Visin Integradora del Tratamiento del Cncer Renal Avanzado














- Slides: 44

Visión Integradora del Tratamiento del Cáncer Renal Avanzado: Situación Actual, Nuevos Fármacos en Investigación y Vías de Progreso M. Guix y J. Bellmunt Hospital del Mar, Barcelona Guadalajara, 18 y 19 Junio 2009

Progress in treatment of m. RCC l Better knowledge of pathology l Better knowledge of molecular biology l Many new options with 4 drugs approved in 18 months!!!! – Sorafenib (Nexavar) – Sunitinib (Sutent) – Temsirolimus (Torisel) – Bevacizumab (Avastin)* with 4 pivotal studies published the same year * In EU, in combination with IFN

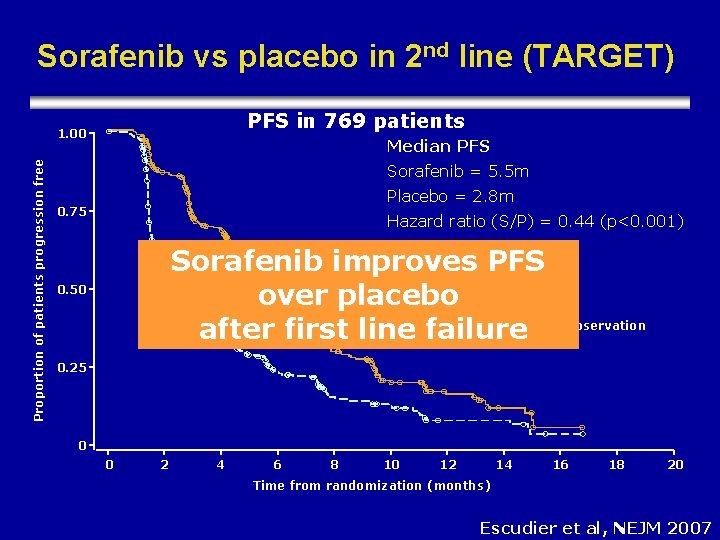
Sorafenib vs placebo in 2 nd line (TARGET) PFS in 769 patients Proportion of patients progression free 1. 00 Median PFS Sorafenib = 5. 5 m Placebo = 2. 8 m 0. 75 Hazard ratio (S/P) = 0. 44 (p<0. 001) Sorafenib improves PFS Sorafenib over placebo Placebo Censored observation after first line failure 0. 50 0. 25 0 0 2 4 6 8 10 12 14 16 18 20 Time from randomization (months) Escudier et al, NEJM 2007

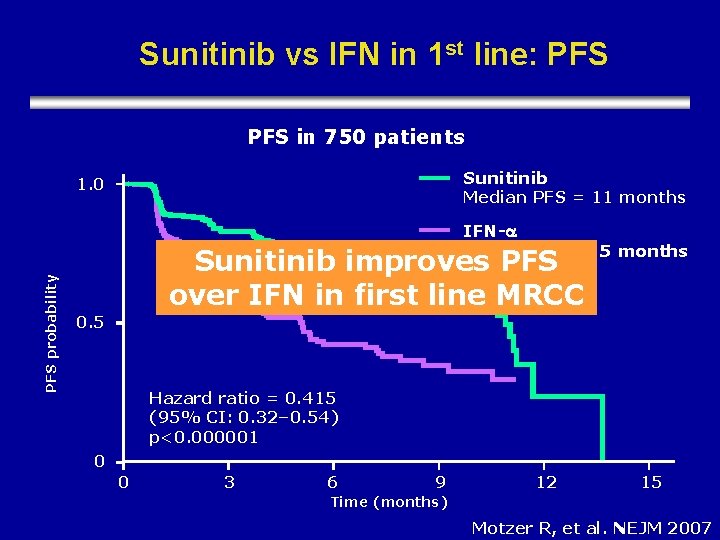
Sunitinib vs IFN in 1 st line: PFS in 750 patients Sunitinib 1. 0 Median PFS = 11 months PFS probability IFN- Median PFS = 5 months Sunitinib improves PFS over IFN in first line MRCC 0. 5 Hazard ratio = 0. 415 (95% CI: 0. 32– 0. 54) p<0. 000001 0 0 3 6 9 12 15 Time (months) Motzer R, et al. NEJM 2007

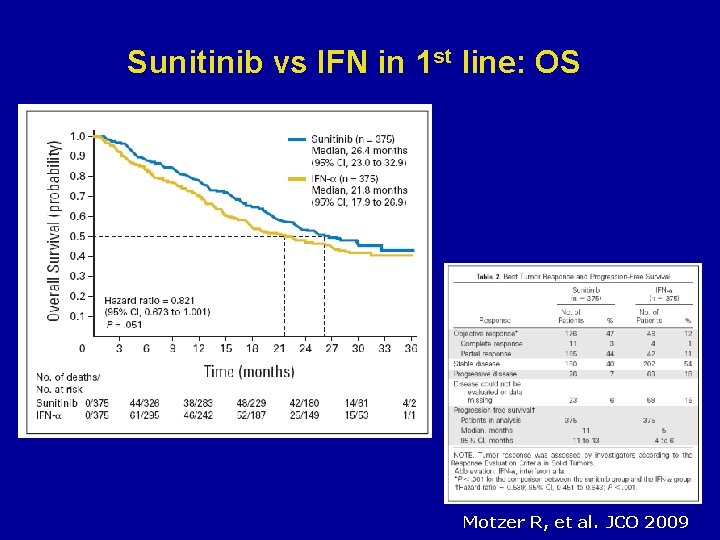
Sunitinib vs IFN in 1 st line: OS Motzer R, et al. JCO 2009

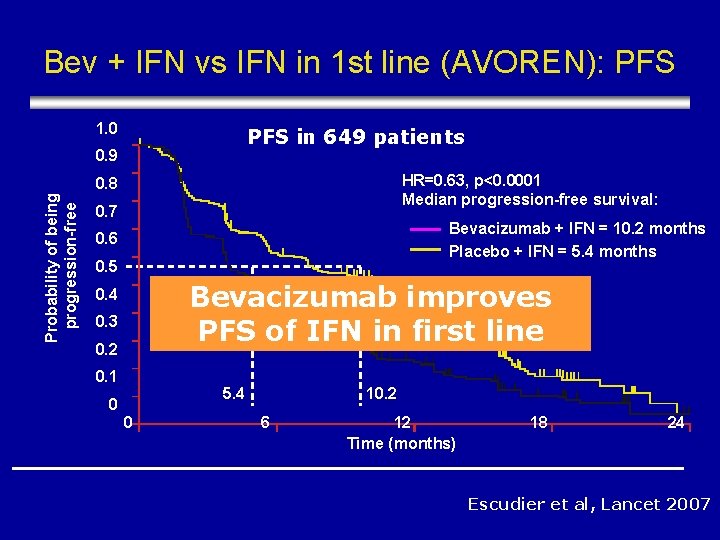
Bev + IFN vs IFN in 1 st line (AVOREN): PFS 1. 0 PFS in 649 patients 0. 9 HR=0. 63, p<0. 0001 Median progression-free survival: Probability of being progression-free 0. 8 0. 7 Bevacizumab + IFN = 10. 2 months Placebo + IFN = 5. 4 months 0. 6 0. 5 Bevacizumab improves PFS of IFN in first line 0. 4 0. 3 0. 2 0. 1 5. 4 0 0 10. 2 6 12 Time (months) 18 24 Escudier et al, Lancet 2007

