PHYSICS Matters for GCE O Level Unit 10




























- Slides: 65

PHYSICS Matters for GCE ‘O’ Level Unit 10: Transfer of Thermal Energy

10. 1 Transfer of Thermal Energy Learning Outcomes In this section, you’ll be able to: • Understand that thermal energy is transferred from a region of higher temperature to a region of lower temperature Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 1 Transfer of Thermal Energy What causes transfer of thermal energy? • Thermal energy is transferred only when there is a difference in temperature. • Thermal energy always flows from a region of higher temperature to a region of lower temperature. • There is no transfer of heat at thermal equilibrium. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

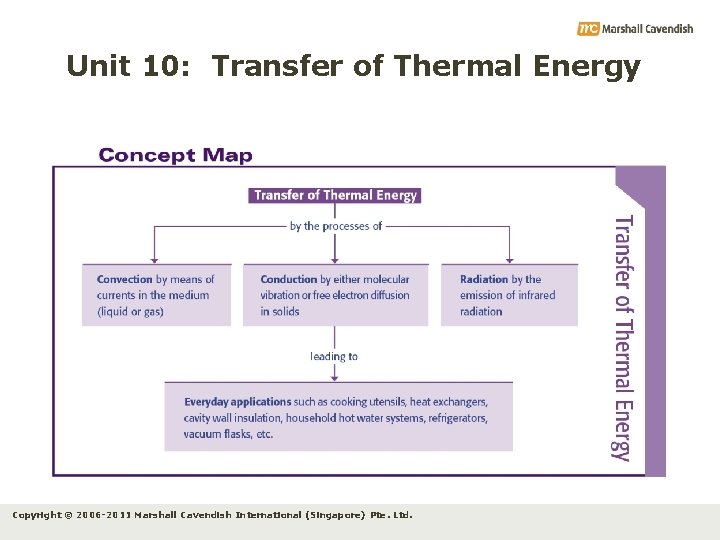
10. 1 Transfer of Thermal Energy How is thermal energy transferred? • Thermal energy is transferred by: • Conduction • Convection • Radiation Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 1 Transfer of Thermal Energy Key Ideas 1. Transfer of thermal energy takes place when there is a temperature difference. Thermal energy is always transferred from a hotter region to a colder region. 2. When thermal equilibrium is reached between two bodies (i. e. both bodies are at the same temperature), there is no net flow of thermal energy between them. 3. There are three different processes of thermal energy transfer: conduction, convection and radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 1 Transfer of Thermal Energy Test Yourself 1. During winter, it is common for people to say ‘keep the cold out of the house’. Is this statement correct? Comment. Answer: The statement ‘keep the cold out of the house’ seems to suggest that ‘the cold tends to move into the house’ which is not true. In fact, it is the transfer of heat energy from the inside of the house to the outside that causes the temperature in the house to drop. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction Learning Outcomes In this section, you’ll be able to: • Describe how energy transfer occurs in solid. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction What is conduction? Definition: Conduction is the process of thermal energy transfer without any flow of the material medium. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

