Tratamiento del carcinoma de ce rvix avanzado Do










- Slides: 49

Tratamiento del carcinoma de ce rvix avanzado. ¿Do nde estamos y hacia do nde vamos? Ana Oaknin, MD Ph. D Head of Gynecologic Cancer Program. Vall d´Hebron Institute of Oncology(VHIO). Hospital Universitario Vall d´Hebron Vice-Chairman GEICO Group Barcelona, Spain #SEOM 2018

My Disclosures I have served on advisory boards for Roche, Astra. Zeneca, Pharma. Mar, Clovis Oncology, and Tesaro and received support for travel and/or accommodation from Roche, Astra. Zeneca, Clovis Oncology and Pharma. Mar. 2 #SEOM 2018

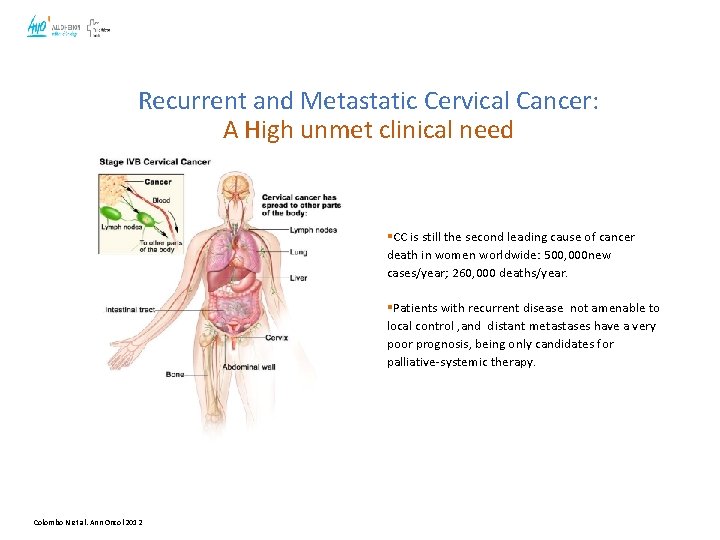
Recurrent and Metastatic Cervical Cancer: A High unmet clinical need §CC is still the second leading cause of cancer death in women worldwide: 500, 000 new cases/year; 260, 000 deaths/year. §Patients with recurrent disease not amenable to local control , and distant metastases have a very poor prognosis, being only candidates for palliative-systemic therapy. Colombo N et al. Ann Oncol 2012

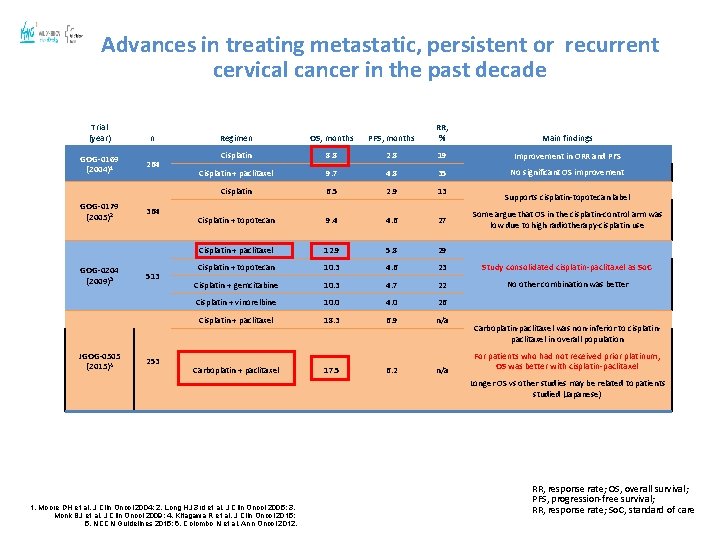
Advances in treating metastatic, persistent or recurrent cervical cancer in the past decade Trial (year) GOG-0169 (2004)1 GOG-0179 (2005)2 GOG-0204 (2009)3 JGOG-0505 (2015)4 n 264 364 513 253 Regimen OS, months PFS, months RR, % Main findings Cisplatin 8. 8 2. 8 19 Improvement in ORR and PFS Cisplatin + paclitaxel 9. 7 4. 8 35 No significant OS improvement Cisplatin 6. 5 2. 9 13 Cisplatin + topotecan 9. 4 4. 6 27 Cisplatin + paclitaxel 12. 9 5. 8 29 Cisplatin + topotecan 10. 3 4. 6 23 Study consolidated cisplatin-paclitaxel as So. C Cisplatin + gemcitabine 10. 3 4. 7 22 No other combination was better Cisplatin + vinorelbine 10. 0 4. 0 26 Cisplatin + paclitaxel 18. 3 6. 9 n/a Carboplatin + paclitaxel 17. 5 6. 2 n/a Supports cisplatin-topotecan label Some argue that OS in the cisplatin-control arm was low due to high radiotherapy-cisplatin use Carboplatin-paclitaxel was non-inferior to cisplatinpaclitaxel in overall population For patients who had not received prior platinum, OS was better with cisplatin-paclitaxel Longer OS vs other studies may be related to patients studied (Japanese) 1. Moore DH et al. J Clin Oncol 2004; 2. Long HJ 3 rd et al. J Clin Oncol 2005; 3. Monk BJ et al. J Clin Oncol 2009; 4. Kitagawa R et al. J Clin Oncol 2015; 5. NCCN Guidelines 2015; 6. Colombo N et al. Ann Oncol 2012. RR, response rate; OS, overall survival; PFS, progression-free survival; RR, response rate; So. C, standard of care

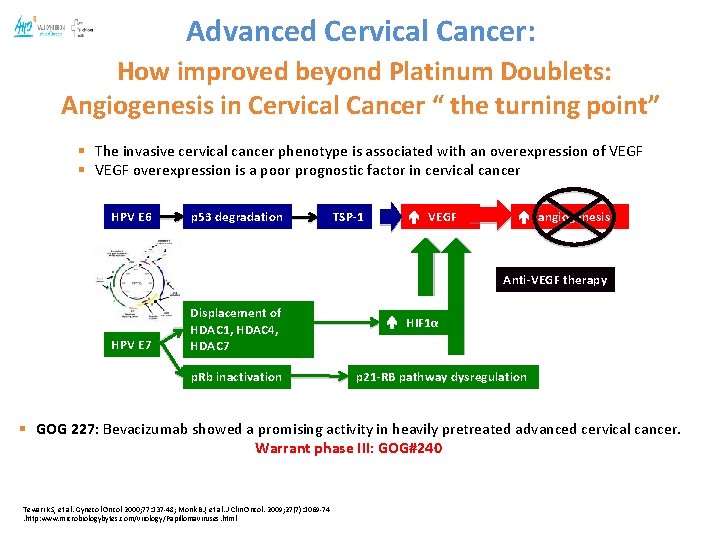
Advanced Cervical Cancer: How improved beyond Platinum Doublets: Angiogenesis in Cervical Cancer “ the turning point” § The invasive cervical cancer phenotype is associated with an overexpression of VEGF § VEGF overexpression is a poor prognostic factor in cervical cancer HPV E 6 p 53 degradation TSP-1 VEGF angiogenesis Anti-VEGF therapy HPV E 7 Displacement of HDAC 1, HDAC 4, HDAC 7 p. Rb inactivation HIF 1α p 21 -RB pathway dysregulation § GOG 227: Bevacizumab showed a promising activity in heavily pretreated advanced cervical cancer. Warrant phase III: GOG#240 Tewari KS, et al. Gynecol Oncol 2000; 77: 137 -48; Monk BJ, et al. J Clin Oncol. 2009; 27(7): 1069 -74. http: www. microbiologybytes. com/virology/Papillomaviruses. html

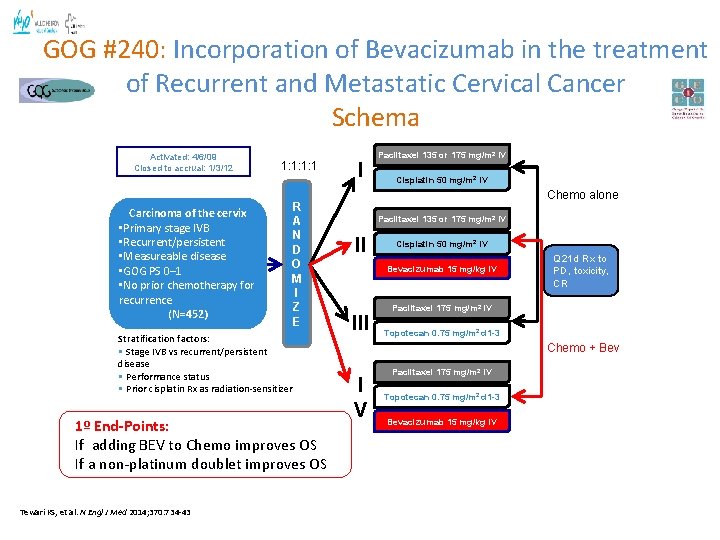
GOG #240: Incorporation of Bevacizumab in the treatment of Recurrent and Metastatic Cervical Cancer Schema Activated: 4/6/09 Closed to accrual: 1/3/12 Carcinoma of the cervix • Primary stage IVB • Recurrent/persistent • Measureable disease • GOG PS 0– 1 • No prior chemotherapy for recurrence (N=452) 1: 1: 1: 1 R A N D O M I Z E Stratification factors: • Stage IVB vs recurrent/persistent disease • Performance status • Prior cisplatin Rx as radiation-sensitizer 1º End-Points: If adding BEV to Chemo improves OS If a non-platinum doublet improves OS Tewari KS, et al. N Engl J Med 2014; 370: 734 -43 I Paclitaxel 135 or 175 mg/m 2 IV Cisplatin 50 mg/m 2 IV Chemo alone Paclitaxel 135 or 175 mg/m 2 IV II Cisplatin 50 mg/m 2 IV Bevacizumab 15 mg/kg IV III Q 21 d Rx to PD, toxicity, CR Paclitaxel 175 mg/m 2 IV Topotecan 0. 75 mg/m 2 d 1 -3 Chemo + Bev I V Paclitaxel 175 mg/m 2 IV Topotecan 0. 75 mg/m 2 d 1 -3 Bevacizumab 15 mg/kg IV

Tewari KS, et al. N Engl J Med 2014; 370: 734 -43 Tewari KS, et al. Lancet. 2017; 390(10103): 1654 -1663.