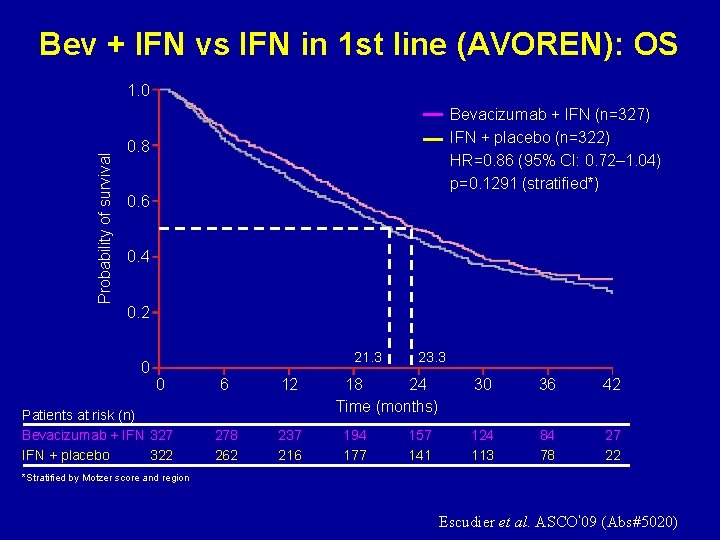
Bev + IFN vs IFN in 1 st line (AVOREN): OS Probability of survival 1. 0 Bevacizumab + IFN (n=327) IFN + placebo (n=322) HR=0. 86 (95% CI: 0. 72– 1. 04) p=0. 1291 (stratified*) 0. 8 0. 6 0. 4 0. 2 0 21. 3 0 Patients at risk (n) Bevacizumab + IFN 327 IFN + placebo 322 6 12 278 262 237 216 23. 3 18 24 Time (months) 194 177 157 141 30 36 42 124 113 84 78 27 22 *Stratified by Motzer score and region Escudier et al. ASCO’ 09 (Abs#5020)

Bev + IFN vs IFN in 1 st line (CALGB 90206): PFS Bevacizumab improves PFS of IFN in first line HR=0. 67, p<0. 0001 Median progression-free survival: Bevacizumab + IFN = 8. 5 months IFN = 5. 2 months Rini BI et al, JCO 2008

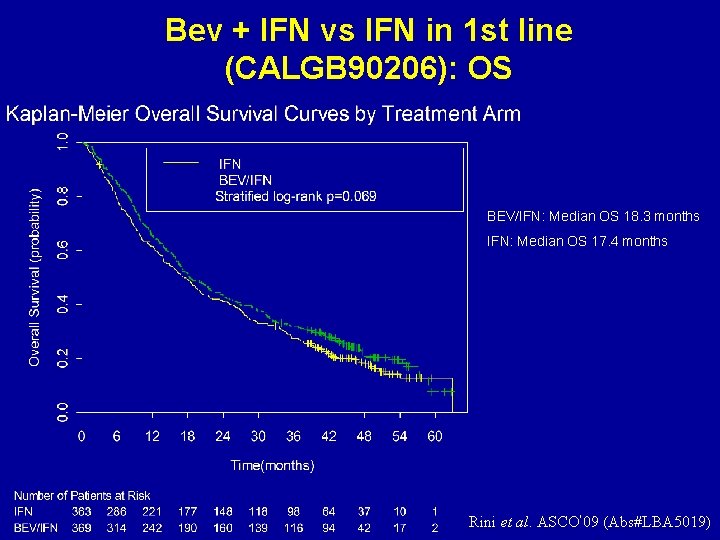
Bev + IFN vs IFN in 1 st line (CALGB 90206): OS BEV/IFN: Median OS 18. 3 months IFN: Median OS 17. 4 months Rini et al. ASCO’ 09 (Abs#LBA 5019)

AVOREN & CALGB 90206 • Mixed news; Neither study exceeded the highwater mark of OS set at ASCO 2008 with sunitinib of 26. 4 months (Figlin et al. ) – patient mix of prognostic factors (and expertise of treating MD’s? ) may explain the difference – Subsequent therapies appear to alter the OS behaviour of m. RCC (ie sorafenib crossover in the TARGET trial)

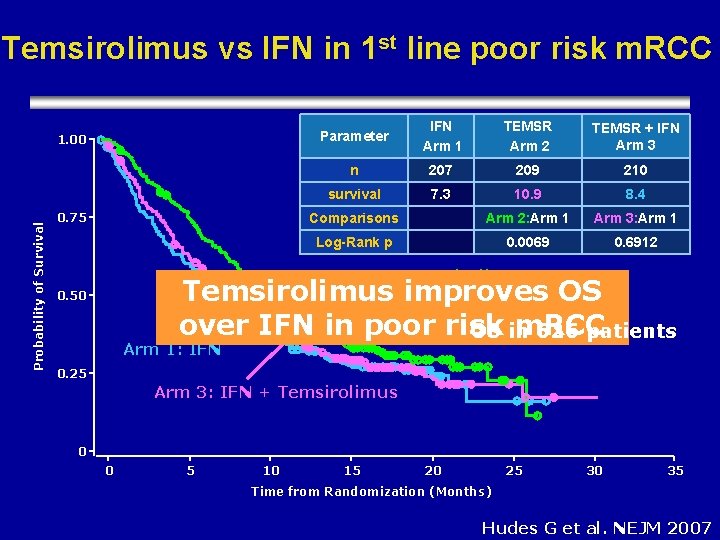
Temsirolimus vs IFN in 1 st line poor risk m. RCC Probability of Survival 1. 00 0. 75 Parameter IFN Arm 1 TEMSR Arm 2 TEMSR + IFN Arm 3 n 207 209 210 survival 7. 3 10. 9 8. 4 Comparisons Arm 2: Arm 1 Arm 3: Arm 1 Log-Rank p 0. 0069 0. 6912 Arm 2: Temsirolimus improves OS over IFN in poor risk m. RCC OS in 626 patients 0. 50 Arm 1: IFN 0. 25 Arm 3: IFN + Temsirolimus 0 0 5 10 15 20 25 30 35 Time from Randomization (Months) Hudes G et al. NEJM 2007

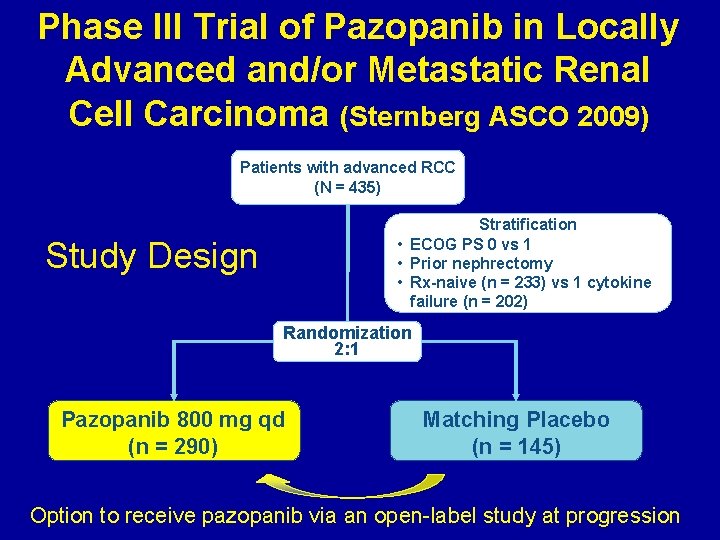
Phase III Trial of Pazopanib in Locally Advanced and/or Metastatic Renal Cell Carcinoma (Sternberg ASCO 2009) Patients with advanced RCC (N = 435) Stratification • ECOG PS 0 vs 1 • Prior nephrectomy • Rx-naive (n = 233) vs 1 cytokine failure (n = 202) Study Design Randomization 2: 1 Pazopanib 800 mg qd (n = 290) Matching Placebo (n = 145) Option to receive pazopanib via an open-label study at progression