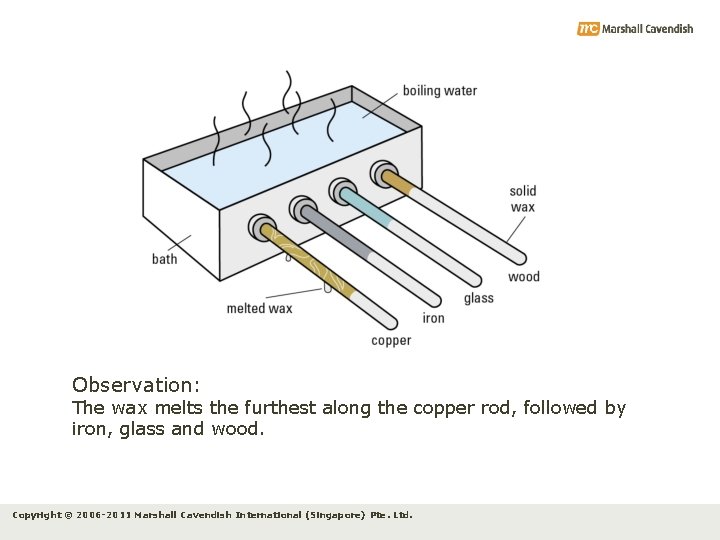
10. 2 Conduction Experiment 10. 1 Objective: To investigate the transfer of thermal energy through solids Apparatus: bath, rods of the same dimensions but of different materials, stopwatch Procedure: 1. Coat the parts of the rods that are on the outside of the tank evenly with melted wax (see figure). 2. Pour boiling water into the bath, so that the ends of the rods are submerged. 3. Record the length of wax that melts in a given interval of time for each of the four rods. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Observation: The wax melts the furthest along the copper rod, followed by iron, glass and wood. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction Experiment 10. 1 Two important conclusions can be drawn: 1. Thermal energy flows through the material of the rods without any flow of the material itself. This process is called conduction. 2. Different materials conduct heat at different rates. Those that conduct faster are called good conductors (e. g. copper) and those slower are called poor conductors (e. g. wood). Note: Poor conductors are also known as insulators. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

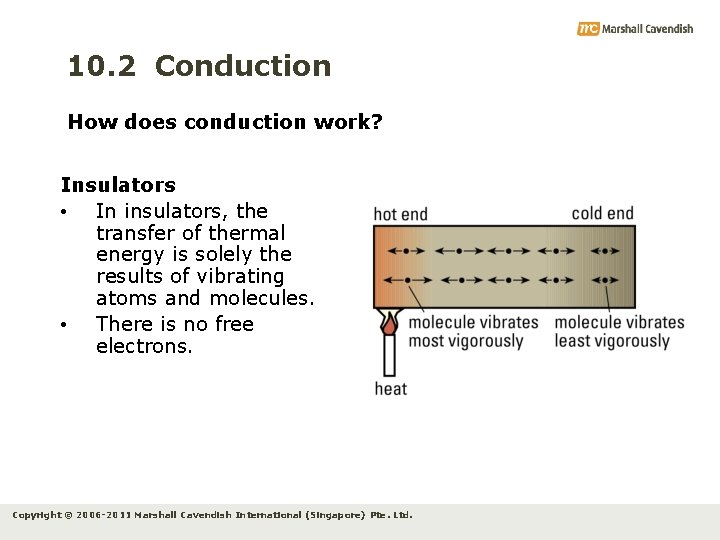
10. 2 Conduction How does conduction work? • • Conductors and insulators have different mechanisms to transfer of thermal energy. All solids are made up of tiny particles called atoms or molecules. Metals contain free electrons which move randomly between the atoms and molecules. Non-metals do not have free electrons. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction How does conduction work? • When thermal energy is supplied to one end of a rod, the particles (atoms and molecules) at the hot end vibrate vigorously. • These particles collide with neighbouring particles, making them vibrate as well. • Kinetic energy of vibrating particles at the hot end is transferred to neighbouring particles. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

