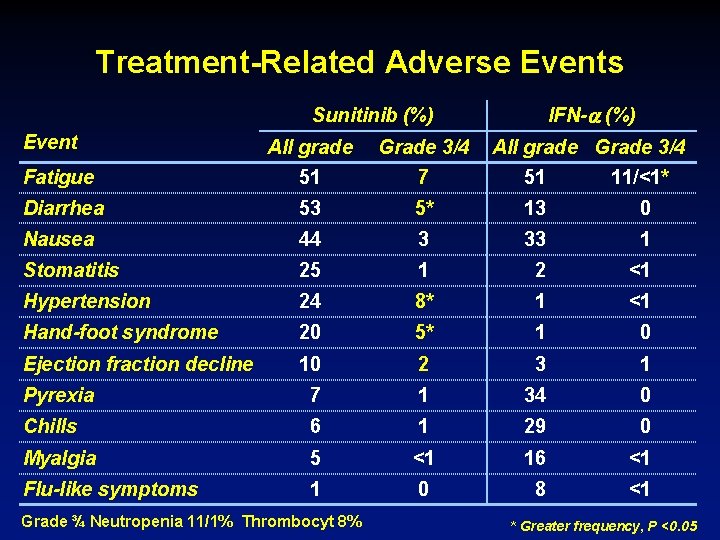
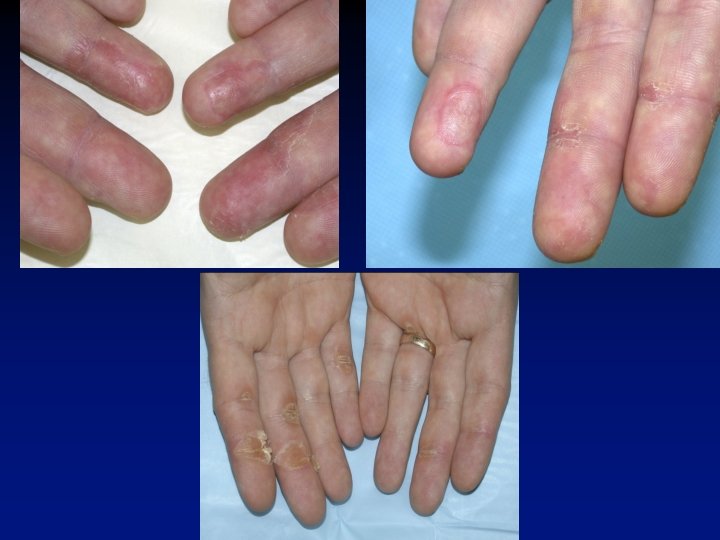
Treatment-Related Adverse Events IFN- (%) Sunitinib (%) Event All grade Grade 3/4 Fatigue 51 7 51 11/<1* Diarrhea 53 5* 13 0 Nausea 44 3 33 1 Stomatitis 25 1 2 <1 Hypertension 24 8* 1 <1 Hand-foot syndrome 20 5* 1 0 Ejection fraction decline 10 2 3 1 Pyrexia 7 1 34 0 Chills 6 1 29 0 Myalgia 5 <1 16 <1 Flu-like symptoms 1 0 8 <1 Grade ¾ Neutropenia 11/1% Thrombocyt 8% All grade Grade 3/4 * Greater frequency, P <0. 05


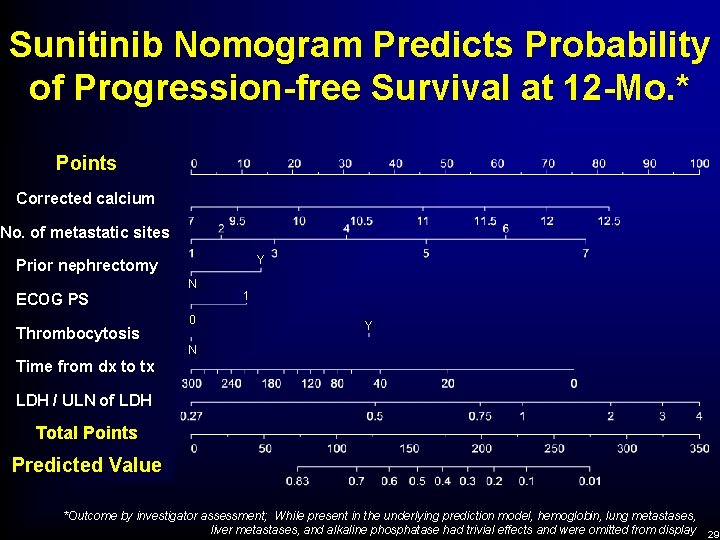
Multivariate Analysis of PFS to Sunitinib Hazard Ratio 95% CI for Hazard Ratio P-value ECOG (0 vs. 1) 0. 700 0. 543 – 0. 901 0. 006 Time since diagnosis to treatment (≥ 1 yr vs. < 1 yr) 0. 618 0. 482 – 0. 792 <0. 001 Corrected calcium (≤ 10 vs. >10 mg/d. L) 0. 503 0. 325 – 0. 778 0. 002 Final Model *Outcome by investigator assessment

Sunitinib Nomogram Predicts Probability of Progression-free Survival at 12 -Mo. * Points Corrected calcium No. of metastatic sites Y Prior nephrectomy N ECOG PS Thrombocytosis 0 1 Y N Time from dx to tx LDH / ULN of LDH Total Points Predicted Value *Outcome by investigator assessment; While present in the underlying prediction model, hemoglobin, lung metastases, liver metastases, and alkaline phosphatase had trivial effects and were omitted from display 29