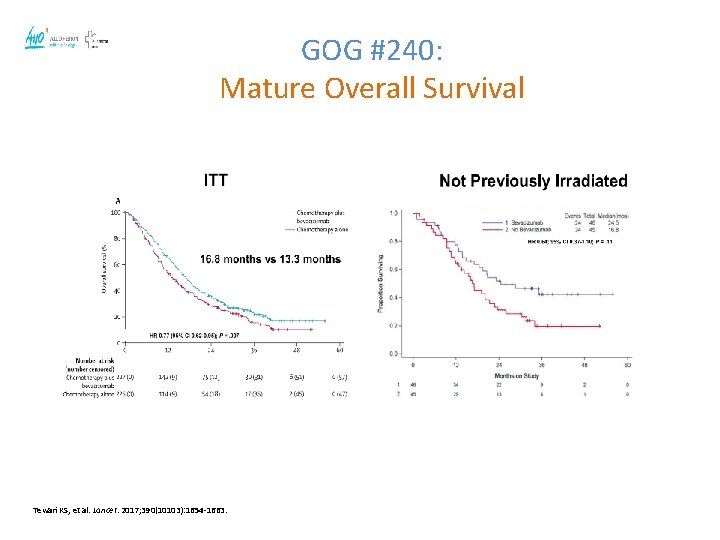
GOG #240: Mature Overall Survival Tewari KS, et al. Lancet. 2017; 390(10103): 1654 -1663.

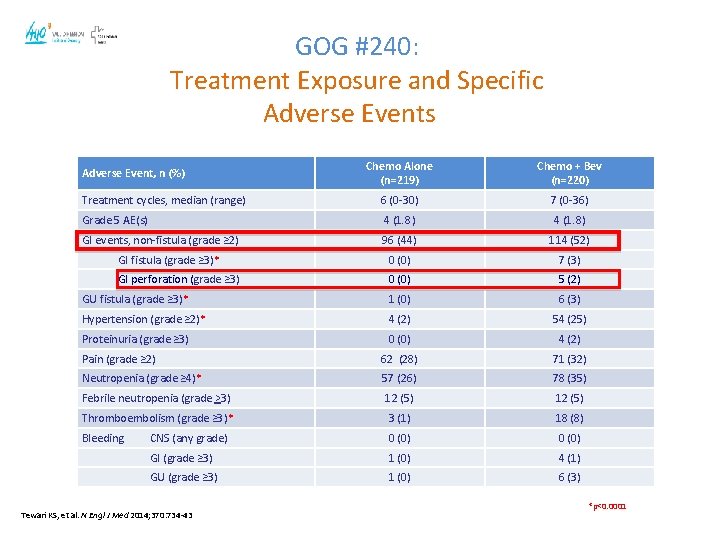
GOG #240: Treatment Exposure and Specific Adverse Events Chemo Alone (n=219) Chemo + Bev (n=220) Treatment cycles, median (range) 6 (0 -30) 7 (0 -36) Grade 5 AE(s) 4 (1. 8) GI events, non-fistula (grade ≥ 2) 96 (44) 114 (52) GI fistula (grade ≥ 3)* 0 (0) 7 (3) GI perforation (grade ≥ 3) 0 (0) 5 (2) GU fistula (grade ≥ 3)* 1 (0) 6 (3) Hypertension (grade ≥ 2)* 4 (2) 54 (25) Proteinuria (grade ≥ 3) 0 (0) 4 (2) Pain (grade ≥ 2) 62 (28) 71 (32) Neutropenia (grade ≥ 4)* 57 (26) 78 (35) Febrile neutropenia (grade >3) 12 (5) Thromboembolism (grade ≥ 3)* 3 (1) 18 (8) Bleeding CNS (any grade) 0 (0) GI (grade ≥ 3) 1 (0) 4 (1) GU (grade ≥ 3) 1 (0) 6 (3) Adverse Event, n (%) Tewari KS, et al. N Engl J Med 2014; 370: 734 -43 *p<0. 0001

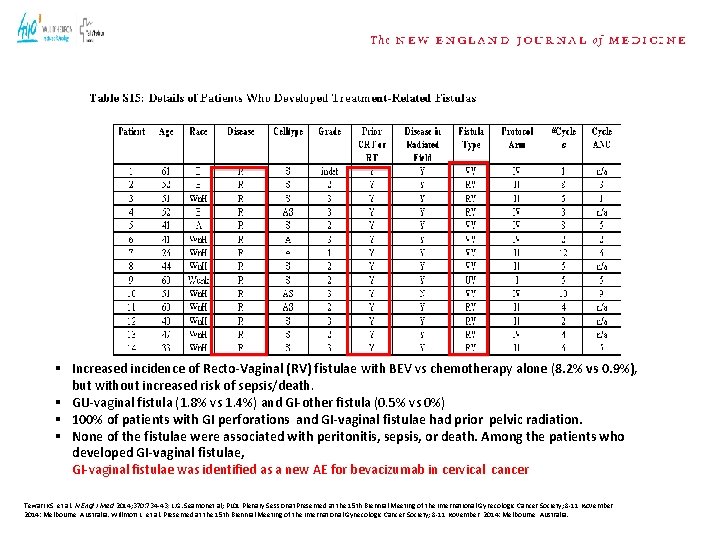
§ Increased incidence of Recto-Vaginal (RV) fistulae with BEV vs chemotherapy alone (8. 2% vs 0. 9%), but without increased risk of sepsis/death. § GU-vaginal fistula (1. 8% vs 1. 4%) and GI-other fistula (0. 5% vs 0%) § 100% of patients with GI perforations and GI-vaginal fistulae had prior pelvic radiation. § None of the fistulae were associated with peritonitis, sepsis, or death. Among the patients who developed GI-vaginal fistulae, GI-vaginal fistulae was identified as a new AE for bevacizumab in cervical cancer Tewari KS, et al. N Engl J Med 2014; 370: 734 -43; L. G. Seamonet al; PL 01 Plenary Sessionat. Presented at the 15 th Biennial Meeting of the International Gynecologic Cancer Society; 8 -11 November, 2014: Melbourne, Australia. Willmott L, et al. Presented at the 15 th Biennial Meeting of the International Gynecologic Cancer Society; 8 -11 November, 2014: Melbourne, Australia.

FDA News : For Immediate Release August 14, 2014 FDA approves Bevacizumab to treat patients with aggressive and late-stage cervical cancer Ref: Brussels, 31. 03. 2015 Auth. Number EU/1/04/300

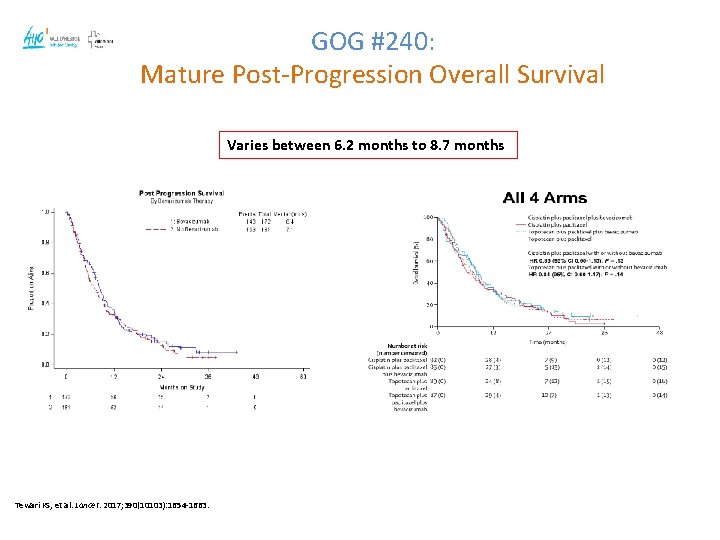
GOG #240: Mature Post-Progression Overall Survival Varies between 6. 2 months to 8. 7 months Tewari KS, et al. Lancet. 2017; 390(10103): 1654 -1663.

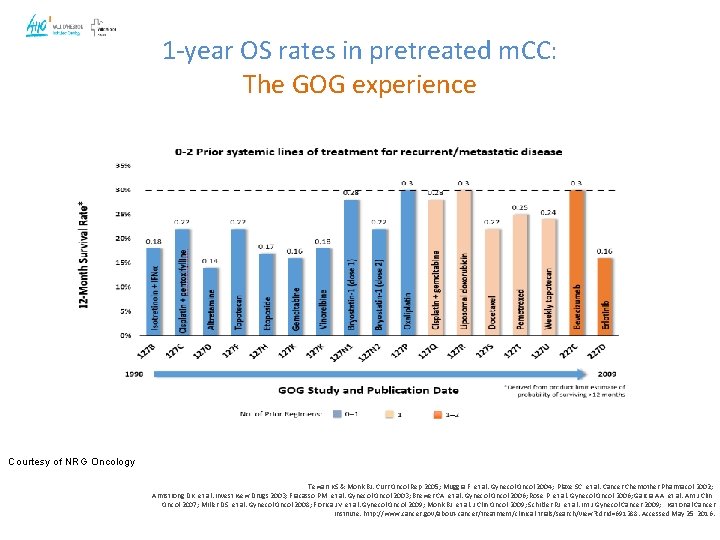
1 -year OS rates in pretreated m. CC: The GOG experience Courtesy of NRG Oncology Tewari KS & Monk BJ. Curr Oncol Rep 2005; Muggia F, et al. Gynecol Oncol 2004; Plaxe SC, et al. Cancer Chemother Pharmacol 2002; Armstrong DK, et al. Invest New Drugs 2003; Fracasso PM, et al. Gynecol Oncol 2003; Brewer CA, et al. Gynecol Oncol 2006; Rose P, et al. Gynecol Oncol 2006; Garcia AA, et al. Am J Clin Oncol 2007; Miller DS, et al. Gynecol Oncol 2008; Fiorica JV, et al. Gynecol Oncol 2009; Monk BJ, et al. J Clin Oncol 2009; Schilder RJ, et al. Int J Gynecol Cancer 2009; National Cancer Institute. http: //www. cancer. gov/about-cancer/treatment/clinical-trials/search/view? cdrid=691288. Accessed May 25, 2016.