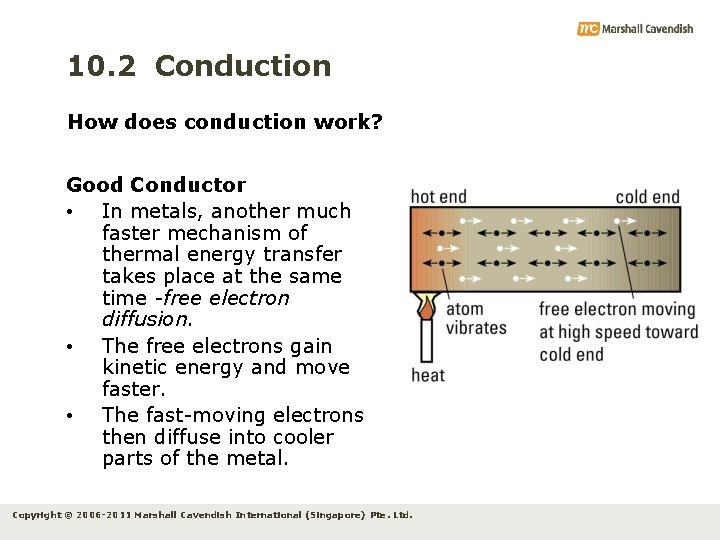
10. 2 Conduction How does conduction work? Good Conductor • In metals, another much faster mechanism of thermal energy transfer takes place at the same time -free electron diffusion. • The free electrons gain kinetic energy and move faster. • The fast-moving electrons then diffuse into cooler parts of the metal. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction How does conduction work? Insulators • In insulators, the transfer of thermal energy is solely the results of vibrating atoms and molecules. • There is no free electrons. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction in liquids and gases • Thermal energy can be conducted from a hotter to a cooler region. • Process of conduction is inefficient. • Liquid particles are further apart and collisions of particles are less frequent and even lesser in gases. • Thus, transfer of kinetic energy from fast-moving molecules to neighbouring molecules is slower. • Hence air is poor conductor of heat compared to water, which is in turn is a poor conductor compared to most solids. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

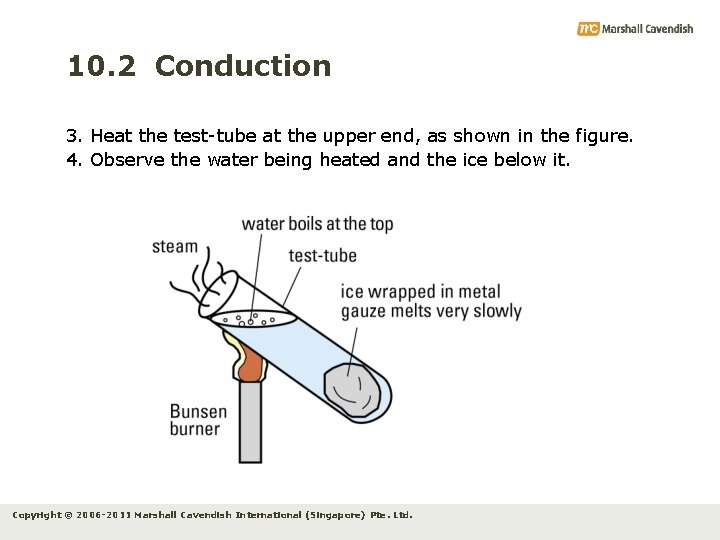
10. 2 Conduction Experiment 10. 1 Objective: To test conduction of thermal energy in water Apparatus: test-tube, ice, metal gauze, Bunsen burner, water Procedure: 1. Wrap a piece of ice with metal gauze and place it at the bottom of a test-tube. 2. Fill the test-tube with tap water till it is almost full. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction 3. Heat the test-tube at the upper end, as shown in the figure. 4. Observe the water being heated and the ice below it. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction Key Ideas 1. Conduction is the transfer of thermal energy without any flow of the material medium. 2. The two mechanisms for conduction are atomic or molecular vibrations (for both metals and non-metals) and free electron diffusion (for metals only). 3. Liquids and gases are poor conductors of heat compared to solids. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction Test Yourself 1. Give an account of thermal energy conduction in metals and non-metals. Answer: In metals, conduction of heat is due mainly to the diffusion of free electrons from a hotter region to a colder region. Conduction of heat can also take place with molecular vibrations. In non-metals, conduction of heat only takes place due to molecular vibrations, where the K. E. of the vibrating molecules at the hot end is transferred to the neighbouring molecules. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction Test Yourself 2. Why are good conductors of thermal energy also good conductor of electricity? Answer: Good conductors such as metals have free electrons. It is the presence of free electrons that enable metals to conduct both thermal energy as well as electricity. Conduction of electric current is the flow of electric charges such as electrons. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 2 Conduction Test Yourself 3. Is the heat transferred from a barbecue fire to a person standing in front of it a good example of heat transfer by conduction? Explain. Answer: A person standing in front of a barbecue fire and feeling hot is not a good example of conduction since air is a poor conductor of heat. In fact, we will learn later that we feel the hotness of the barbecue fire due to radiation of the heat energy. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3 Convection Learning Outcomes In this section, you’ll be able to: • Describe how energy transfer occurs in fluids. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3 Convection What is convection? Definition: Convection is the transfer of thermal energy by means of currents in a fluid (liquids or gases). Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