SU 11248 versus Interferon- in First-Line Treatment for m. RCC Conclusions - ASCO 2007 • Updated results of phase 3 trial show superior efficacy of sunitinib compared to IFN- in first-line treatment of m. RCC • Prognostic factors for PFS to sunitinib firstline therapy are identified • Analysis of survival for sunitinib compared to IFN- awaits longer follow-up

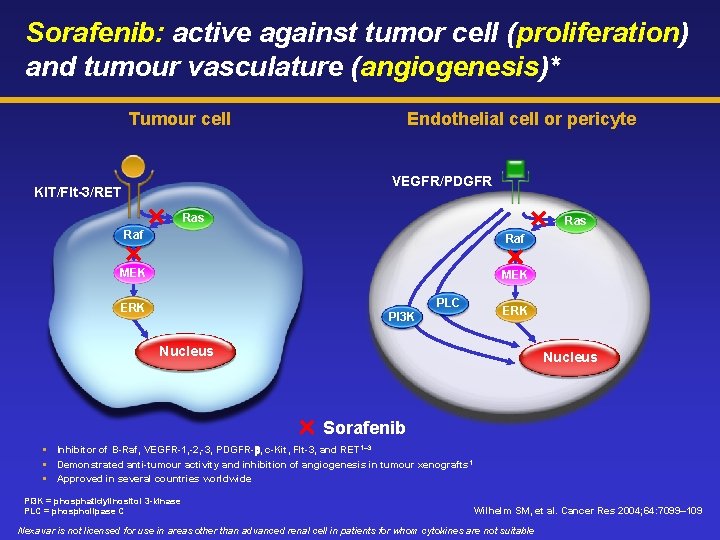
Sorafenib: active against tumor cell (proliferation) and tumour vasculature (angiogenesis)* Tumour cell Endothelial cell or pericyte VEGFR/PDGFR KIT/Flt-3/RET Ras Raf MEK PLC ERK PI 3 K ERK Nucleus Sorafenib Inhibitor of B-Raf, VEGFR-1, -2, -3, PDGFR-b, c-Kit, Flt-3, and RET 1– 3 Demonstrated anti-tumour activity and inhibition of angiogenesis in tumour xenografts 1 Approved in several countries worldwide PI 3 K = phosphatidylinositol 3 -kinase PLC = phospholipase C Wilhelm SM, et al. Cancer Res 2004; 64: 7099– 109 Nexavar is not licensed for use in areas other than advanced renal cell in patients for whom cytokines are not suitable

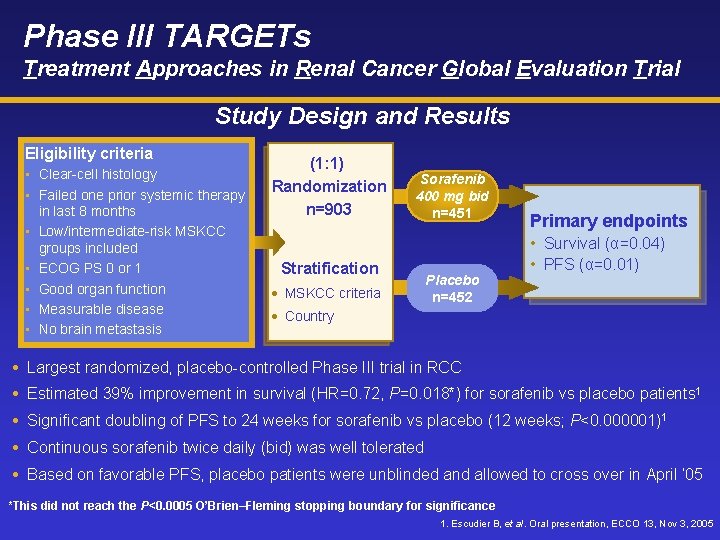
Phase III TARGETs Treatment Approaches in Renal Cancer Global Evaluation Trial Study Design and Results Eligibility criteria • Clear-cell histology • Failed one prior systemic therapy in last 8 months • Low/intermediate-risk MSKCC groups included • ECOG PS 0 or 1 • Good organ function • Measurable disease • No brain metastasis (1: 1) Randomization n=903 Sorafenib 400 mg bid n=451 Stratification • MSKCC criteria Placebo n=452 Primary endpoints • Survival (α=0. 04) • PFS (α=0. 01) • Country Largest randomized, placebo-controlled Phase III trial in RCC Estimated 39% improvement in survival (HR=0. 72, P=0. 018*) for sorafenib vs placebo patients 1 Significant doubling of PFS to 24 weeks for sorafenib vs placebo (12 weeks; P<0. 000001)1 Continuous sorafenib twice daily (bid) was well tolerated Based on favorable PFS, placebo patients were unblinded and allowed to cross over in April ’ 05 *This did not reach the P<0. 0005 O’Brien–Fleming stopping boundary for significance 1. Escudier B, et al. Oral presentation, ECCO 13, Nov 3, 2005