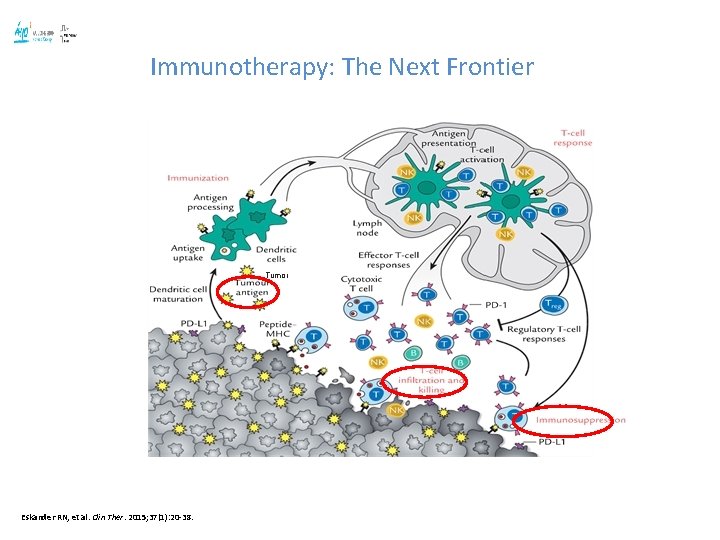
Immunotherapy: The Next Frontier Tumor Eskander RN, et al. Clin Ther. 2015; 37(1): 20 -38.

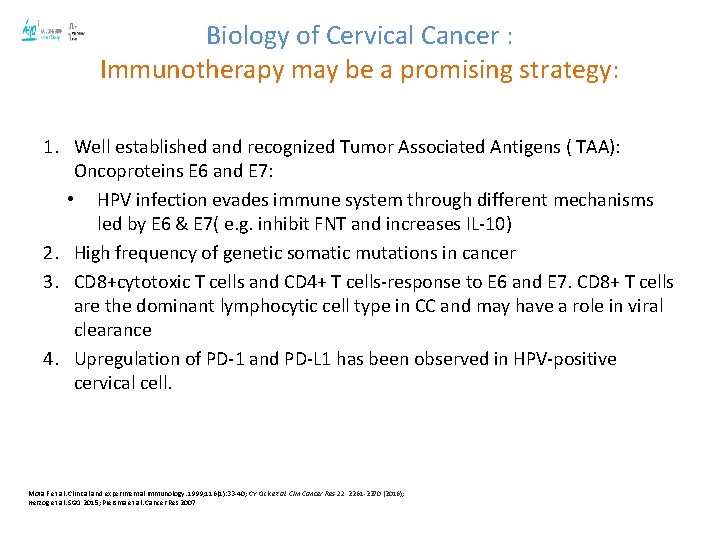
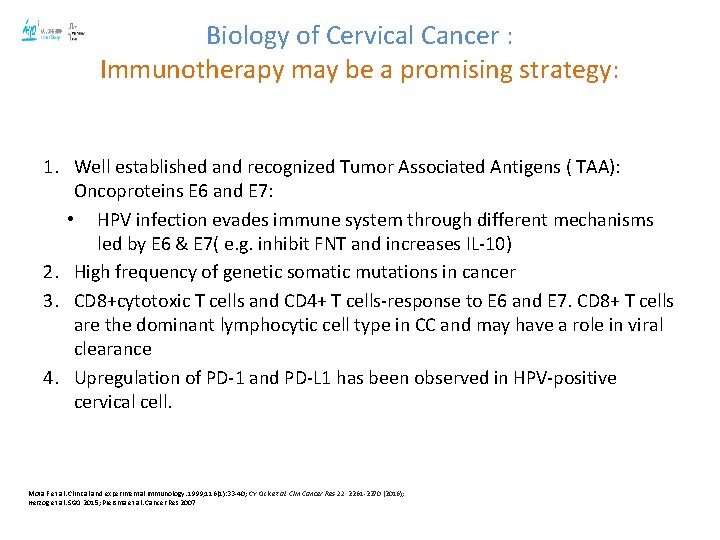
Biology of Cervical Cancer : Immunotherapy may be a promising strategy: 1. Well established and recognized Tumor Associated Antigens ( TAA): Oncoproteins E 6 and E 7: • HPV infection evades immune system through different mechanisms led by E 6 & E 7( e. g. inhibit FNT and increases IL-10) 2. High frequency of genetic somatic mutations in cancer 3. CD 8+cytotoxic T cells and CD 4+ T cells-response to E 6 and E 7. CD 8+ T cells are the dominant lymphocytic cell type in CC and may have a role in viral clearance 4. Upregulation of PD-1 and PD-L 1 has been observed in HPV-positive cervical cell. Mota F et al. Clinical and experimental immunology. 1999; 116(1): 33 -40; CY Ock et al. Clin Cancer Res 22, 2261 -2270 (2016); Herzog et al. SGO 2015; Piersma et al. Cancer Res 2007

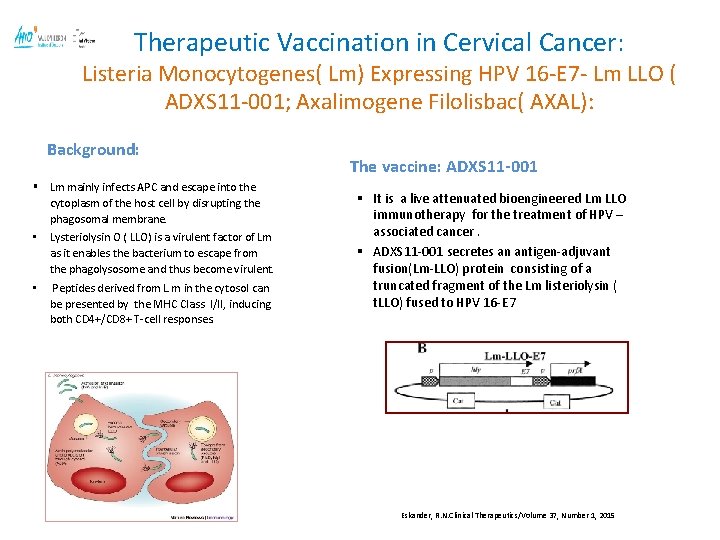
Therapeutic Vaccination in Cervical Cancer: Listeria Monocytogenes( Lm) Expressing HPV 16 -E 7 - Lm LLO ( ADXS 11 -001; Axalimogene Filolisbac( AXAL): Background: § Lm mainly infects APC and escape into the cytoplasm of the host cell by disrupting the phagosomal membrane. • Lysteriolysin O ( LLO) is a virulent factor of Lm as it enables the bacterium to escape from the phagolysosome and thus become virulent. • Peptides derived from L. m in the cytosol can be presented by the MHC Class I/II, inducing both CD 4+/CD 8+ T-cell responses. The vaccine: ADXS 11 -001 § It is a live attenuated bioengineered Lm LLO immunotherapy for the treatment of HPV – associated cancer. § ADXS 11 -001 secretes an antigen-adjuvant fusion(Lm-LLO) protein consisting of a truncated fragment of the Lm listeriolysin ( t. LLO) fused to HPV 16 -E 7 Eskander, R. N. Clinical Therapeutics/Volume 37, Number 1, 2015

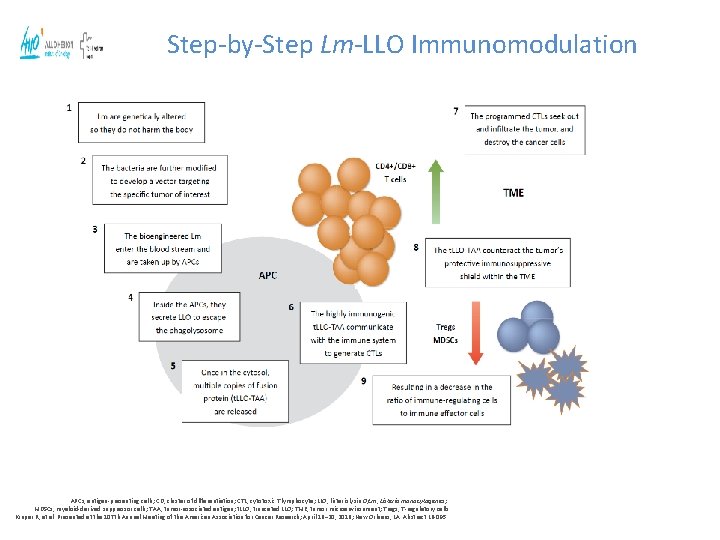
Step-by-Step Lm-LLO Immunomodulation APCs, antigen-presenting cells; CD, cluster of differentiation; CTL, cytotoxic T lymphocyte; LLO, listeriolysin O; Lm, Listeria monocytogenes; MDSCs, myeloid-derived suppressor cells; TAA, tumor-associated antigen; t. LLO, truncated LLO; TME, tumor microenvironment; Tregs, T-regulatory cells. Krupar R, et al. Presented at the 107 th Annual Meeting of the American Association for Cancer Research; April 16– 20, 2016; New Orleans, LA. Abstract LB-095.