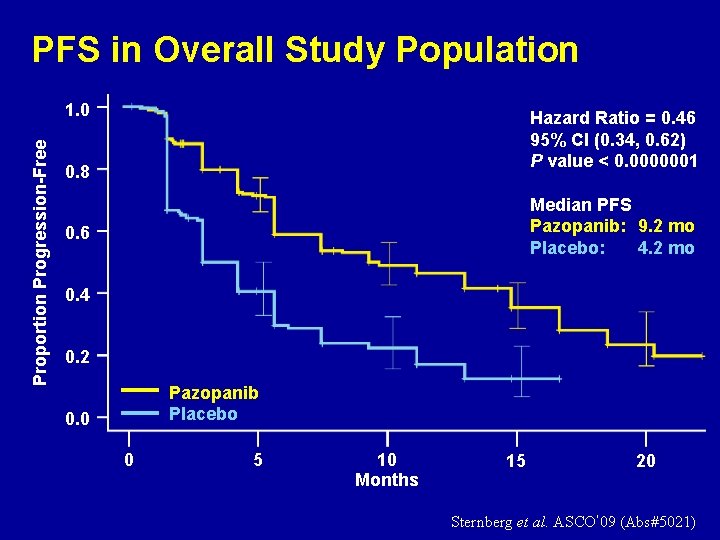
PFS in Overall Study Population Proportion Progression-Free 1. 0 Hazard Ratio = 0. 46 95% CI (0. 34, 0. 62) P value < 0. 0000001 0. 8 Median PFS Pazopanib: 9. 2 mo Placebo: 4. 2 mo 0. 6 0. 4 0. 2 Pazopanib Placebo 0. 0 0 5 10 Months 15 20 Sternberg et al. ASCO’ 09 (Abs#5021)

But now, how to deal with all these options in real life? l Can we establish a treatment algorithm? l Should we use MSKCC in routine? l Is histology important to decide therapy? l Should we use combination or sequential therapy?

Algorithm: 2008/2009

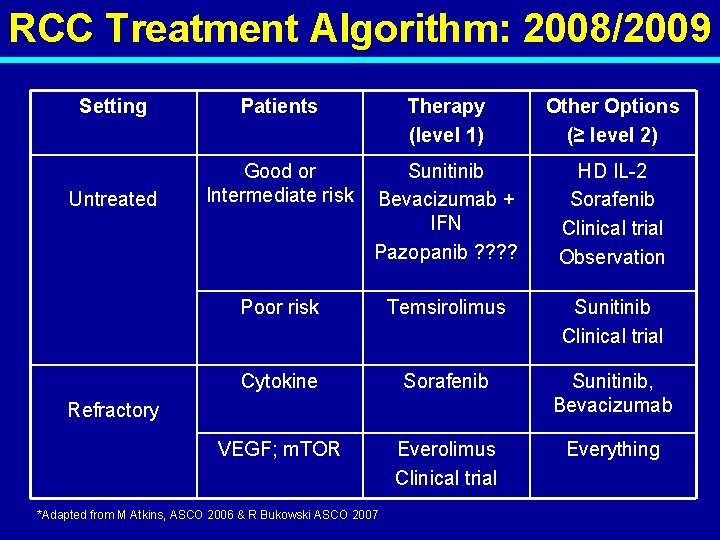
RCC Treatment Algorithm: 2008/2009 Setting Patients Therapy (level 1) Other Options (≥ level 2) Untreated Good or Intermediate risk Sunitinib Bevacizumab + IFN Pazopanib ? ? HD IL-2 Sorafenib Clinical trial Observation Poor risk Temsirolimus Sunitinib Clinical trial Cytokine Sorafenib Sunitinib, Bevacizumab VEGF; m. TOR Everolimus Clinical trial Everything Refractory *Adapted from M Atkins, ASCO 2006 & R Bukowski ASCO 2007

But now, how to deal with all these options in real life? • • Can we establish a treatment algorithm? Should we use MSKCC in routine? Is histology important to decide therapy? Should we use combination or sequential therapy?

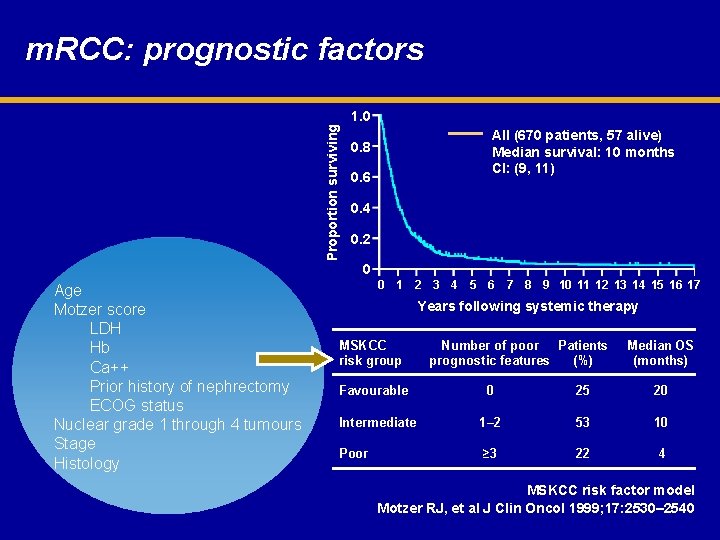
Proportion surviving m. RCC: prognostic factors 1. 0 All (670 patients, 57 alive) Median survival: 10 months CI: (9, 11) 0. 8 0. 6 0. 4 0. 2 0 Age Motzer score LDH Hb Ca++ Prior history of nephrectomy ECOG status Nuclear grade 1 through 4 tumours Stage Histology 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Years following systemic therapy MSKCC risk group Favourable Number of poor Patients prognostic features (%) Median OS (months) 0 25 20 Intermediate 1– 2 53 10 Poor ≥ 3 22 4 MSKCC risk factor model Motzer RJ, et al J Clin Oncol 1999; 17: 2530– 2540

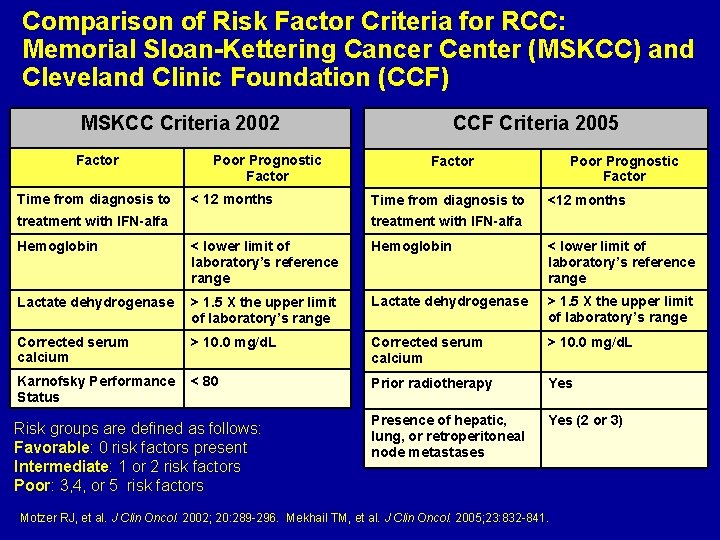
Comparison of Risk Factor Criteria for RCC: Memorial Sloan-Kettering Cancer Center (MSKCC) and Cleveland Clinic Foundation (CCF) MSKCC Criteria 2002 Factor Time from diagnosis to Poor Prognostic Factor < 12 months treatment with IFN-alfa CCF Criteria 2005 Factor Time from diagnosis to Poor Prognostic Factor <12 months treatment with IFN-alfa Hemoglobin < lower limit of laboratory’s reference range Lactate dehydrogenase > 1. 5 X the upper limit of laboratory’s range Corrected serum calcium > 10. 0 mg/d. L Karnofsky Performance Status < 80 Prior radiotherapy Yes Presence of hepatic, lung, or retroperitoneal node metastases Yes (2 or 3) Risk groups are defined as follows: Favorable: 0 risk factors present Intermediate: 1 or 2 risk factors Poor: 3, 4, or 5 risk factors Motzer RJ, et al. J Clin Oncol. 2002; 20: 289 -296. Mekhail TM, et al. J Clin Oncol. 2005; 23: 832 -841.