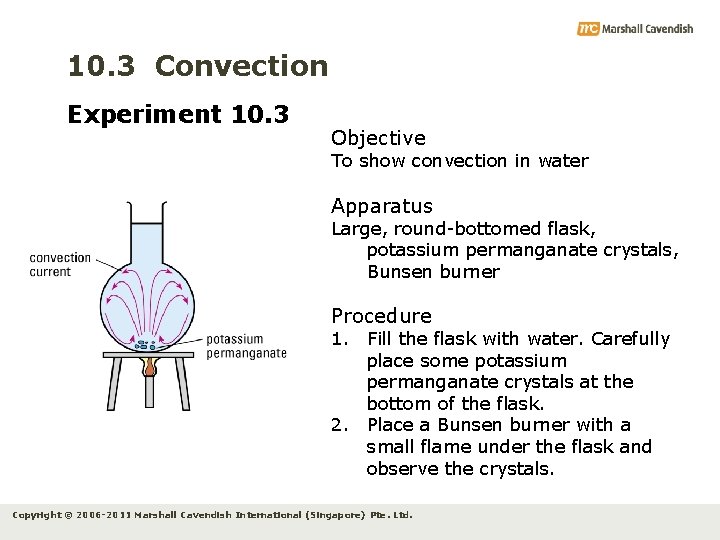
10. 3 Convection Experiment 10. 3 Objective To show convection in water Apparatus Large, round-bottomed flask, potassium permanganate crystals, Bunsen burner Procedure 1. Fill the flask with water. Carefully place some potassium permanganate crystals at the bottom of the flask. 2. Place a Bunsen burner with a small flame under the flask and observe the crystals. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