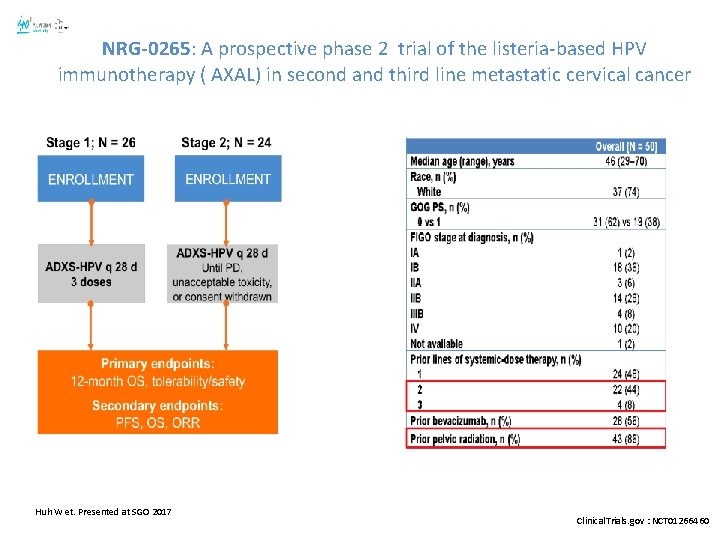
NRG-0265: A prospective phase 2 trial of the listeria-based HPV immunotherapy ( AXAL) in second and third line metastatic cervical cancer Huh W et. Presented at SGO 2017 Clinical. Trials. gov : NCT 01266460

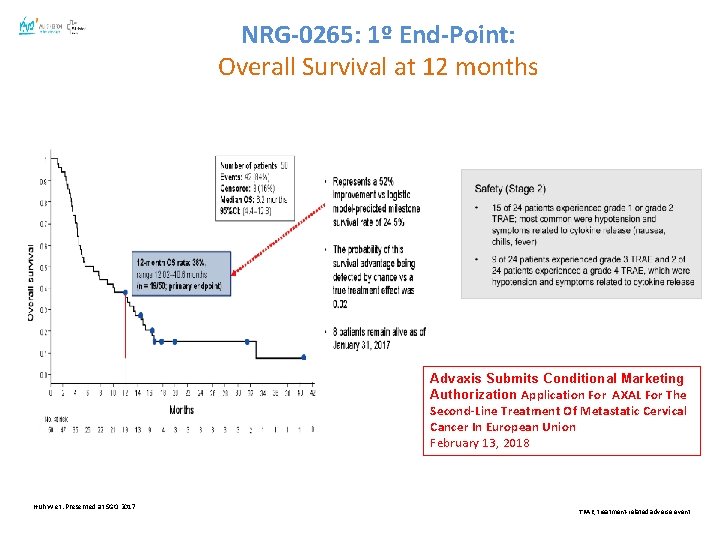
NRG-0265: 1º End-Point: Overall Survival at 12 months Advaxis Submits Conditional Marketing Authorization Application For AXAL For The Second-Line Treatment Of Metastatic Cervical Cancer In European Union February 13, 2018 Huh W et. Presented at SGO 2017 TRAE, treatment-related adverse event.

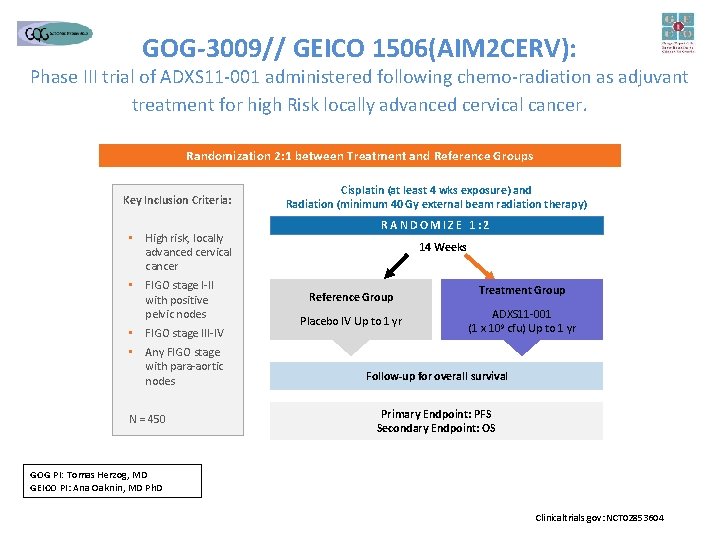
GOG-3009// GEICO 1506(AIM 2 CERV): Phase III trial of ADXS 11 -001 administered following chemo-radiation as adjuvant treatment for high Risk locally advanced cervical cancer. Randomization 2: 1 between Treatment and Reference Groups Key Inclusion Criteria: • High risk, locally advanced cervical cancer • FIGO stage I-II with positive pelvic nodes • FIGO stage III-IV • Any FIGO stage with para-aortic nodes N = 450 Cisplatin (at least 4 wks exposure) and Radiation (minimum 40 Gy external beam radiation therapy) RANDOMIZE 1: 2 14 Weeks Reference Group Placebo IV Up to 1 yr Treatment Group ADXS 11 -001 (1 x 109 cfu) Up to 1 yr Follow-up for overall survival Primary Endpoint: PFS Secondary Endpoint: OS GOG PI: Tomas Herzog, MD GEICO PI: Ana Oaknin, MD Ph. D Clinicaltrials. gov: NCT 02853604

Biology of Cervical Cancer : Immunotherapy may be a promising strategy: 1. Well established and recognized Tumor Associated Antigens ( TAA): Oncoproteins E 6 and E 7: • HPV infection evades immune system through different mechanisms led by E 6 & E 7( e. g. inhibit FNT and increases IL-10) 2. High frequency of genetic somatic mutations in cancer 3. CD 8+cytotoxic T cells and CD 4+ T cells-response to E 6 and E 7. CD 8+ T cells are the dominant lymphocytic cell type in CC and may have a role in viral clearance 4. Upregulation of PD-1 and PD-L 1 has been observed in HPV-positive cervical cell. Mota F et al. Clinical and experimental immunology. 1999; 116(1): 33 -40; CY Ock et al. Clin Cancer Res 22, 2261 -2270 (2016); Herzog et al. SGO 2015; Piersma et al. Cancer Res 2007

Presented By Christian Hinrichs at 2018 ASCO Annual Meeting

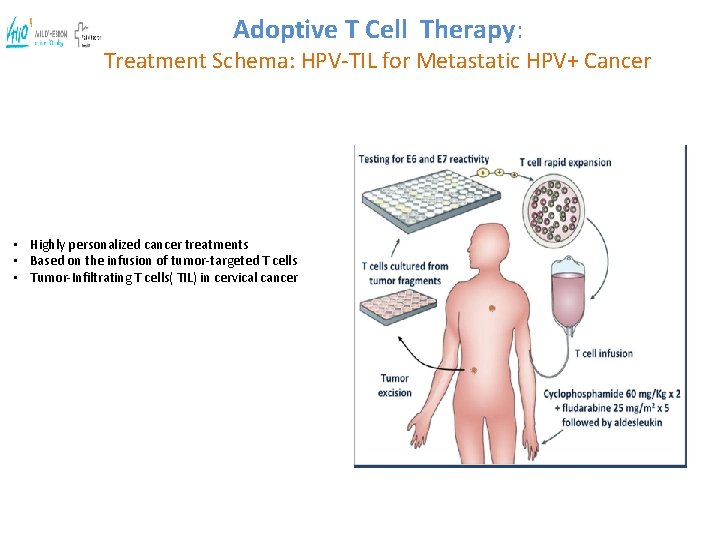
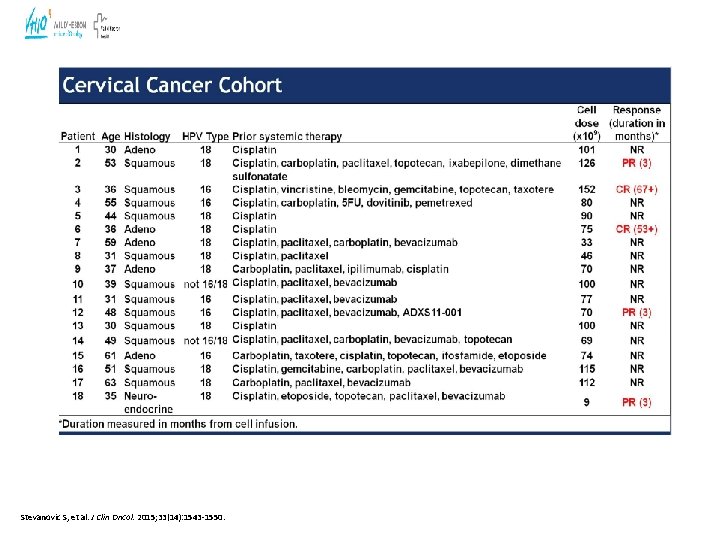
Adoptive T Cell Therapy: Treatment Schema: HPV-TIL for Metastatic HPV+ Cancer • Highly personalized cancer treatments • Based on the infusion of tumor-targeted T cells • Tumor-Infiltrating T cells( TIL) in cervical cancer

Stevanovic S, et al. J Clin Oncol. 2015; 33(14): 1543 -1550.

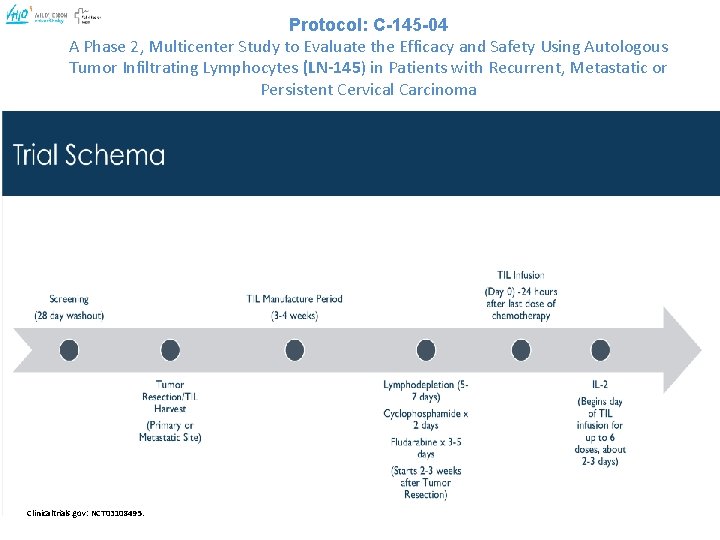
Protocol: C-145 -04 A Phase 2, Multicenter Study to Evaluate the Efficacy and Safety Using Autologous Tumor Infiltrating Lymphocytes (LN-145) in Patients with Recurrent, Metastatic or Persistent Cervical Carcinoma Clinicaltrials. gov: NCT 03108495.