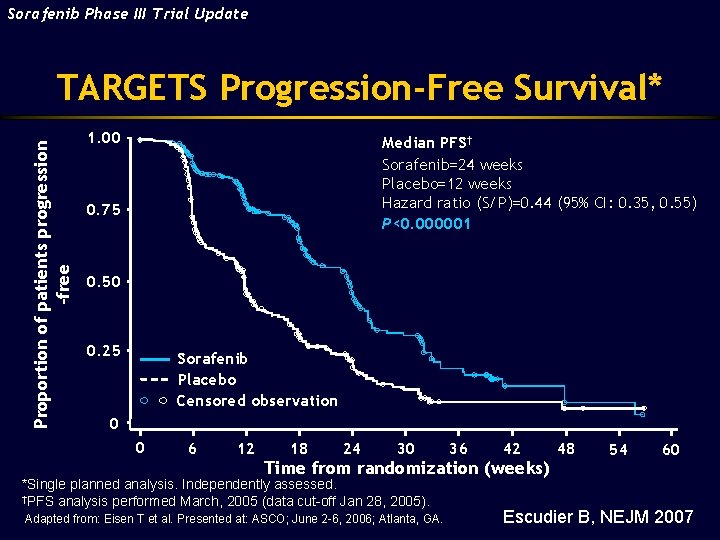
Sorafenib Phase III Trial Update Proportion of patients progression -free TARGETS Progression-Free Survival* 1. 00 Median PFS† Sorafenib=24 weeks Placebo=12 weeks Hazard ratio (S/P)=0. 44 (95% CI: 0. 35, 0. 55) P<0. 000001 0. 75 0. 50 0. 25 Sorafenib Placebo Censored observation 0 0 6 12 18 24 30 36 42 Time from randomization (weeks) *Single planned analysis. Independently assessed. †PFS analysis performed March, 2005 (data cut-off Jan 28, 2005). Adapted from: Eisen T et al. Presented at: ASCO; June 2 -6, 2006; Atlanta, GA. 48 54 60 Escudier B, NEJM 2007

TARGETs Incidence of Grade 3/4 Treatment-Emergent Adverse Events* in 2% patients Sorafenib (n=451) Any grade Grades 3/4 Decreased hemoglobin 34 (8%) 12 (3%) Hypertension 76 (17%) 16 (4%) Placebo (n=451) Any grade Grades 3/4 33 (7%) 20 (4%) 8 (2%) 2 (<1%) Fatigue 165 (37%) 22 (5%) 125 (28%) 16 (4%) Diarrhea 195 (43%) 11 (2%) 58 (13%) 3 (1%) Hand–foot skin reaction 134 (30%) 25 (6%) 30 (7%) – Tumor pain Bone pain 29 (6%) 13 (3%) 34 (8%) 3 (1%) 24 (5%) 8 (2%) 35 (8%) 15 (3%) Dyspnea 65 (14%) 16 (4%) 52 (12%) 11 (2%) *NCI-CTC Version 3. 0

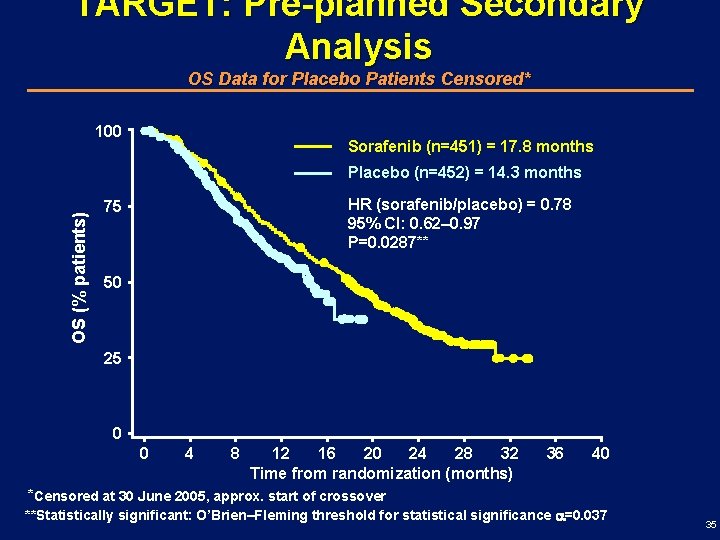
TARGET: Pre-planned Secondary Analysis OS Data for Placebo Patients Censored* 100 Sorafenib (n=451) = 17. 8 months OS (% patients) Placebo (n=452) = 14. 3 months HR (sorafenib/placebo) = 0. 78 95% CI: 0. 62– 0. 97 P=0. 0287** 75 50 25 0 0 4 8 12 16 20 24 28 32 Time from randomization (months) 36 40 *Censored at 30 June 2005, approx. start of crossover **Statistically significant: O’Brien–Fleming threshold for statistical significance =0. 037 35

Final Conclusions of TARGET - ASCO 2007 • Sorafenib significantly increased PFS versus placebo leading to protocol amendment to allow for placebo crossover – 216/452 (48%) of placebo patients crossed over to sorafenib • 12. 0 weeks exposure to placebo • 40. 1 weeks exposure to sorafenib • OS results confounded due to cross over – The pre-planned secondary analysis censoring placebo data demonstrated a significant survival advantage for sorafenib • Baseline VEGF was an independent prognostic factor for PFS • Sorafenib benefited patients with both low and high baseline VEGF • Sorafenib-associated changes in VEGF and s. VEGFR 2 are consistent with inhibition of VEGF signaling 37