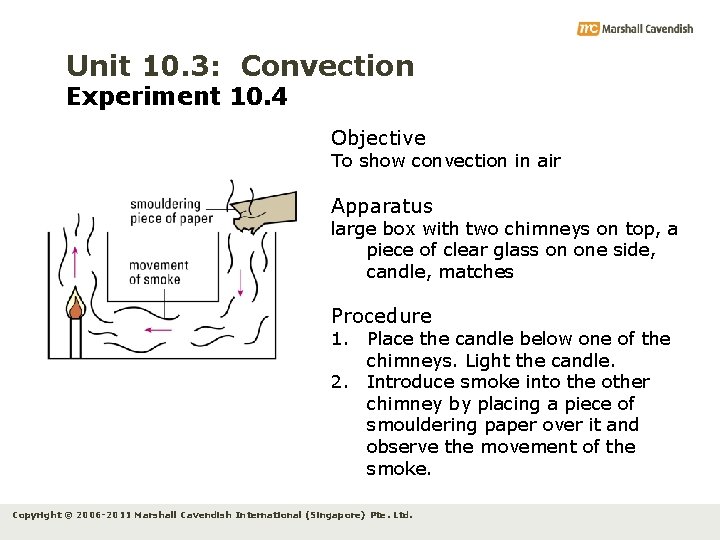
Unit 10. 3: Convection Experiment 10. 4 Objective To show convection in air Apparatus large box with two chimneys on top, a piece of clear glass on one side, candle, matches Procedure 1. Place the candle below one of the chimneys. Light the candle. 2. Introduce smoke into the other chimney by placing a piece of smouldering paper over it and observe the movement of the smoke. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3: Convection How does convection work? • When fluids (liquids and gases) are heated, they expand become less dense. • The less dense fluids tend to rise from the heating source. • Cooler fluids, being more dense, sink to replace the less dense fluids. • This movement of fluid due to a difference in its density sets up a convection current. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3: Convection How does convection work? • Convection currents occur only in fluids such as liquids and gases but not in solids. • Convection involves the bulk movement of the fluids which carry with them thermal energy. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3: Convection Key Ideas 1. Convection is the transfer of thermal energy by means of currents in a fluid (liquid or gas). 2. A convection current is the movement of fluid caused by the change in density in various parts of the fluid. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3: Convection Test Yourself 1. Why does it feel hot when you put your hands above a small burning candle? Answer: The hand feels hot because of convection. The air around the flame is being heated and becomes less dense and rises. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 3: Convection Test Yourself 2. Describe briefly the mechanism for the transfer of thermal energy in fluids. Answer: • When fluids are heated, they expand become less dense. The less dense fluid rises. • The cooler, denser fluids will replace the less dense fluids. • This sets up a convection current. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation Learning Outcomes In this section, you’ll be able to: • Explain energy transfer of a body by radiation. • State the factors affecting the rate of energy transfer by radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation What is radiation? Definition: Radiation is the continual emission of infrared waves from the surface of all bodies, transmitted without the aid of a medium. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation What is radiation? • Radiation does not require a medium for energy transfer. • It can take place in vacuum. For example, the Sun is a major source of radiant heat. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation What is radiation? • The sun emits electromagnetic waves. • Part of this electromagnetic waves, called infrared waves, make us feel warm. • Thermal energy from infrared waves is called radiant heat. • All objects emit some radiant heat. • The hotter the object, the greater the radiant heat emitted. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation Absorption of infrared radiation • Infrared radiation is absorbed by all objects and surfaces. • The absorption of radiant heat causes a temperature rise. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation Emission of infrared radiation • Infrared radiation is emitted by all objects and surfaces. • This emission causes the temperature of the objects themselves to fall. • In general, good emitter of radiant heat is also a good absorber of radiant heat. • Conversely, poor emitter of radiant heat is also a poor absorber of radiant heat. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 4: Radiation Experiment 10. 6 Objective To investigate the emission of infrared radiation Apparatus Two temperature sensors, data logger, two identical tins (one black and one shiny), boiling water from two electric kettles Procedure 1. Connect the temperature sensors A and B to the data logger 2. Set the sampling rate to ten seconds 3. Pour boiling water into both tins at the same time until both are filled to the brim. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

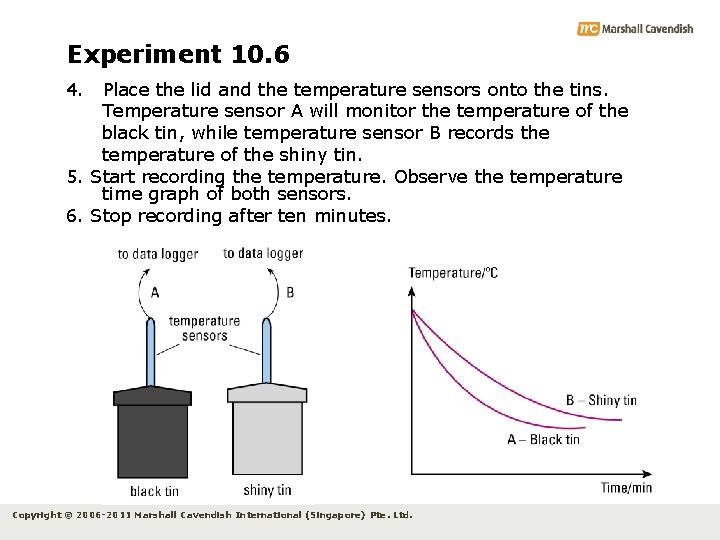
Experiment 10. 6 4. Place the lid and the temperature sensors onto the tins. Temperature sensor A will monitor the temperature of the black tin, while temperature sensor B records the temperature of the shiny tin. 5. Start recording the temperature. Observe the temperature time graph of both sensors. 6. Stop recording after ten minutes. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