Biology of Cervical Cancer : Immunotherapy may be a promising strategy: 1. Well established and recognized Tumor Associated Antigens ( TAA): Oncoproteins E 6 and E 7: • HPV infection evades immune system through different mechanisms led by E 6 & E 7( e. g. inhibit FNT and increases IL-10) 2. High frequency of genetic somatic mutations in cancer 3. CD 8+cytotoxic T cells and CD 4+ T cells-response to E 6 and E 7. CD 8+ T cells are the dominant lymphocytic cell type in CC and may have a role in viral clearance 4. Upregulation of PD-1 and PD-L 1 has been observed in HPV-positive cervical cell. Mota F et al. Clinical and experimental immunology. 1999; 116(1): 33 -40; CY Ock et al. Clin Cancer Res 22, 2261 -2270 (2016); Herzog et al. SGO 2015; Piersma et al. Cancer Res 2007

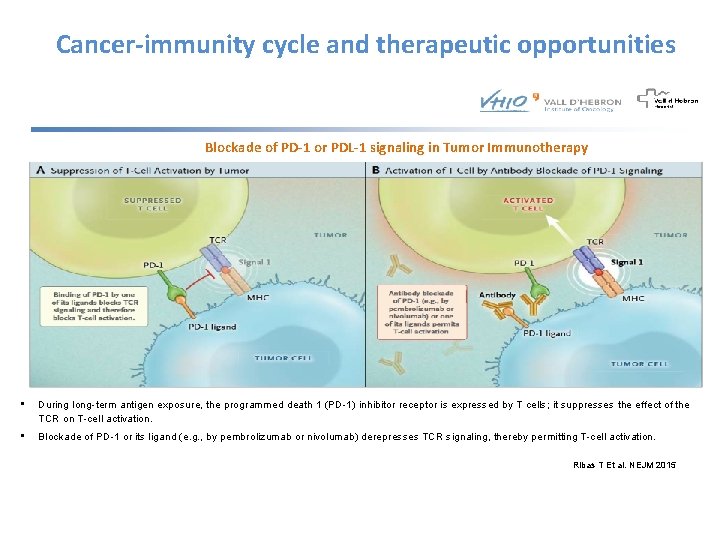
Cancer-immunity cycle and therapeutic opportunities Blockade of PD-1 or PDL-1 signaling in Tumor Immunotherapy • During long-term antigen exposure, the programmed death 1 (PD-1) inhibitor receptor is expressed by T cells; it suppresses the effect of the TCR on T-cell activation. • Blockade of PD-1 or its ligand (e. g. , by pembrolizumab or nivolumab) derepresses TCR signaling, thereby permitting T-cell activation. Ribas T Et al. NEJM 2015

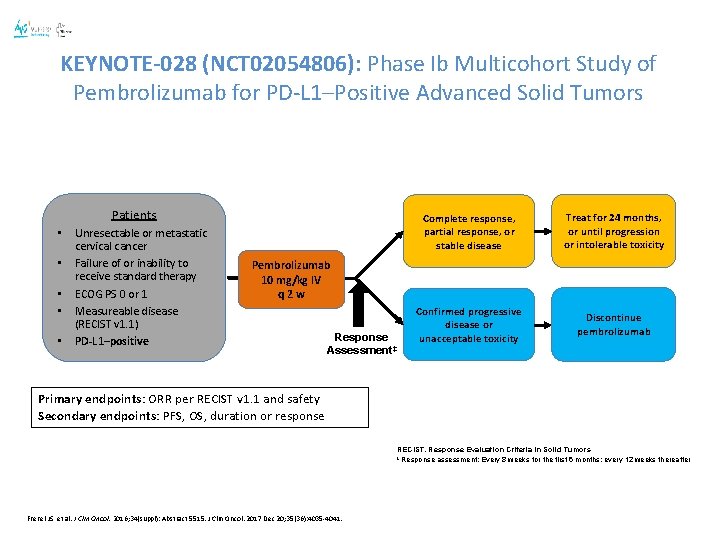
KEYNOTE-028 (NCT 02054806): Phase Ib Multicohort Study of Pembrolizumab for PD-L 1–Positive Advanced Solid Tumors Patients • • • Unresectable or metastatic cervical cancer Failure of or inability to receive standard therapy ECOG PS 0 or 1 Measureable disease (RECIST v 1. 1) PD-L 1–positive Complete response, partial response, or stable disease Treat for 24 months, or until progression or intolerable toxicity Confirmed progressive disease or unacceptable toxicity Discontinue pembrolizumab Pembrolizumab 10 mg/kg IV q 2 w Response Assessment‡ Primary endpoints: ORR per RECIST v 1. 1 and safety Secondary endpoints: PFS, OS, duration or response RECIST, Response Evaluation Criteria in Solid Tumors ‡ Response assessment: Every 8 weeks for the first 6 months; every 12 weeks thereafter Frenel JS, et al. J Clin Oncol. 2016; 34(suppl): Abstract 5515. J Clin Oncol. 2017 Dec 20; 35(36): 4035 -4041.

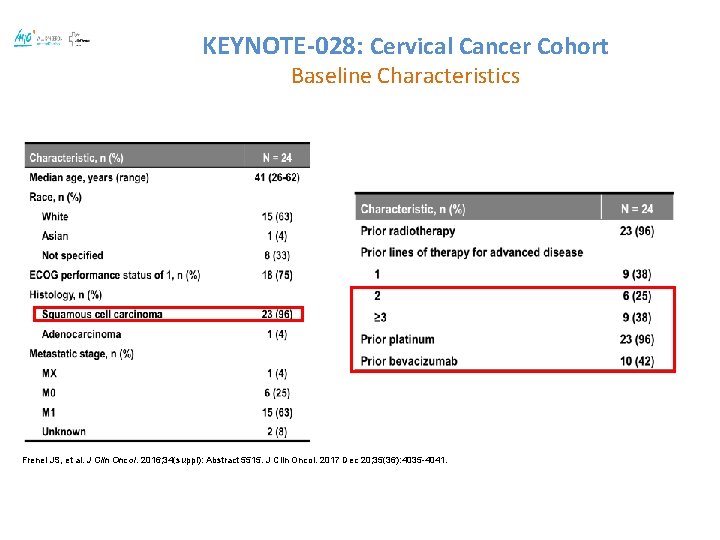
KEYNOTE-028: Cervical Cancer Cohort Baseline Characteristics Frenel JS, et al. J Clin Oncol. 2016; 34(suppl): Abstract 5515. J Clin Oncol. 2017 Dec 20; 35(36): 4035 -4041.

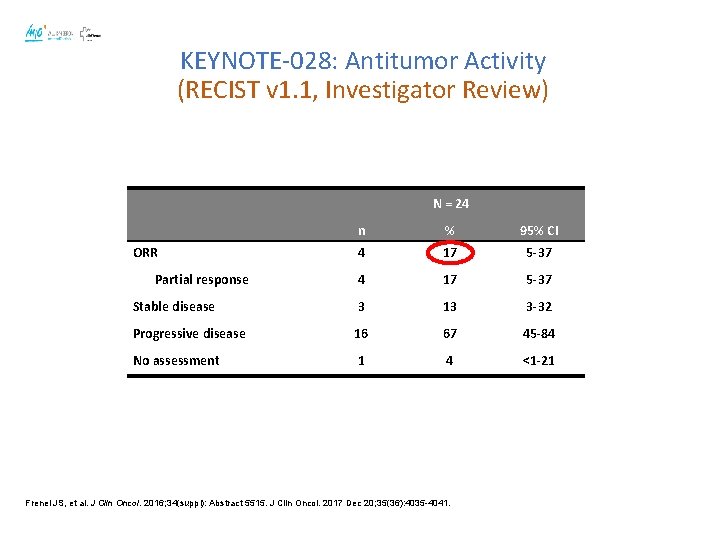
KEYNOTE-028: Antitumor Activity (RECIST v 1. 1, Investigator Review) N = 24 n % 95% CI 4 17 5 -37 Stable disease 3 13 3 -32 Progressive disease 16 67 45 -84 No assessment 1 4 <1 -21 ORR Partial response Frenel JS, et al. J Clin Oncol. 2016; 34(suppl): Abstract 5515. J Clin Oncol. 2017 Dec 20; 35(36): 4035 -4041.

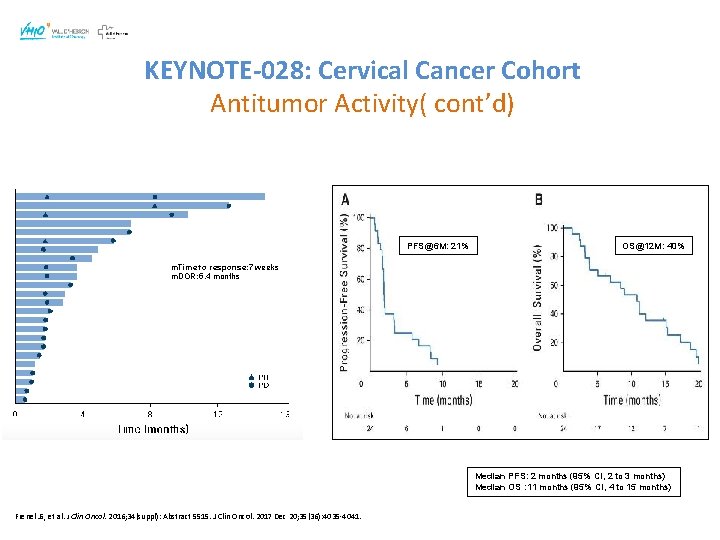
KEYNOTE-028: Cervical Cancer Cohort Antitumor Activity( cont’d) PFS@6 M: 21% OS@12 M: 40% m. Time to response: 7 weeks m. DOR: 5. 4 months Median PFS: 2 months (95% CI, 2 to 3 months) Median OS : 11 months (95% CI, 4 to 15 months) Frenel JS, et al. J Clin Oncol. 2016; 34(suppl): Abstract 5515. J Clin Oncol. 2017 Dec 20; 35(36): 4035 -4041.