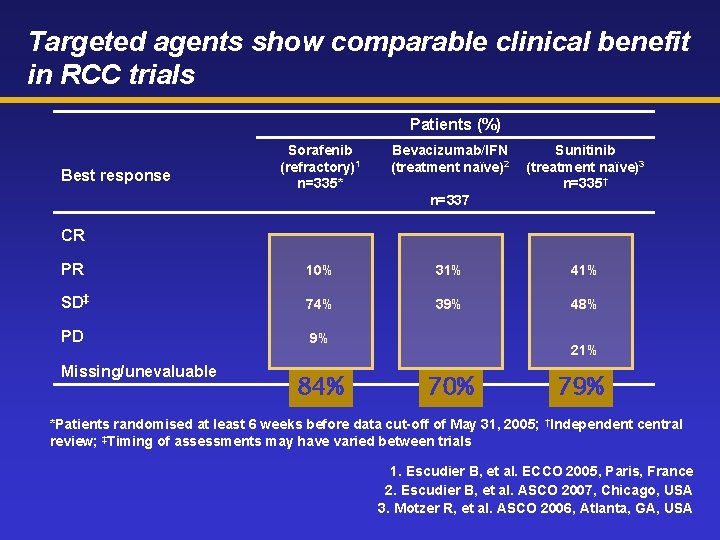
Targeted agents show comparable clinical benefit in RCC trials Patients (%) Best response Sorafenib (refractory)1 n=335* Bevacizumab/IFN (treatment naïve)2 Sunitinib (treatment naïve)3 n=335† n=337 CR PR 10% 31% 41% SD‡ 74% 39% 48% PD 9% Missing/unevaluable 11% 84% 21% 70% 79% *Patients randomised at least 6 weeks before data cut-off of May 31, 2005; †Independent central review; ‡Timing of assessments may have varied between trials 1. Escudier B, et al. ECCO 2005, Paris, France 2. Escudier B, et al. ASCO 2007, Chicago, USA 3. Motzer R, et al. ASCO 2006, Atlanta, GA, USA

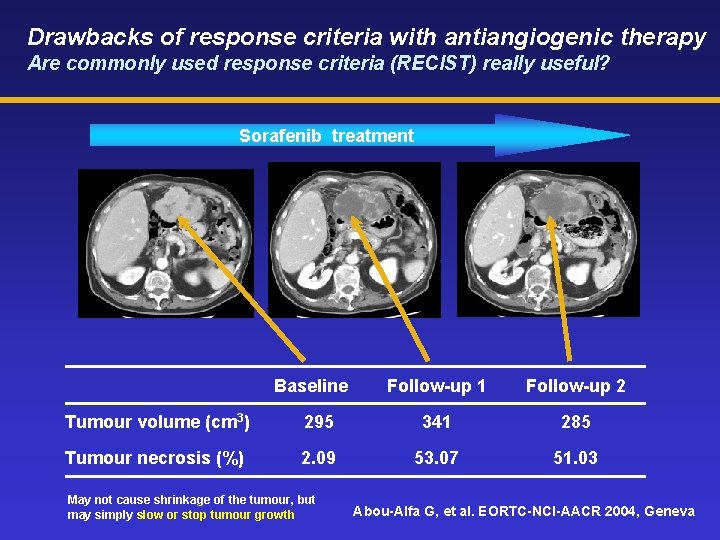
Drawbacks of response criteria with antiangiogenic therapy Are commonly used response criteria (RECIST) really useful? Sorafenib treatment Baseline Follow-up 1 Follow-up 2 Tumour volume (cm 3) 295 341 285 Tumour necrosis (%) 2. 09 53. 07 51. 03 May not cause shrinkage of the tumour, but may simply slow or stop tumour growth Abou-Alfa G, et al. EORTC-NCI-AACR 2004, Geneva


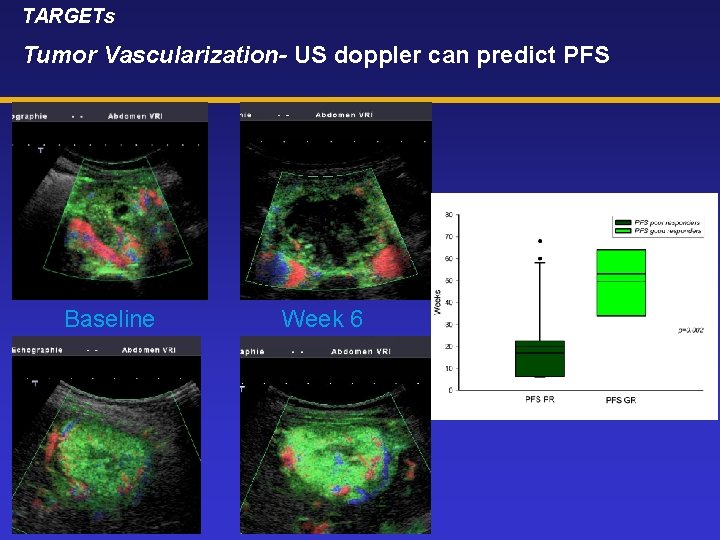
TARGETs Tumor Vascularization- US doppler can predict PFS Baseline Week 6