10. 4: Radiation Factors affecting rate of infrared radiation 1. Colour and texture of the surface • Dull, black surfaces are good absorbers of infrared radiation than shiny, white surfaces • Dull, black surfaces are better emitters of infrared radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

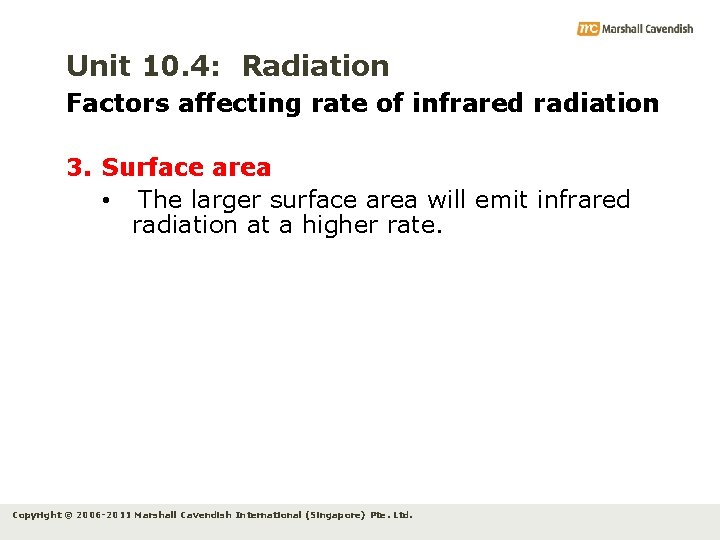
Unit 10. 4: Radiation Factors affecting rate of infrared radiation 2. Surface temperature • Rate of infrared radiation also depends on surface temperature • The higher the temperature of the surface of the object relative to the surrounding temperature, the higher the rate of infrared radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 4: Radiation Factors affecting rate of infrared radiation 3. Surface area • The larger surface area will emit infrared radiation at a higher rate. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 4: Radiation Key Ideas 1. Radiation is the continual emission of thermal energy in the form of infrared waves. 2. Radiation is emitted from the surface of all bodies and does not require a medium of thermal transfer. 3. Dull, black surfaces are better emitters of infrared radiation than shiny, white surface. 4. The factors affecting rate of energy transfer by radiation are: colour, and texture of the surface, surface temperature and surface area. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 4: Radiation Test Yourself 1. Give two everyday examples of thermal energy transfer by radiation. Answer: a. Feeling the hotness of Sun’s radiation – Sun’s thermal energy reaches earth by radiation. b. Standing next the a BBQ fire will make you feel hot. The thermal energy reaches you by radiation. c. Placing your hand next to a hot object such a jar of hot water. Thermal energy reaches your hand by radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 4: Radiation Test Yourself 2. State briefly how thermal energy is transferred by radiation. Answer: Hot objects emit thermal energy in the form of infrared radiation, which is a type of electromagnetic waves. The hotter the object, the higher the rate of radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 4: Radiation Test Yourself 3. State three factors that affect the rate of transfer of thermal energy by radiation. Answer: • Colour and texture of the surface. • Surface temperature. • Surface area. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Learning Outcomes In this section, you’ll be able to: • Understand identify how thermal energy is transferred by conduction, convection and radiation in everyday life. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of good conductors of heat 1. Cooking utensils – made of metals eg. Stainless steel or aluminium Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of good conductors of heat 2. Soldering iron rods – the tip is made of copper Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