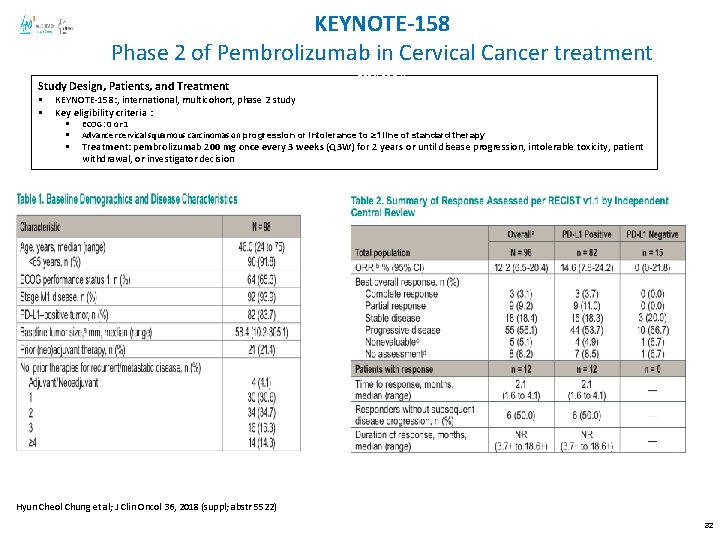
KEYNOTE-158 Phase 2 of Pembrolizumab in Cervical Cancer treatment Study Design, Patients, and Treatment § § abstr 5514) KEYNOTE-158: , international, multicohort, phase 2 study Key eligibility criteria : § § § ECOG: 0 or 1 Advancer cervical squamous carcinomas on progression or intolerance to ≥ 1 line of standard therapy Treatment: pembrolizumab 200 mg once every 3 weeks (Q 3 W) for 2 years or until disease progression, intolerable toxicity, patient withdrawal, or investigator decision Hyun Cheol Chung et al; J Clin Oncol 36, 2018 (suppl; abstr 5522) 32

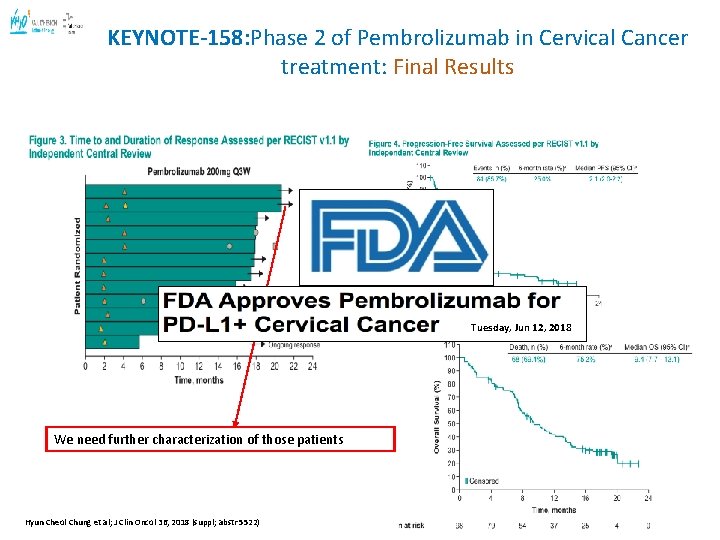
KEYNOTE-158: Phase 2 of Pembrolizumab in Cervical Cancer treatment: Final Results abstr 5514) Tuesday, Jun 12, 2018 We need further characterization of those patients Hyun Cheol Chung et al; J Clin Oncol 36, 2018 (suppl; abstr 5522) 33

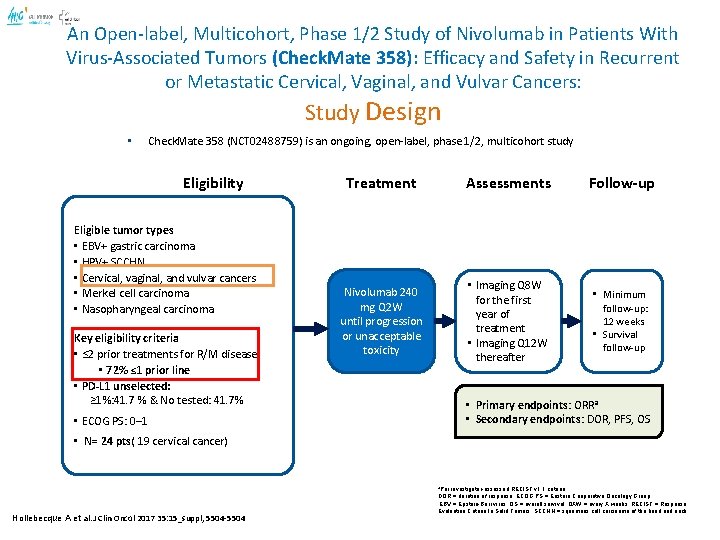
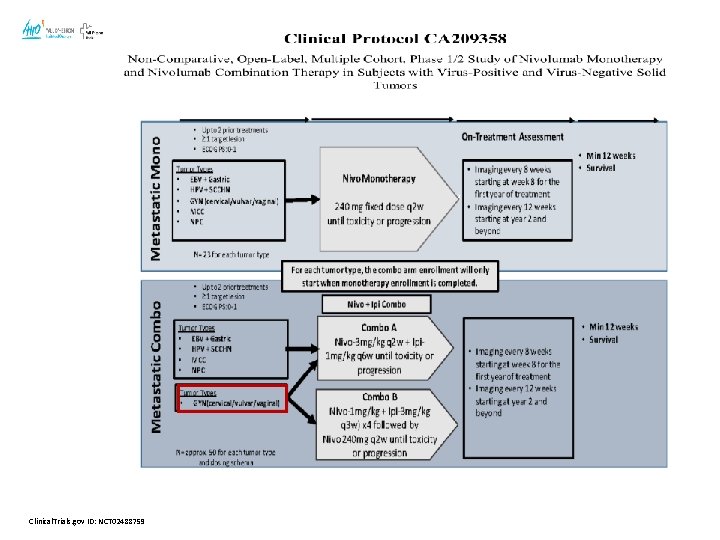
An Open-label, Multicohort, Phase 1/2 Study of Nivolumab in Patients With Virus-Associated Tumors (Check. Mate 358): Efficacy and Safety in Recurrent or Metastatic Cervical, Vaginal, and Vulvar Cancers: Study Design • Check. Mate 358 (NCT 02488759) is an ongoing, open-label, phase 1/2, multicohort study Eligibility Eligible tumor types • EBV+ gastric carcinoma • HPV+ SCCHN • Cervical, vaginal, and vulvar cancers • Merkel cell carcinoma • Nasopharyngeal carcinoma Key eligibility criteria • ≤ 2 prior treatments for R/M disease • 72% ≤ 1 prior line • PD-L 1 unselected: ≥ 1%: 41. 7 % & No tested: 41. 7% Treatment Assessments Follow-up Nivolumab 240 mg Q 2 W until progression or unacceptable toxicity • Imaging Q 8 W for the first year of treatment • Imaging Q 12 W thereafter • Minimum follow-up: 12 weeks • Survival follow-up • Primary endpoints: ORRa • Secondary endpoints: DOR, PFS, OS • ECOG PS: 0– 1 • N= 24 pts( 19 cervical cancer) a. Per Hollebecque A et al. J Clin Oncol 2017 35: 15_suppl, 5504 -5504 investigator-assessed RECIST v 1. 1 criteria DOR = duration of response; ECOG PS = Eastern Cooperative Oncology Group; EBV = Epstein-Barr virus; OS = overall survival; QXW = every X weeks; RECIST = Response Evaluation Criteria In Solid Tumors; SCCHN = squamous cell carcinoma of the head and neck

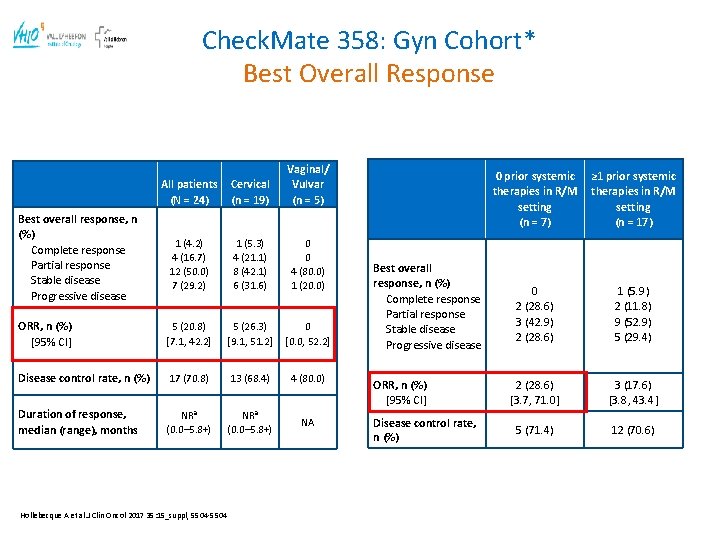
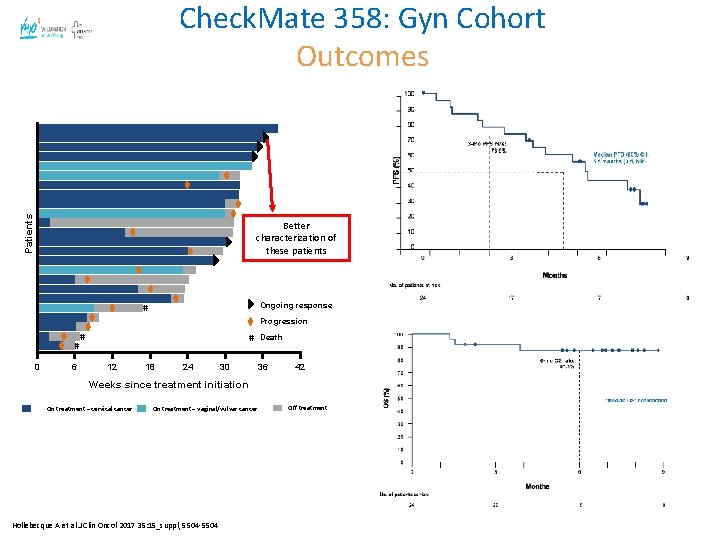
Check. Mate 358: Gyn Cohort* Best Overall Response All patients (N = 24) Cervical (n = 19) Vaginal/ Vulvar (n = 5) 1 (4. 2) 4 (16. 7) 12 (50. 0) 7 (29. 2) 1 (5. 3) 4 (21. 1) 8 (42. 1) 6 (31. 6) 0 0 4 (80. 0) 1 (20. 0) ORR, n (%) [95% CI] 5 (20. 8) [7. 1, 42. 2] 5 (26. 3) [9. 1, 51. 2] 0 [0. 0, 52. 2] Disease control rate, n (%) 17 (70. 8) 13 (68. 4) 4 (80. 0) Duration of response, median (range), months NRa (0. 0– 5. 8+) NA Best overall response, n (%) Complete response Partial response Stable disease Progressive disease Hollebecque A et al. J Clin Oncol 2017 35: 15_suppl, 5504 -5504 Best overall response, n (%) Complete response Partial response Stable disease Progressive disease ORR, n (%) [95% CI] Disease control rate, n (%) 0 prior systemic therapies in R/M setting (n = 7) ≥ 1 prior systemic therapies in R/M setting (n = 17) 0 2 (28. 6) 3 (42. 9) 2 (28. 6) 1 (5. 9) 2 (11. 8) 9 (52. 9) 5 (29. 4) 2 (28. 6) [3. 7, 71. 0] 3 (17. 6) [3. 8, 43. 4] 5 (71. 4) 12 (70. 6)