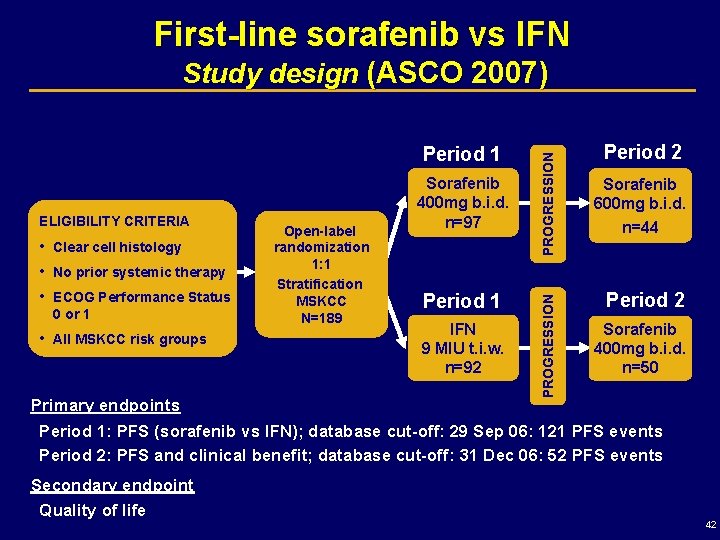
First-line sorafenib vs IFN ELIGIBILITY CRITERIA • • • Clear cell histology • All MSKCC risk groups No prior systemic therapy ECOG Performance Status 0 or 1 Primary endpoints Open-label randomization 1: 1 Stratification MSKCC N=189 Sorafenib 400 mg b. i. d. n=97 Period 1 IFN 9 MIU t. i. w. n=92 PROGRESSION Period 1 PROGRESSION Study design (ASCO 2007) Period 2 Sorafenib 600 mg b. i. d. n=44 Period 2 Sorafenib 400 mg b. i. d. n=50 Period 1: PFS (sorafenib vs IFN); database cut-off: 29 Sep 06: 121 PFS events Period 2: PFS and clinical benefit; database cut-off: 31 Dec 06: 52 PFS events Secondary endpoint Quality of life 42

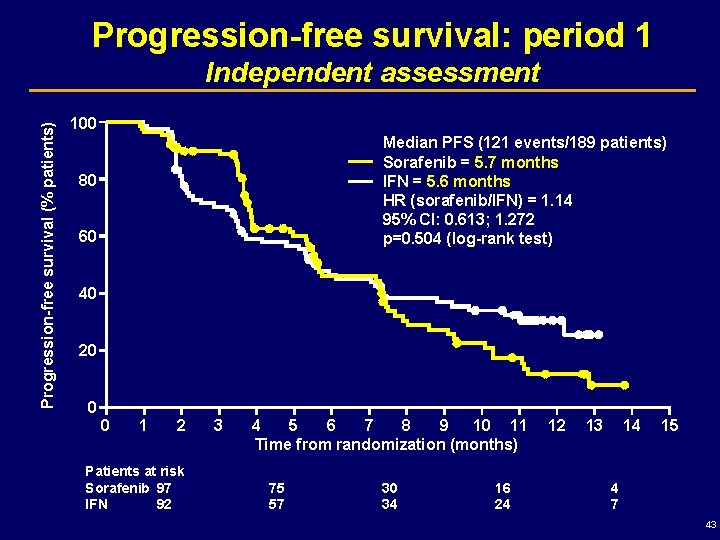
Progression-free survival: period 1 Progression-free survival (% patients) Independent assessment 100 Median PFS (121 events/189 patients) Sorafenib = 5. 7 months IFN = 5. 6 months HR (sorafenib/IFN) = 1. 14 95% CI: 0. 613; 1. 272 p=0. 504 (log-rank test) 80 60 40 20 0 0 1 2 Patients at risk Sorafenib 97 IFN 92 3 4 5 6 7 8 9 10 11 Time from randomization (months) 75 57 30 34 16 24 12 13 14 15 4 7 43

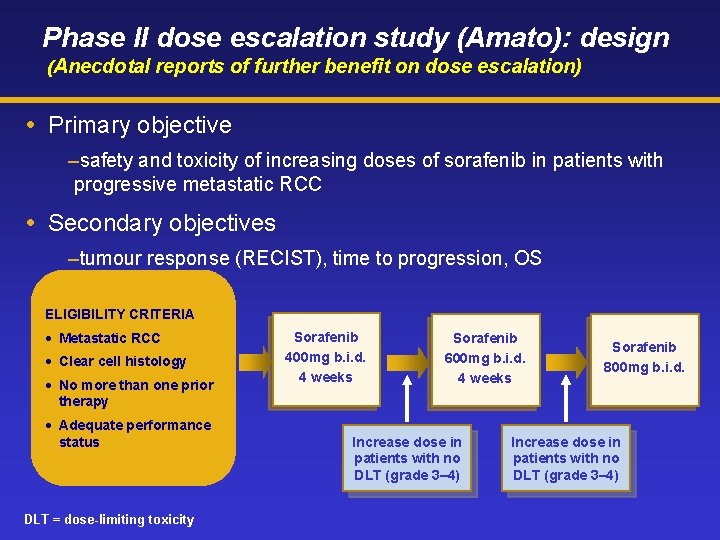
Phase II dose escalation study (Amato): design (Anecdotal reports of further benefit on dose escalation) Primary objective –safety and toxicity of increasing doses of sorafenib in patients with progressive metastatic RCC Secondary objectives –tumour response (RECIST), time to progression, OS ELIGIBILITY CRITERIA • Metastatic RCC • Clear cell histology • No more than one prior therapy • Adequate performance status DLT = dose-limiting toxicity Sorafenib 400 mg b. i. d. 4 weeks Sorafenib 600 mg b. i. d. 4 weeks Increase dose in patients with no DLT (grade 3– 4) Sorafenib 800 mg b. i. d. Increase dose in patients with no DLT (grade 3– 4)