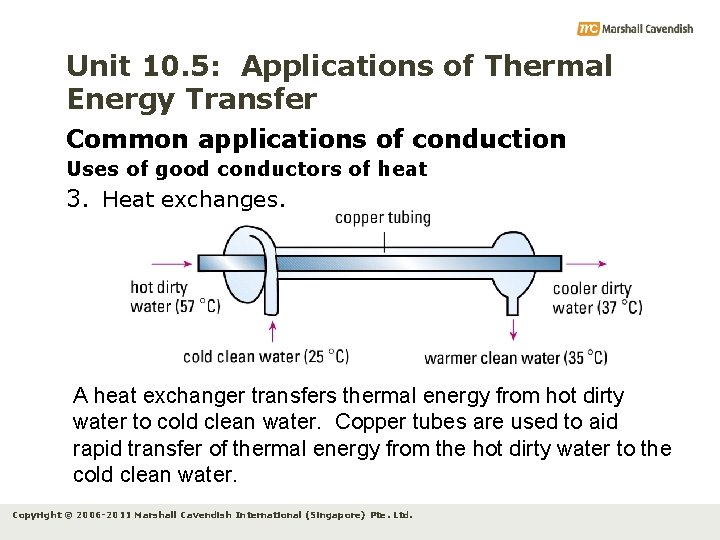
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of good conductors of heat 3. Heat exchanges. A heat exchanger transfers thermal energy from hot dirty water to cold clean water. Copper tubes are used to aid rapid transfer of thermal energy from the hot dirty water to the cold clean water. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of bad conductors of heat (insulators) 1. Handles of appliances and utensils – made of plastics or wood Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of bad conductors of heat (insulators) 2. Table mats – made of cork Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of bad conductors of heat (insulators) 3. Sawdust. • Used to cover ice blocks because of its insulating property 4. Wooden ladles • Useful for stirring or scooping hot soup. 5. Woolen clothes • Used to keep body warm Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of conduction Uses of bad conductors of heat (insulators) 6. Fiberglass, felt and expanded polystyrene foam. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

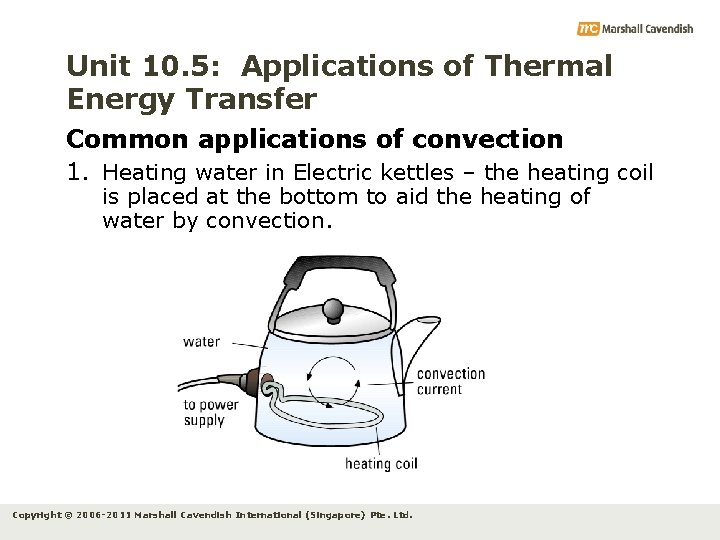
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of convection 1. Heating water in Electric kettles – the heating coil is placed at the bottom to aid the heating of water by convection. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