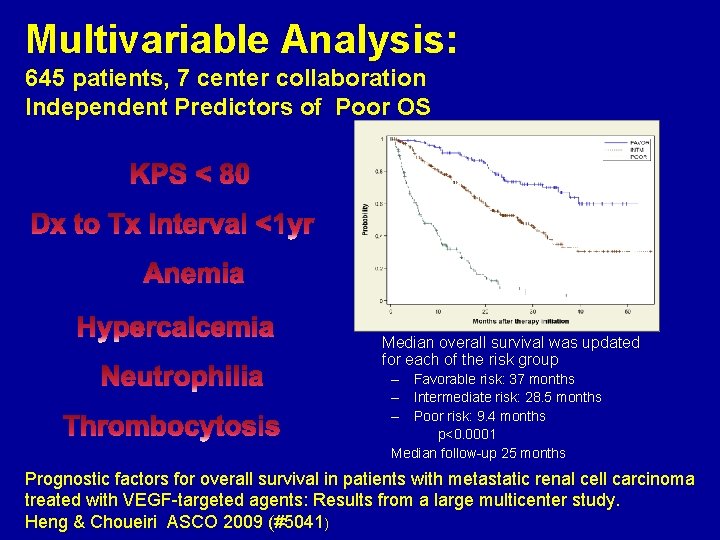
Multivariable Analysis: 645 patients, 7 center collaboration Independent Predictors of Poor OS Median overall survival was updated for each of the risk group – Favorable risk: 37 months – Intermediate risk: 28. 5 months – Poor risk: 9. 4 months p<0. 0001 Median follow-up 25 months Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with VEGF-targeted agents: Results from a large multicenter study. Heng & Choueiri ASCO 2009 (#5041)

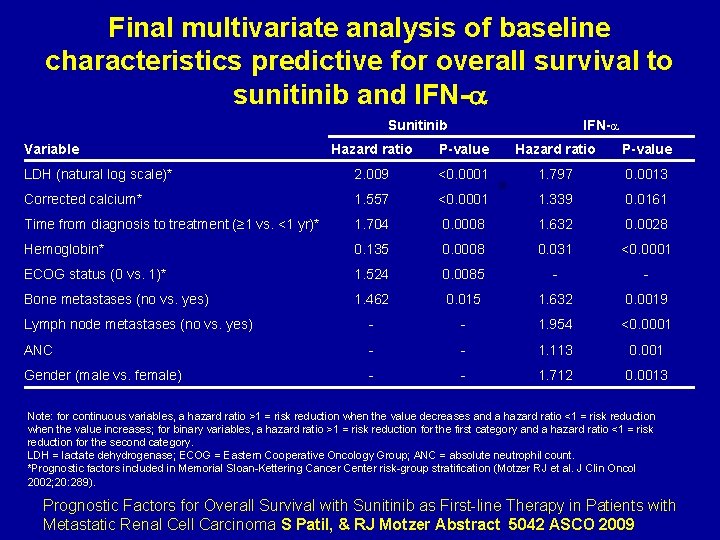
Final multivariate analysis of baseline characteristics predictive for overall survival to sunitinib and IFN- Sunitinib Variable IFN- Hazard ratio P-value LDH (natural log scale)* 2. 009 <0. 0001 1. 797 0. 0013 Corrected calcium* 1. 557 <0. 0001 1. 339 0. 0161 Time from diagnosis to treatment (≥ 1 vs. <1 yr)* 1. 704 0. 0008 1. 632 0. 0028 Hemoglobin* 0. 135 0. 0008 0. 031 <0. 0001 ECOG status (0 vs. 1)* 1. 524 0. 0085 - - Bone metastases (no vs. yes) 1. 462 0. 015 1. 632 0. 0019 Lymph node metastases (no vs. yes) - - 1. 954 <0. 0001 ANC - - 1. 113 0. 001 Gender (male vs. female) - - 1. 712 0. 0013 Note: for continuous variables, a hazard ratio >1 = risk reduction when the value decreases and a hazard ratio <1 = risk reduction when the value increases; for binary variables, a hazard ratio >1 = risk reduction for the first category and a hazard ratio <1 = risk reduction for the second category. LDH = lactate dehydrogenase; ECOG = Eastern Cooperative Oncology Group; ANC = absolute neutrophil count. *Prognostic factors included in Memorial Sloan-Kettering Cancer Center risk-group stratification (Motzer RJ et al. J Clin Oncol 2002; 20: 289). Prognostic Factors for Overall Survival with Sunitinib as First-line Therapy in Patients with Metastatic Renal Cell Carcinoma S Patil, & RJ Motzer Abstract 5042 ASCO 2009

But now, how to deal with all these options in real life? l Can we establish a treatment algorithm? l Should we use MSKCC in routine? l Is histology important to decide therapy? l Should we use combination or sequential therapy?

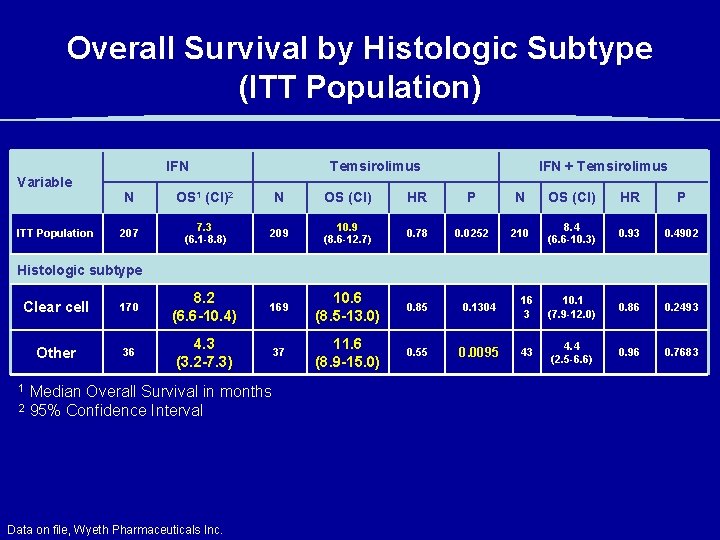
Overall Survival by Histologic Subtype (ITT Population) IFN Temsirolimus IFN + Temsirolimus Variable ITT Population N OS 1 (CI)2 N OS (CI) HR P 207 7. 3 (6. 1 -8. 8) 209 10. 9 (8. 6 -12. 7) 0. 78 0. 0252 210 8. 4 (6. 6 -10. 3) 0. 93 0. 4902 Histologic subtype Clear cell 170 8. 2 (6. 6 -10. 4) 169 10. 6 (8. 5 -13. 0) 0. 85 0. 1304 16 3 10. 1 (7. 9 -12. 0) 0. 86 0. 2493 Other 36 4. 3 (3. 2 -7. 3) 37 11. 6 (8. 9 -15. 0) 0. 55 0. 0095 43 4. 4 (2. 5 -6. 6) 0. 96 0. 7683 1 2 Median Overall Survival in months 95% Confidence Interval Data on file, Wyeth Pharmaceuticals Inc.