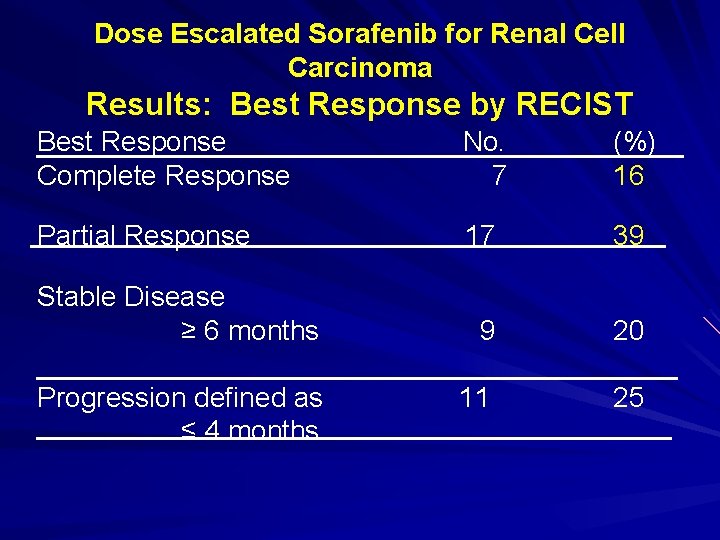
Dose Escalated Sorafenib for Renal Cell Carcinoma Results: Best Response by RECIST Best Response Complete Response No. 7 (%) 16 Partial Response 17 39 9 20 11 25 Stable Disease ≥ 6 months Progression defined as ≤ 4 months

What have we learnt in ASCO 2007 ? How many new questions we have?

Is Any Agent Better ? No comparative studies “Objective response rates” and TTP patient benefit § RCC can have highly variable natural history With multiple agents will we be able to demonstrate survival advantage? What other phase III definitive endpoints should be utilized?

Is Dose and Schedule Optimal? Sunitinib with continuous dosing (abstract 5040) § Possible lower “response rate” (ORR 19%) Sorafenib at higher doses (abstract 5026, Amato, GU oral) § Possible higher “response rate” § Dose escalation “effective” in “resistant” disease Temsirolimus no dose response (JCO, 22: 909, 2004) Response rate patient benefit

Histology as a Predictive Biomarker: Activity in Non-Clear Cell RCC Sorafenib or Sunitinib in papillary and chromophobe (abstracts 5036 (Stadler), 5037 (Plantade) Temsirolimus in non-clear (abstract 5033(JP Dutcher) How is non-clear cell defined? § Lack of uniform expertise on histologic grounds § Are molecular markers necessary? § Do current classification schemes capture entire spectrum of heterogeneity/subtypes?

Other Predictive Biomarkers (VEGF Pathway Directed Therapies) Lower s. VEGFR 3 and VEGF-C in responders to Su (abstract 5035 BVZ->SU, DJ George). Sorafenib active in low and high (Bukowsky) Pharmacokinetic parameters Su-AUC(abstract 5027, Houk, GU oral) VHL mutation status (abstract 5012, Choueiri, RCC Clinical Sci. RR↑ if mut) Retrospective Multiple testing and hypothesis generating Prognostic, predictive, or spurious?

Can Combination Therapy Improve Outcome? VEGF pathway + m. TOR § Temsirolimus/bevacizumab (abstract 5034, JR Merchan 7 PR 3 SD/12 eval) § Valatinib(PTK-ZK)/everolimus (abstract 5039, JC Speca n=13 PR 15%/SD 62%) § Many others VEGF binding + VEGFR/PDGFR inhibitor § Bevacizumab/Sorafenib (ASCO 2006) § Bevacizumab/Sunitinib (abstract 5099 DR Feldman, GU poster n=13 4 PR/7 SD) VEGF binding + IFNA § Plenary session

Clinical Resistance Responses to alternative VEGF pathway targeting in “resistant” disease § Sunitinib in bevacizumab resistant (abstract 5035. DJ George) § Sunitinib or sorafenib following the alternative (abstract 5038 MP Sablin. So-> Su better) § Axitinib in sorafenib resistant (abstract 5032, BI Rini PR 14%+SD 36%) § Sorafenib in bevacizumab resistant (ARCCS) (abstract 5041, HA Drabkin n=195 eval PR 2, 5% SD 77, 5%) Mechanism completely unknown