Patients Check. Mate 358: Gyn Cohort Outcomes Better characterization of these patients Ongoing response # Progression # 0 6 # # 12 18 24 30 Death 36 42 Weeks since treatment initiation On treatment – cervical cancer On treatment – vaginal/vulvar cancer Hollebecque A et al. J Clin Oncol 2017 35: 15_suppl, 5504 -5504 Off treatment

Clinical. Trials. gov ID: NCT 02488759

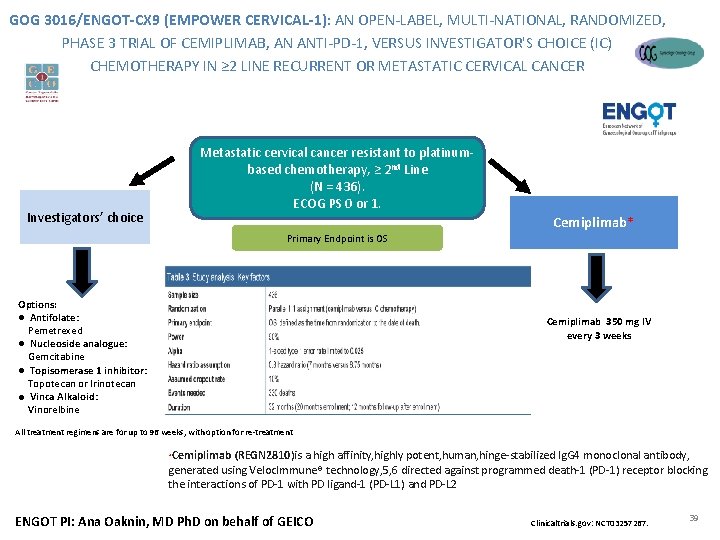
GOG 3016/ENGOT-CX 9 (EMPOWER CERVICAL-1): AN OPEN-LABEL, MULTI-NATIONAL, RANDOMIZED, PHASE 3 TRIAL OF CEMIPLIMAB, AN ANTI-PD-1, VERSUS INVESTIGATOR'S CHOICE (IC) CHEMOTHERAPY IN ≥ 2 LINE RECURRENT OR METASTATIC CERVICAL CANCER Metastatic cervical cancer resistant to platinumbased chemotherapy, ≥ 2 nd Line (N = 436). ECOG PS 0 or 1. Investigators’ choice Primary Endpoint is OS Options: ● Antifolate: Pemetrexed ● Nucleoside analogue: Gemcitabine ● Topisomerase 1 inhibitor: Topotecan or Irinotecan ● Vinca Alkaloid: Vinorelbine Cemiplimab* Cemiplimab 350 mg IV every 3 weeks All treatment regimens are for up to 96 weeks, with option for re-treatment Cemiplimab (REGN 2810)is a high affinity, highly potent, human, hinge-stabilized Ig. G 4 monoclonal antibody, Confidential generated using Veloc. Immune® technology, 5, 6 directed against programmed death-1 (PD-1) receptor blocking the interactions of PD-1 with PD ligand-1 (PD-L 1) and PD-L 2 * ENGOT PI: Ana Oaknin, MD Ph. D on behalf of GEICO Clinicaltrials. gov: NCT 03257267. 39

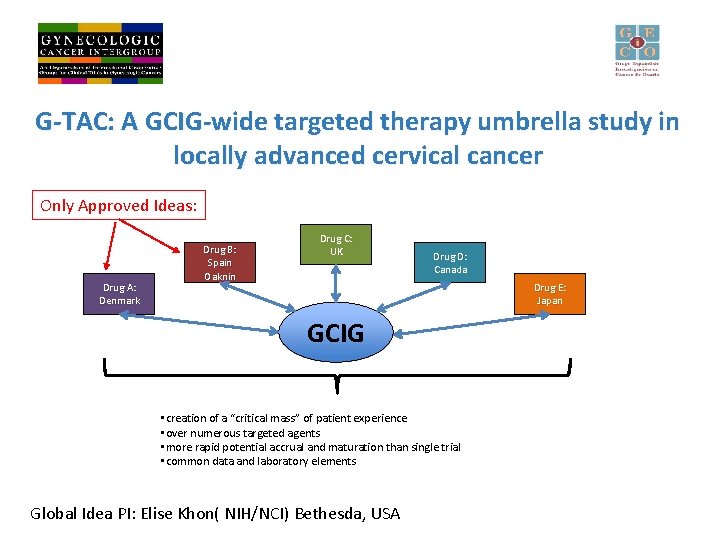
G-TAC: A GCIG-wide targeted therapy umbrella study in locally advanced cervical cancer Only Approved Ideas: Drug A: Denmark Drug B: Spain Oaknin Drug C: UK Drug D: Canada Drug E: Japan GCIG • creation of a “critical mass” of patient experience • over numerous targeted agents • more rapid potential accrual and maturation than single trial • common data and laboratory elements Global Idea PI: Elise Khon( NIH/NCI) Bethesda, USA

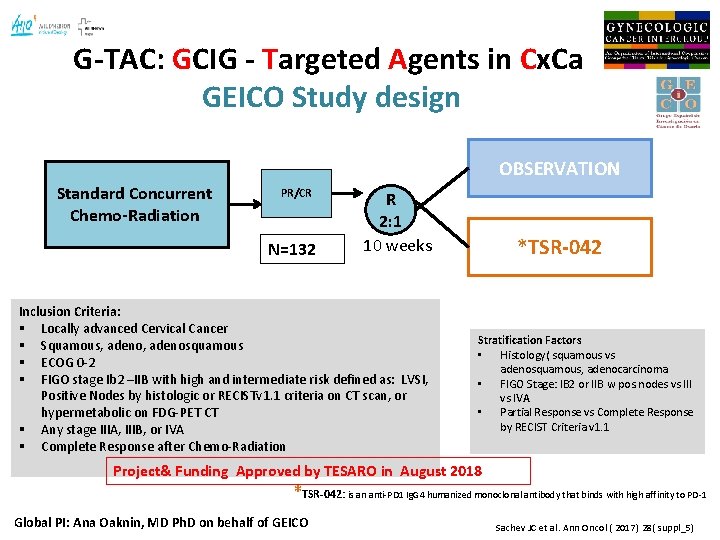
G-TAC: GCIG - Targeted Agents in Cx. Ca GEICO Study design OBSERVATION Standard Concurrent Chemo-Radiation PR/CR N=132 R 2: 1 10 weeks Inclusion Criteria: § Locally advanced Cervical Cancer § Squamous, adenosquamous § ECOG 0 -2 § FIGO stage Ib 2 –IIB with high and intermediate risk defined as: LVSI, Positive Nodes by histologic or RECISTv 1. 1 criteria on CT scan, or hypermetabolic on FDG-PET CT § Any stage IIIA, IIIB, or IVA § Complete Response after Chemo-Radiation *TSR-042 Stratification Factors • Histology( squamous vs adenosquamous, adenocarcinoma • FIGO Stage: IB 2 or IIB w pos. nodes vs III vs IVA • Partial Response vs Complete Response by RECIST Criteria v 1. 1 Project& Funding Approved by TESARO in August 2018 *TSR-042: is an anti-PD 1 Ig. G 4 humanized monoclonal antibody that binds with high affinity to PD-1 Global PI: Ana Oaknin, MD Ph. D on behalf of GEICO Sachev JC et al. Ann Oncol ( 2017) 28( suppl_5)

BEATcc: Protocol Overview Study Design This is a phase III, randomized, open-label, multi-center study to assess the efficacy of Atezolizumab administered concurrent to the combination of Cisplatin and Paclitaxel plus Bevacizumab in previously untreated patients with metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix. Global PI: Ana Oaknin, MD Ph. D on behalf of GEICO Version 1. 0, 6 Mar 2018 Clinical. Trials. gov Identifier: NCT 03556839

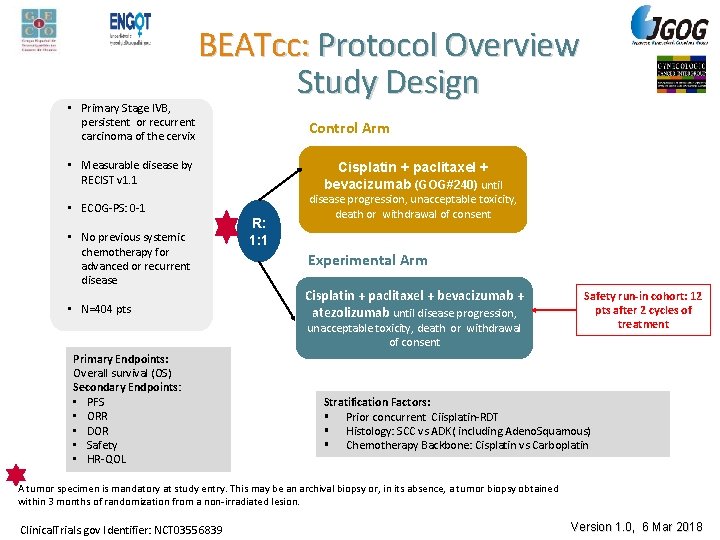
• Primary Stage IVB, persistent or recurrent carcinoma of the cervix BEATcc: Protocol Overview Study Design Control Arm • Measurable disease by RECIST v 1. 1 • ECOG-PS: 0 -1 • No previous systemic chemotherapy for advanced or recurrent disease • N=404 pts Cisplatin + paclitaxel + bevacizumab (GOG#240) until R: 1: 1 disease progression, unacceptable toxicity, death or withdrawal of consent Experimental Arm Cisplatin + paclitaxel + bevacizumab + atezolizumab until disease progression, unacceptable toxicity, death or withdrawal of consent Primary Endpoints: Overall survival (OS) Secondary Endpoints: • PFS • ORR • DOR • Safety • HR-QOL Safety run-in cohort: 12 pts after 2 cycles of treatment Stratification Factors: § Prior concurrent Ciisplatin-RDT § Histology: SCC vs ADK( including Adeno. Squamous) § Chemotherapy Backbone: Cisplatin vs Carboplatin A tumor specimen is mandatory at study entry. This may be an archival biopsy or, in its absence, a tumor biopsy obtained within 3 months of randomization from a non-irradiated lesion. Clinical. Trials. gov Identifier: NCT 03556839 Version 1. 0, 6 Mar 2018