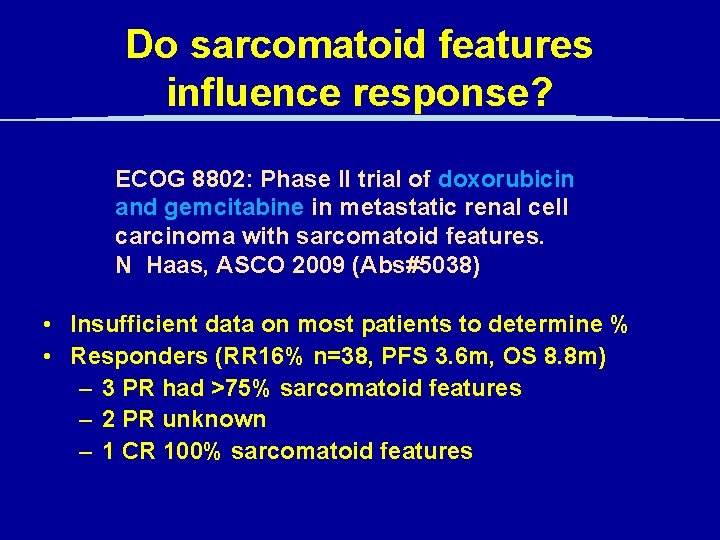
Do sarcomatoid features influence response? ECOG 8802: Phase II trial of doxorubicin and gemcitabine in metastatic renal cell carcinoma with sarcomatoid features. N Haas, ASCO 2009 (Abs#5038) • Insufficient data on most patients to determine % • Responders (RR 16% n=38, PFS 3. 6 m, OS 8. 8 m) – 3 PR had >75% sarcomatoid features – 2 PR unknown – 1 CR 100% sarcomatoid features

But now, how to deal with all these options in real life? l Which is the best first line in good or intermediate risk? l Should we use MSKCC in routine? l Is histology important to decide therapy? l Should we use combination or sequential therapy?

Is sequential better than combination? • Sequential allows full dose of each drug • Avoids safety issues • Has been shown active in different situations: – TKIs post cytokines – TKIs post bevacizumab – m. TOR post TKIs • But design and feasibility of such studies are difficult

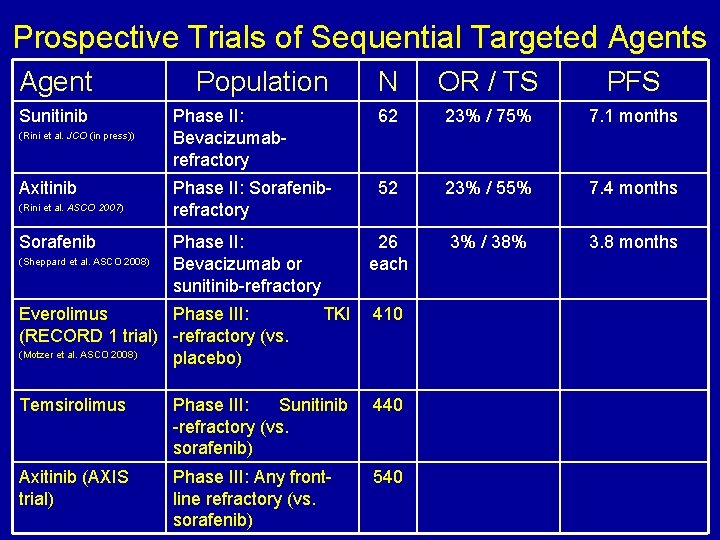
Prospective Trials of Sequential Targeted Agents Agent Sunitinib (Rini et al. JCO (in press)) Axitinib (Rini et al. ASCO 2007) Sorafenib (Sheppard et al. ASCO 2008) Population N OR / TS PFS Phase II: Bevacizumabrefractory 62 23% / 75% 7. 1 months Phase II: Sorafenibrefractory 52 23% / 55% 7. 4 months 26 each 3% / 38% 3. 8 months Phase II: Bevacizumab or sunitinib-refractory Everolimus Phase III: (RECORD 1 trial) -refractory (vs. (Motzer et al. ASCO 2008) placebo) TKI 410 Temsirolimus Phase III: Sunitinib -refractory (vs. sorafenib) 440 Axitinib (AXIS trial) Phase III: Any frontline refractory (vs. sorafenib) 540

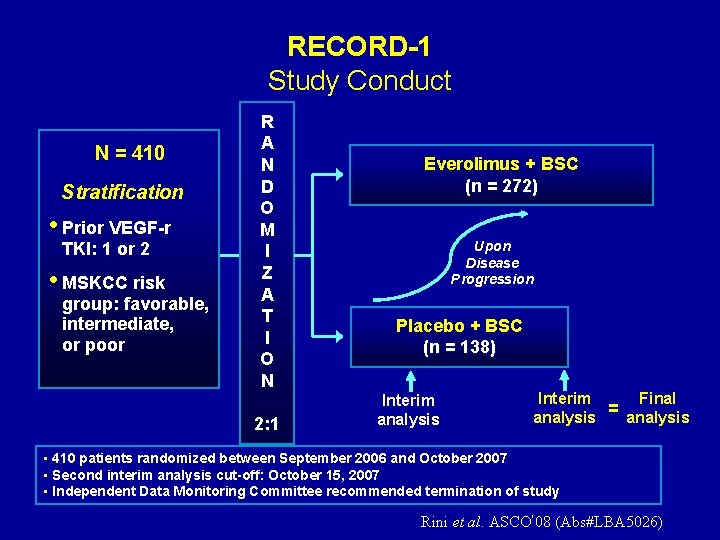
RECORD-1 Study Conduct N = 410 Stratification • Prior VEGF-r TKI: 1 or 2 • MSKCC risk group: favorable, intermediate, or poor R A N D O M I Z A T I O N 2: 1 Everolimus + BSC (n = 272) Upon Disease Progression Placebo + BSC (n = 138) Interim analysis Final Interim = analysis • 410 patients randomized between September 2006 and October 2007 • Second interim analysis cut-off: October 15, 2007 • Independent Data Monitoring Committee recommended termination of study Rini et al. ASCO’ 08 (Abs#LBA 5026)

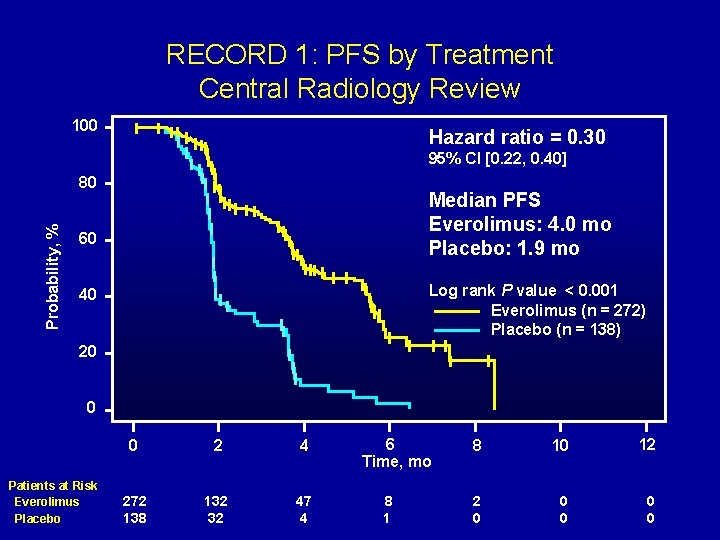
RECORD 1: PFS by Treatment Central Radiology Review 100 Hazard ratio = 0. 30 95% CI [0. 22, 0. 40] Probability, % 80 Median PFS Everolimus: 4. 0 mo Placebo: 1. 9 mo 60 Log rank P value < 0. 001 Everolimus (n = 272) Placebo (n = 138) 40 20 0 Patients at Risk Everolimus Placebo 0 2 4 272 138 132 32 47 4 6 Time, mo 8 10 12 2 0 0 0