MOVING TO ADJUVANT

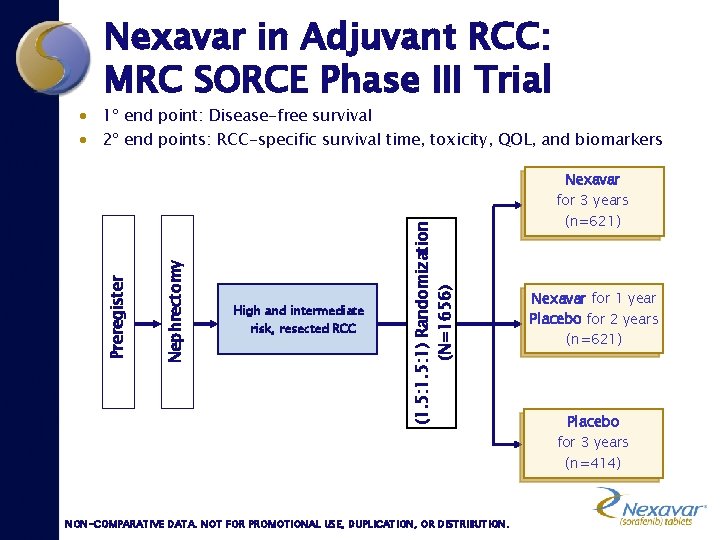
Nexavar in Adjuvant RCC: MRC SORCE Phase III Trial · 1º end point: Disease-free survival · 2º end points: RCC-specific survival time, toxicity, QOL, and biomarkers Nexavar High and intermediate risk, resected RCC (1. 5: 1) Randomization (N=1656) Nephrectomy Preregister for 3 years (n=621) Nexavar for 1 year Placebo for 2 years (n=621) Placebo for 3 years (n=414) NON-COMPARATIVE DATA. NOT FOR PROMOTIONAL USE, DUPLICATION, OR DISTRIBUTION.

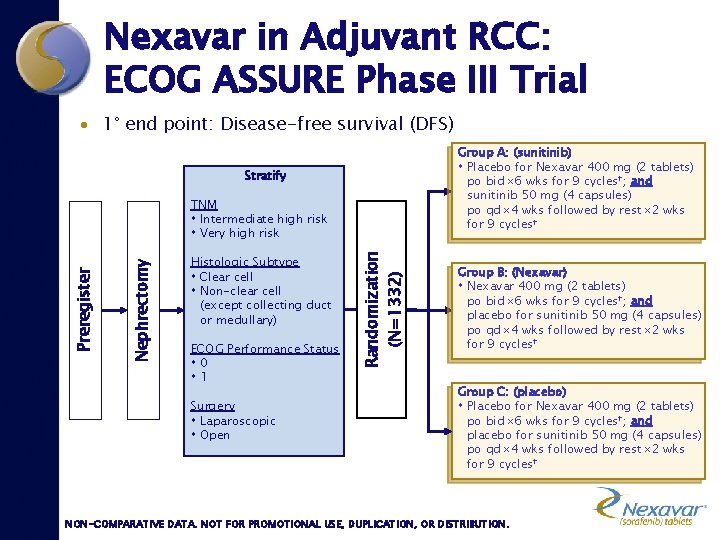
Nexavar in Adjuvant RCC: ECOG ASSURE Phase III Trial · 1° end point: Disease-free survival (DFS) Group A: (sunitinib) Placebo for Nexavar 400 mg (2 tablets) po bid 6 wks for 9 cycles†; and sunitinib 50 mg (4 capsules) po qd 4 wks followed by rest 2 wks for 9 cycles† Stratify Histologic Subtype Clear cell Non-clear cell (except collecting duct or medullary) ECOG Performance Status 0 1 Surgery Laparoscopic Open Randomization (N=1332) Nephrectomy Preregister TNM Intermediate high risk Very high risk Group B: (Nexavar) Nexavar 400 mg (2 tablets) po bid 6 wks for 9 cycles†; and placebo for sunitinib 50 mg (4 capsules) po qd 4 wks followed by rest 2 wks for 9 cycles† Group C: (placebo) Placebo for Nexavar 400 mg (2 tablets) po bid 6 wks for 9 cycles†; and placebo for sunitinib 50 mg (4 capsules) po qd 4 wks followed by rest 2 wks for 9 cycles† NON-COMPARATIVE DATA. NOT FOR PROMOTIONAL USE, DUPLICATION, OR DISTRIBUTION.

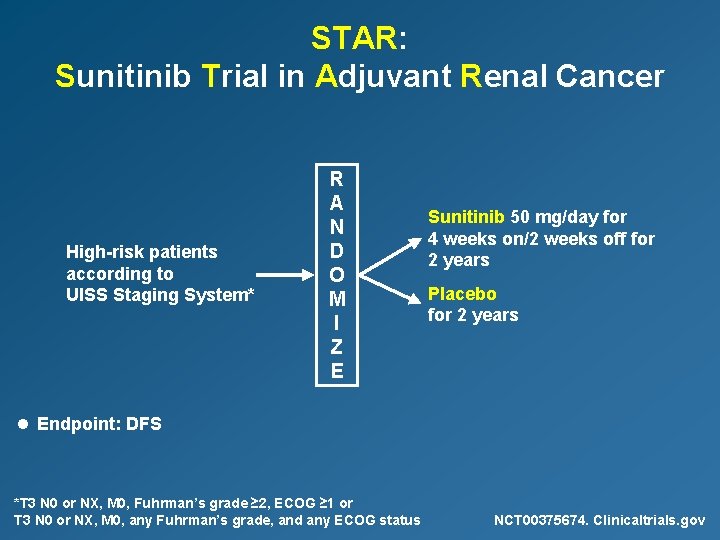
STAR: Sunitinib Trial in Adjuvant Renal Cancer High-risk patients according to UISS Staging System* R A N D O M I Z E Sunitinib 50 mg/day for 4 weeks on/2 weeks off for 2 years Placebo for 2 years l Endpoint: DFS *T 3 N 0 or NX, M 0, Fuhrman’s grade ≥ 2, ECOG ≥ 1 or T 3 N 0 or NX, M 0, any Fuhrman’s grade, and any ECOG status NCT 00375674. Clinicaltrials. gov