Beyond Immunotherapy

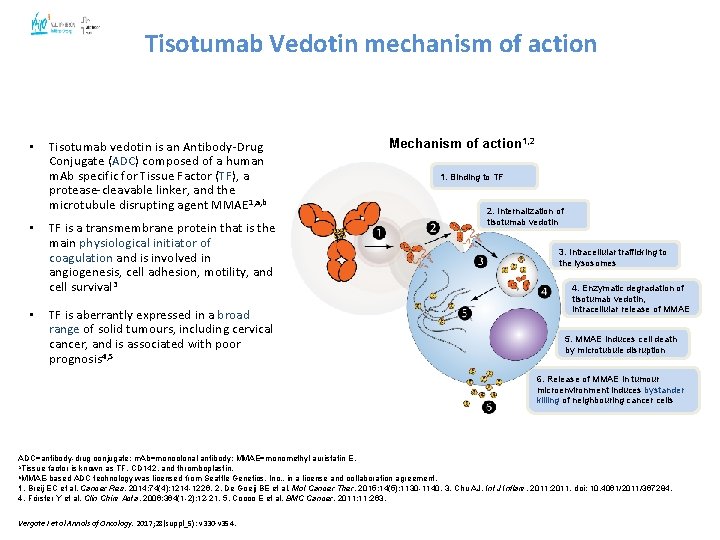
4 5 Tisotumab Vedotin mechanism of action • • • Tisotumab vedotin is an Antibody-Drug Conjugate (ADC) composed of a human m. Ab specific for Tissue Factor (TF), a protease-cleavable linker, and the microtubule disrupting agent MMAE 1, a, b TF is a transmembrane protein that is the main physiological initiator of coagulation and is involved in angiogenesis, cell adhesion, motility, and cell survival 3 TF is aberrantly expressed in a broad range of solid tumours, including cervical cancer, and is associated with poor prognosis 4, 5 Mechanism of action 1, 2 1. Binding to TF 2. Internalization of tisotumab vedotin 3. Intracellular trafficking to the lysosomes 4. Enzymatic degradation of tisotumab vedotin, intracellular release of MMAE 5. MMAE induces cell death by microtubule disruption 6. Release of MMAE in tumour microenvironment induces bystander killing of neighbouring cancer cells ADC=antibody-drug conjugate; m. Ab=monoclonal antibody; MMAE=monomethyl auristatin E. a. Tissue factor is known as TF, CD 142, and thromboplastin. b. MMAE-based ADC technology was licensed from Seattle Genetics, Inc. , in a license and collaboration agreement. 1. Breij EC et al. Cancer Res. 2014; 74(4): 1214 -1226. 2. De Goeij BE et al. Mol Cancer Ther. 2015; 14(5): 1130 -1140. 3. Chu AJ. Int J Inflam. 2011; 2011. doi: 10. 4061/2011/367284. 4. Förster Y et al. Clin Chim Acta. 2006; 364(1 -2): 12 -21. 5. Cocco E et al. BMC Cancer. 2011; 11: 263. . Vergote I et al Annals of Oncology. 2017; 28(suppl_5): v 330 -v 354

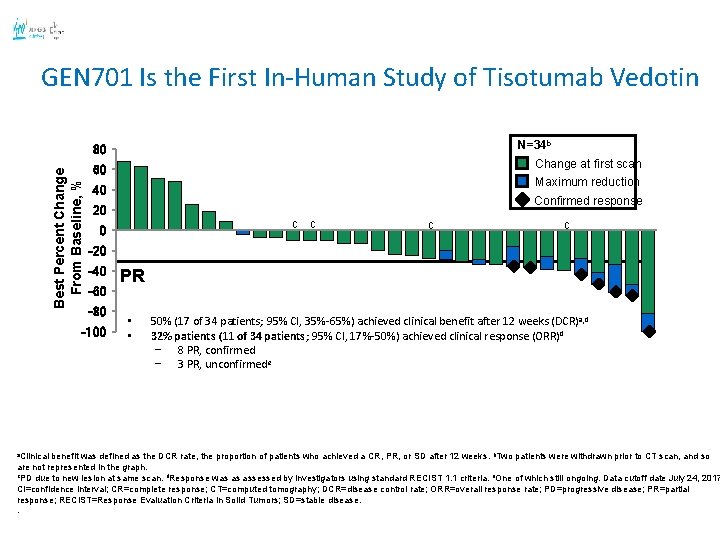
GEN 701 Is the First In-Human Study of Tisotumab Vedotin N=34 b Best Percent Change From Baseline, % 80 Maximum reduction 40 Confirmed response 20 c c c -20 -40 -60 -80 -100 a. Clinical Change at first scan 60 PR • • 50% (17 of 34 patients; 95% CI, 35%-65%) achieved clinical benefit after 12 weeks (DCR) a, d 32% patients (11 of 34 patients; 95% CI, 17%-50%) achieved clinical response (ORR)d − 8 PR, confirmed − 3 PR, unconfirmede 46 benefit was defined as the DCR rate, the proportion of patients who achieved a CR, PR, or SD after 12 weeks. b. Two patients were withdrawn prior to CT scan, and so are not represented in the graph. c. PD due to new lesion at same scan. d. Response was as assessed by investigators using standard RECIST 1. 1 criteria. e. One of which still ongoing. Data cutoff date July 24, 2017 CI=confidence interval; CR=complete response; CT=computed tomography; DCR=disease control rate; ORR=overall response rate; PD=progressive disease; PR=partial response; RECIST=Response Evaluation Criteria in Solid Tumors; SD=stable disease. .

ENGOT-cx 6/GCT 1015 -04: A Single arm, Multicenter, Phase 2 b Trial Investigating the Efficacy of Tisotumab Vedotin (Hu. Max®-TF-ADC) Therapy in Patients with Recurrent or Metastatic Cervical Cancer with Disease Progression after First Line Therapy"


Metastatic Cervical Cancer: Conclusions q. Metastatic Cervical Cancer is a devastating disease affecting young women. q. The incorporation of BEV as part of first line chemotherapy treatment improves the overall survival beyond 1 year( 17 months): A new Standard of Care: FDA& EMA Approved. q. Immunotherapy is our next step in Cervical Cancer: • Incorporating in 1 ST Line Regimen: BEATcc Trial • Pembrolizumab approved by FDA in 2 nd line in PDL-1 positive patients Clinical Research is a MUST

GRACIAS!!
 Semestre avanzado universidad de cundinamarca
Semestre avanzado universidad de cundinamarca Discipulado avanzado para líderes
Discipulado avanzado para líderes Ajax avanzado
Ajax avanzado Fin de tubo
Fin de tubo Fases del tratamiento periodontal
Fases del tratamiento periodontal Cervix carcinoma
Cervix carcinoma Renal cell carcinoma
Renal cell carcinoma Neuro derm
Neuro derm Neoplasia
Neoplasia Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Breast tnm staging
Breast tnm staging Carcinoma de mama
Carcinoma de mama Carcinoma in situ
Carcinoma in situ Carcinoma in situ
Carcinoma in situ Kode icd 10 oat
Kode icd 10 oat Wikipedia
Wikipedia Squamous cell carcinoma louisiana
Squamous cell carcinoma louisiana Follicular adenoma
Follicular adenoma Breast papillary carcinoma
Breast papillary carcinoma Hormone
Hormone Carcinoma micropapilar invasivo de mama
Carcinoma micropapilar invasivo de mama Epithelial component
Epithelial component Carcinoma of stomach
Carcinoma of stomach Carcinoma
Carcinoma Haggit evrelemesi
Haggit evrelemesi Nodular melanoma
Nodular melanoma Mucoepidermoid carcinoma pathology outlines
Mucoepidermoid carcinoma pathology outlines Venacavogram
Venacavogram Premalignant lesions of esophagus
Premalignant lesions of esophagus Carcinoma comedonico
Carcinoma comedonico Tumor carcinoide
Tumor carcinoide Squamous cell carcinoma
Squamous cell carcinoma Carcinoma on scalp
Carcinoma on scalp Carcinoma renal de células claras fuhrman
Carcinoma renal de células claras fuhrman Squamous cell carcinoma
Squamous cell carcinoma Tnm cáncer de pulmón 2021
Tnm cáncer de pulmón 2021 Carcinoma epidermoide microinfiltrante
Carcinoma epidermoide microinfiltrante Menopausa precoce sintomi iniziali
Menopausa precoce sintomi iniziali Basal cell carcinoma
Basal cell carcinoma Serosan
Serosan Pleomorphic adenomas
Pleomorphic adenomas Carcinoma squamoso polmone
Carcinoma squamoso polmone Carcinoma squamoso polmone
Carcinoma squamoso polmone Dr. aldo azael garza galindo
Dr. aldo azael garza galindo Thyroid cancer
Thyroid cancer Endocirne glands
Endocirne glands Plasmocitoma
Plasmocitoma Stdis
Stdis Hedgehog mites
Hedgehog mites Indice de breslow y clark
Indice de breslow y clark