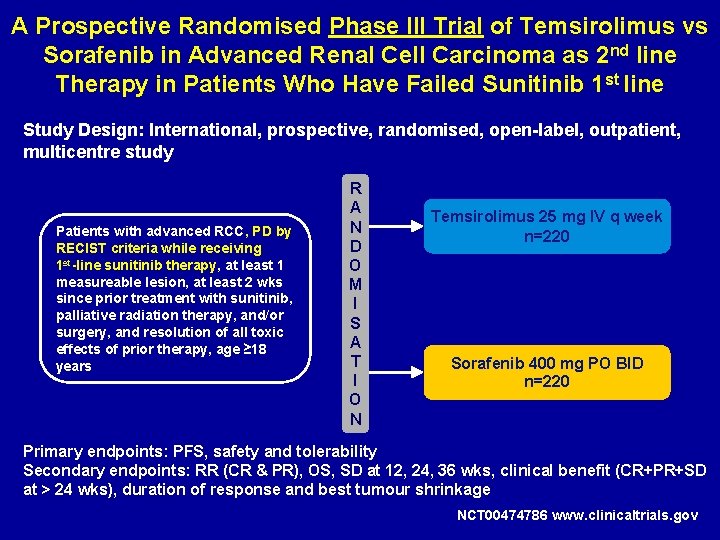
A Prospective Randomised Phase III Trial of Temsirolimus vs Sorafenib in Advanced Renal Cell Carcinoma as 2 nd line Therapy in Patients Who Have Failed Sunitinib 1 st line Study Design: International, prospective, randomised, open-label, outpatient, multicentre study Patients with advanced RCC, PD by RECIST criteria while receiving 1 st-line sunitinib therapy, at least 1 measureable lesion, at least 2 wks since prior treatment with sunitinib, palliative radiation therapy, and/or surgery, and resolution of all toxic effects of prior therapy, age ≥ 18 years R A N D O M I S A T I O N Temsirolimus 25 mg IV q week n=220 Sorafenib 400 mg PO BID n=220 Primary endpoints: PFS, safety and tolerability Secondary endpoints: RR (CR & PR), OS, SD at 12, 24, 36 wks, clinical benefit (CR+PR+SD at > 24 wks), duration of response and best tumour shrinkage NCT 00474786 www. clinicaltrials. gov

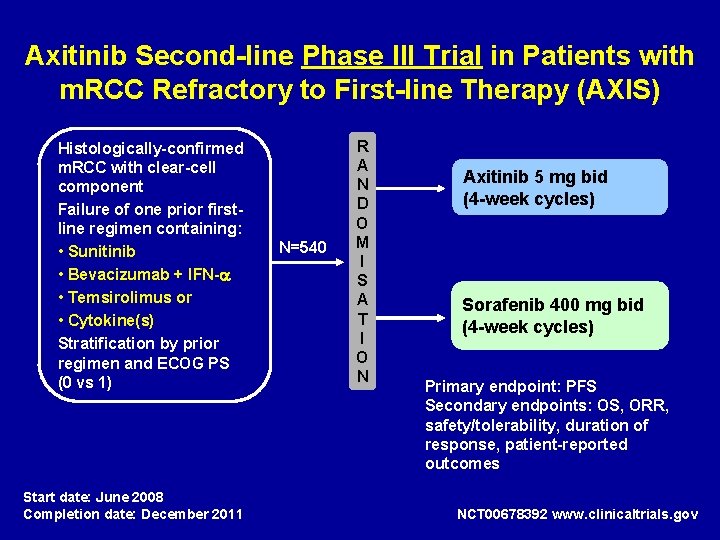
Axitinib Second-line Phase III Trial in Patients with m. RCC Refractory to First-line Therapy (AXIS) Histologically-confirmed m. RCC with clear-cell component Failure of one prior firstline regimen containing: • Sunitinib • Bevacizumab + IFN- • Temsirolimus or • Cytokine(s) Stratification by prior regimen and ECOG PS (0 vs 1) Start date: June 2008 Completion date: December 2011 N=540 R A N D O M I S A T I O N Axitinib 5 mg bid (4 -week cycles) Sorafenib 400 mg bid (4 -week cycles) Primary endpoint: PFS Secondary endpoints: OS, ORR, safety/tolerability, duration of response, patient-reported outcomes NCT 00678392 www. clinicaltrials. gov

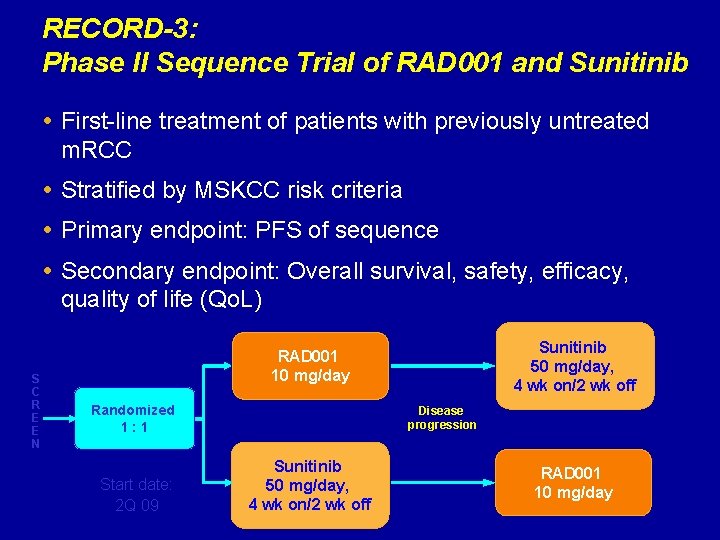
RECORD-3: Phase II Sequence Trial of RAD 001 and Sunitinib First-line treatment of patients with previously untreated m. RCC Stratified by MSKCC risk criteria Primary endpoint: PFS of sequence Secondary endpoint: Overall survival, safety, efficacy, quality of life (Qo. L) S C R E E N Sunitinib 50 mg/day, 4 wk on/2 wk off RAD 001 10 mg/day Randomized 1: 1 Start date: 2 Q 09 Disease progression Sunitinib 50 mg/day, 4 wk on/2 wk off RAD 001 10 mg/day

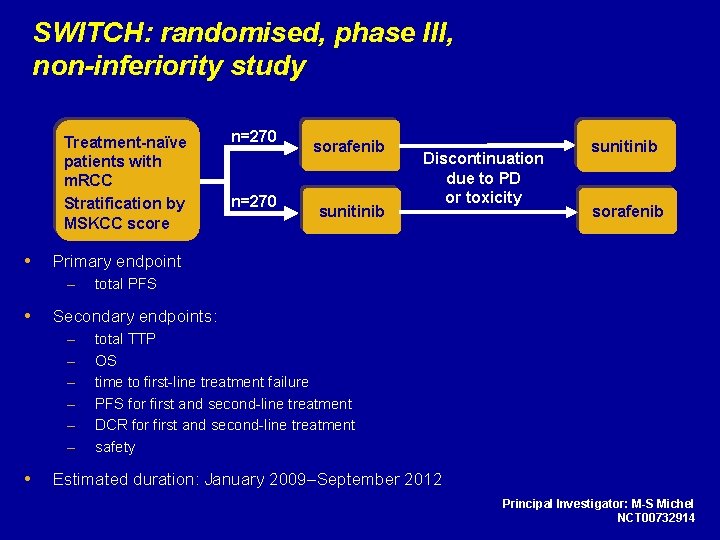
SWITCH: randomised, phase III, non-inferiority study Treatment-naïve patients with m. RCC Stratification by MSKCC score sunitinib Discontinuation due to PD or toxicity sunitinib sorafenib total PFS Secondary endpoints: – – – n=270 sorafenib Primary endpoint – n=270 total TTP OS time to first-line treatment failure PFS for first and second-line treatment DCR for first and second-line treatment safety Estimated duration: January 2009–September 2012 Principal Investigator: M-S Michel NCT 00732914