Conclusions, Adjuvant therapy l No standard therapy → inclusion in clinical trials l Rationale to test antiangiogenic therapies – three phase III trials started in 2007 l Opportunity = commitment to participate Results available after 2010

Phase III data supporting the use of targeted agents in RCC Randomised phase III study Sorafenib vs Placebo 1 Sunitinib vs IFN 2 Temsirolimus* vs IFN vs Temsirolimus + IFN 3 Bevacizumab* + IFN vs Placebo + IFN 4 Line of treatment ORR (%) Impact on survival 10 Second-line PFS 2 2. 8 months 41† 11 months† PFS First-line (poor-risk patients only) 6 5 months 9 10. 9 months‡ 5 OS 7. 3 months 8 8. 4 months 31† 10. 2 months† First-line *not approved for use in the EU for RCC †statistically significant difference vs comparator arm (p<0. 001) ‡statistically significant difference vs IFN alone (p<0. 01) ORR = objective response rate; PFS = progression-free survival OS = overall survival 5. 5 months† PFS 13 5. 4 months 1. Escudier B, et al. N Engl J Med 2007; 356: 125– 34 2. Motzer RJ, et al. N Engl J Med 2007; 356: 115– 24 3. Hudes G, et al. N Engl J Med 2007; 356: 2271– 81 4. Escudier B, et al. ASCO 2007, Chicago, IL, USA

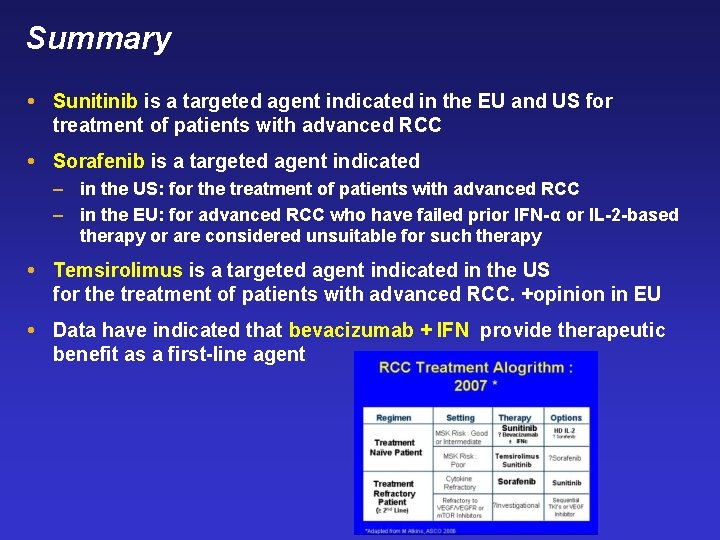
Summary Sunitinib is a targeted agent indicated in the EU and US for treatment of patients with advanced RCC Sorafenib is a targeted agent indicated – in the US: for the treatment of patients with advanced RCC – in the EU: for advanced RCC who have failed prior IFN-α or IL-2 -based therapy or are considered unsuitable for such therapy Temsirolimus is a targeted agent indicated in the US for the treatment of patients with advanced RCC. +opinion in EU Data have indicated that bevacizumab + IFN provide therapeutic benefit as a first-line agent

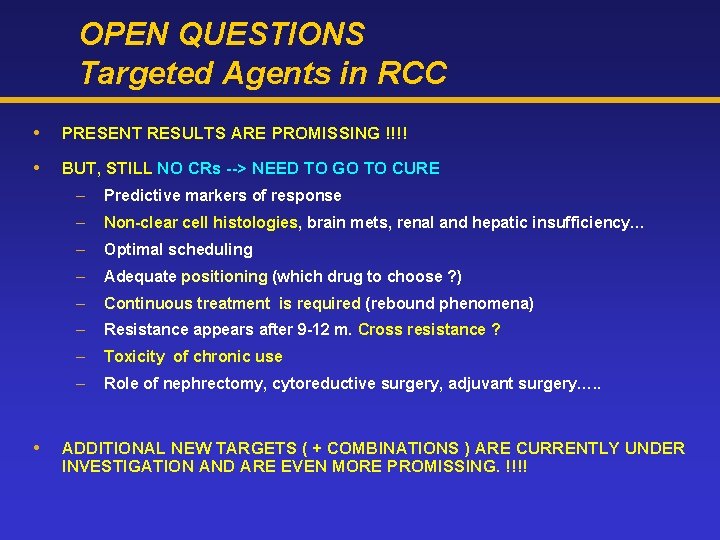
OPEN QUESTIONS Targeted Agents in RCC PRESENT RESULTS ARE PROMISSING !!!! BUT, STILL NO CRs --> NEED TO GO TO CURE – Predictive markers of response – Non-clear cell histologies, brain mets, renal and hepatic insufficiency… – Optimal scheduling – Adequate positioning (which drug to choose ? ) – Continuous treatment is required (rebound phenomena) – Resistance appears after 9 -12 m. Cross resistance ? – Toxicity of chronic use – Role of nephrectomy, cytoreductive surgery, adjuvant surgery…. . ADDITIONAL NEW TARGETS ( + COMBINATIONS ) ARE CURRENTLY UNDER INVESTIGATION AND ARE EVEN MORE PROMISSING. !!!!