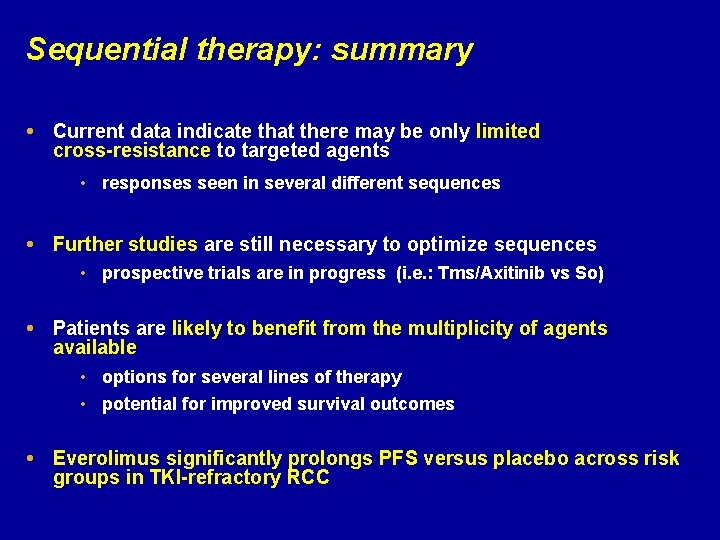
Sequential therapy: summary Current data indicate that there may be only limited cross-resistance to targeted agents • responses seen in several different sequences Further studies are still necessary to optimize sequences • prospective trials are in progress (i. e. : Tms/Axitinib vs So) Patients are likely to benefit from the multiplicity of agents available • options for several lines of therapy • potential for improved survival outcomes Everolimus significantly prolongs PFS versus placebo across risk groups in TKI-refractory RCC

Combination therapy

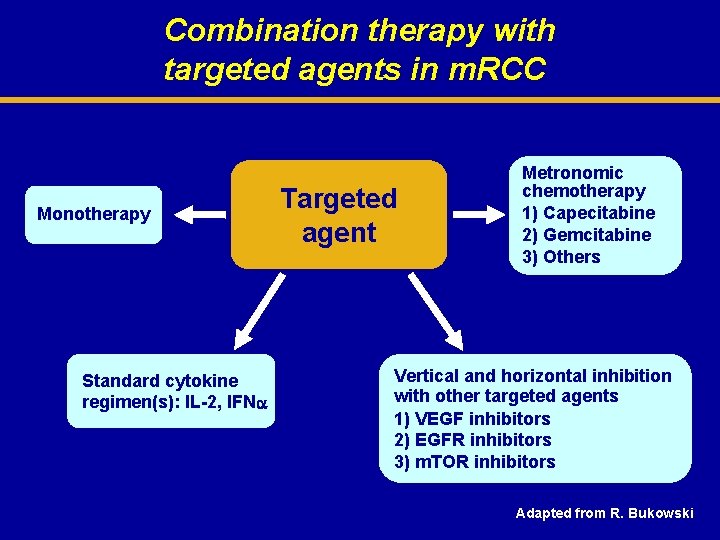
Combination therapy with targeted agents in m. RCC Monotherapy Standard cytokine regimen(s): IL-2, IFN Targeted agent Metronomic chemotherapy 1) Capecitabine 2) Gemcitabine 3) Others Vertical and horizontal inhibition with other targeted agents 1) VEGF inhibitors 2) EGFR inhibitors 3) m. TOR inhibitors Adapted from R. Bukowski

Combination with approved drugs in randomized trials • 4 important comparative (II/III) studies: – Be. ST study phase II (6 arm trial of Combination Targeted Therapy With Bevacizumab, Sorafenib and Temsirolimus) – TORAVA study phase II – IFN-Bev vs TEMS-Bev phase III – IFN-Bev vs EVER-Bev phase II

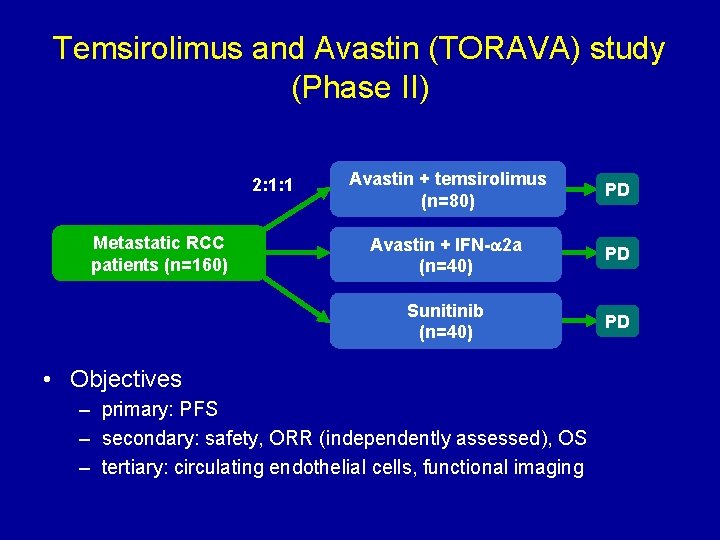
Temsirolimus and Avastin (TORAVA) study (Phase II) 2: 1: 1 Metastatic RCC patients (n=160) Avastin + temsirolimus (n=80) PD Avastin + IFN- 2 a (n=40) PD Sunitinib (n=40) PD • Objectives – primary: PFS – secondary: safety, ORR (independently assessed), OS – tertiary: circulating endothelial cells, functional imaging

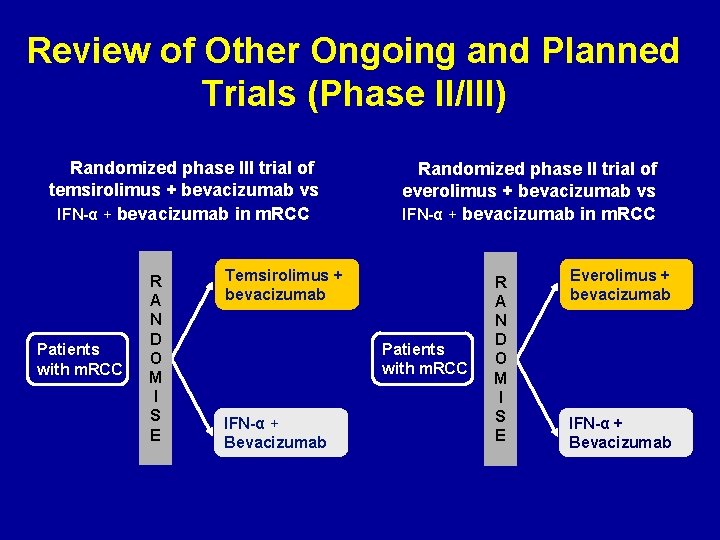
Review of Other Ongoing and Planned Trials (Phase II/III) Randomized phase III trial of temsirolimus + bevacizumab vs IFN-α + bevacizumab in m. RCC Patients with m. RCC R A N D O M I S E Randomized phase II trial of everolimus + bevacizumab vs IFN-α + bevacizumab in m. RCC Temsirolimus + bevacizumab Patients with m. RCC IFN-α + Bevacizumab R A N D O M I S E Everolimus + bevacizumab IFN-α + Bevacizumab

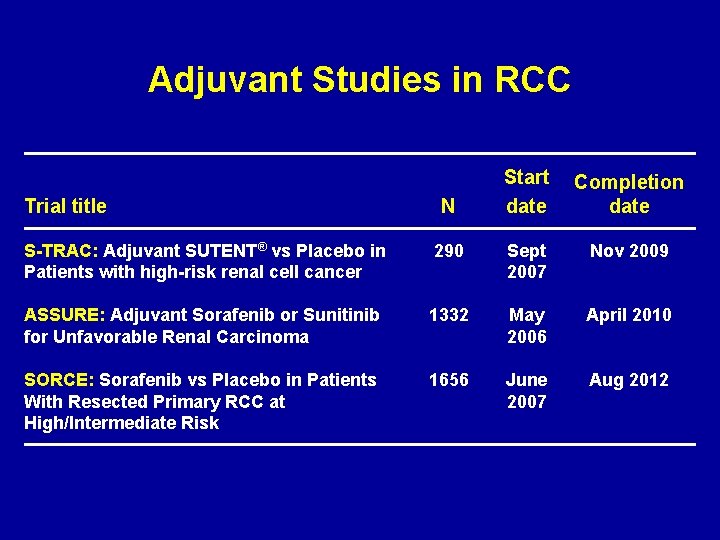
Adjuvant Studies in RCC Trial title N Start date Completion date S-TRAC: Adjuvant SUTENT® vs Placebo in Patients with high-risk renal cell cancer 290 Sept 2007 Nov 2009 ASSURE: Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma 1332 May 2006 April 2010 SORCE: Sorafenib vs Placebo in Patients With Resected Primary RCC at High/Intermediate Risk 1656 June 2007 Aug 2012

Any other News at ASCO 2009 in RCC? • Combinations of Targeted Agents – Sunitinib + everolimus (VEGF + m. TOR) • • Kroog GS Abs#5037 Phase I 20 mg RAD 001 weekly + sunitinib 37. 5 mg 4 w on 2 w off 3/5 pts PR, responses both in clear cell and other histologies – bevacizumab + temsirolimus (VEGF + m. TOR) • Merchan JR Abs#5039 • Phase II in RTKI refractory pts • N=35 pts RR 16% – sorafenib/gem/capecitabine (VEGF + chemo) • Bellmunt J Abs#5040 • Phase II 1 st line • n=40 pts RR 47% PFS 10. 2 m

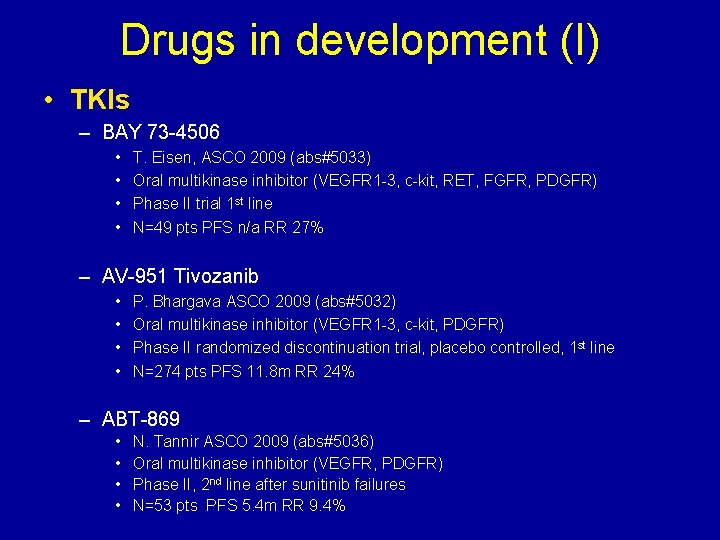
Drugs in development (I) • TKIs – BAY 73 -4506 • • T. Eisen, ASCO 2009 (abs#5033) Oral multikinase inhibitor (VEGFR 1 -3, c-kit, RET, FGFR, PDGFR) Phase II trial 1 st line N=49 pts PFS n/a RR 27% – AV-951 Tivozanib • • P. Bhargava ASCO 2009 (abs#5032) Oral multikinase inhibitor (VEGFR 1 -3, c-kit, PDGFR) Phase II randomized discontinuation trial, placebo controlled, 1 st line N=274 pts PFS 11. 8 m RR 24% – ABT-869 • • N. Tannir ASCO 2009 (abs#5036) Oral multikinase inhibitor (VEGFR, PDGFR) Phase II, 2 nd line after sunitinib failures N=53 pts PFS 5. 4 m RR 9. 4%

Drugs in development (II) • Other targeted agents: – Integrin inhibitors: • Volociximab (M 200) – c MET inhibitors • AMG 102 • XL 880 • ARQ 197 – Akt / MAPK / JNK pathway inhibitors • Perifosine – NJ Vogelzang, ASCO 2009 abs#5034 – Phase II 2 nd line after VEGFR inhib or m. TOR inhib – N=46 pts PFS 15 w RR 5%

Conclusions • RCC is a very active area for clinical trials • Many drugs have been approved recently, and many more drugs are in development • There is an urgent need for better understanding mechanisms of resistance and synergy to better develop current and future drugs
 Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Teoria do nefron intacto
Teoria do nefron intacto Semestre avanzado universidad de cundinamarca
Semestre avanzado universidad de cundinamarca Discipulado avanzado para líderes
Discipulado avanzado para líderes Ajax avanzado
Ajax avanzado Cncer
Cncer Investigacionales
Investigacionales Cortical and juxtamedullary nephrons difference
Cortical and juxtamedullary nephrons difference Anne eid
Anne eid Emetropia
Emetropia Sed visin
Sed visin Sade visin
Sade visin Visiones de mundo en literatura
Visiones de mundo en literatura Tratamiento al final del tubo
Tratamiento al final del tubo Fases del tratamiento periodontal
Fases del tratamiento periodontal Nicho ecologico definicion
Nicho ecologico definicion Definición de actividad
Definición de actividad Módulo 23 prepa en línea semana 1
Módulo 23 prepa en línea semana 1 Material potencialmente significativo
Material potencialmente significativo Reconciliación integradora ejemplos
Reconciliación integradora ejemplos Modulo 22 semana 1 fase 2
Modulo 22 semana 1 fase 2 Perspectiva integradora
Perspectiva integradora Fase 6: control. medir y corregir
Fase 6: control. medir y corregir Módulo 23 semana 3 fase 6: control medir y corregir
Módulo 23 semana 3 fase 6: control medir y corregir Fase 6 control. medir y corregir
Fase 6 control. medir y corregir Dirección
Dirección Actividad integradora fase 3 módulo 23 semana 2
Actividad integradora fase 3 módulo 23 semana 2 Actividad integradora fase 6: control. medir y corregir
Actividad integradora fase 6: control. medir y corregir Actividad integradora 6. encuesta para mi comunidad
Actividad integradora 6. encuesta para mi comunidad Sifilis congenita radiografia de huesos largos
Sifilis congenita radiografia de huesos largos Organos diana en emergencia hipertensiva
Organos diana en emergencia hipertensiva Relaciones anatomicas de la glandula tiroides
Relaciones anatomicas de la glandula tiroides Tromboxitopenia
Tromboxitopenia Tuberculosis patogenia
Tuberculosis patogenia Diagnostico diferencial tvp
Diagnostico diferencial tvp Bronquiectasias tratamiento
Bronquiectasias tratamiento Hiperkalemia
Hiperkalemia Hollín en la garganta
Hollín en la garganta Disalimentacion
Disalimentacion Tratamiento primario acortado de la tuberculosis
Tratamiento primario acortado de la tuberculosis Rickettsias y clamidias
Rickettsias y clamidias Actividad electrica sin pulso
Actividad electrica sin pulso Nadrenalina
Nadrenalina Clasificacion de quemaduras por superficie corporal
Clasificacion de quemaduras por superficie corporal Secuencia de intubacion
Secuencia de intubacion