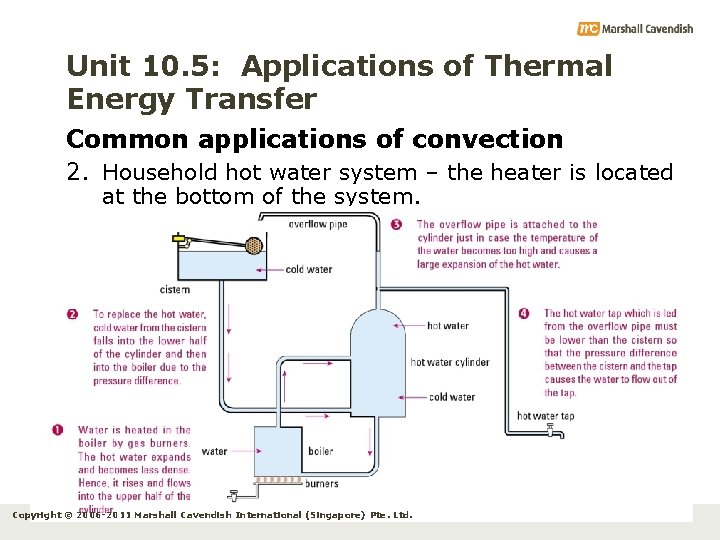
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of convection 2. Household hot water system – the heater is located at the bottom of the system. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

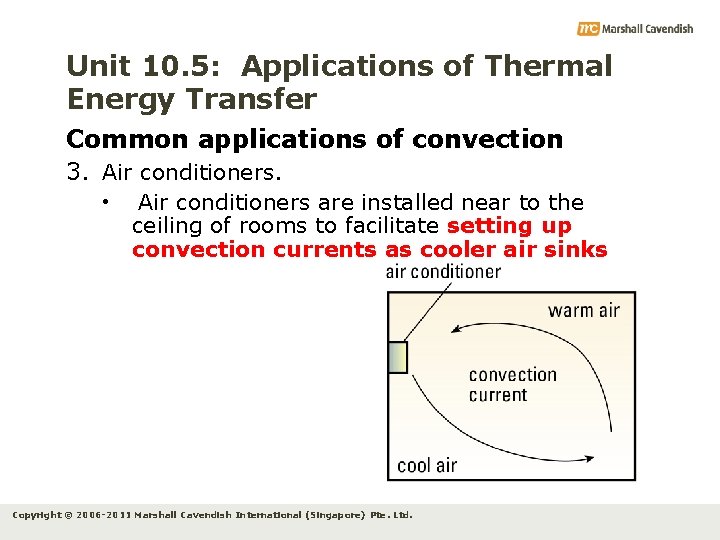
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of convection 3. Air conditioners. • Air conditioners are installed near to the ceiling of rooms to facilitate setting up convection currents as cooler air sinks Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

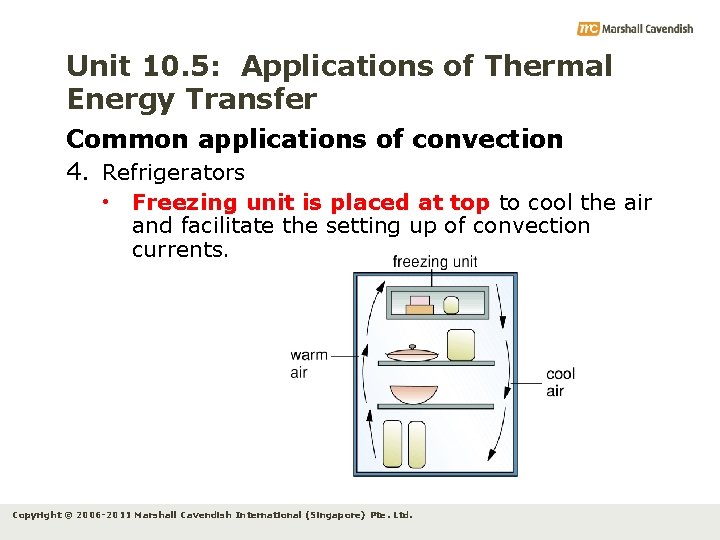
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of convection 4. Refrigerators • Freezing unit is placed at top to cool the air and facilitate the setting up of convection currents. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Common applications of radiation 1. Teapots • Shiny teapots can keep tea warm for a loner time than black teapots. • It can also keep cold liquids cool for a longer time than black containers. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

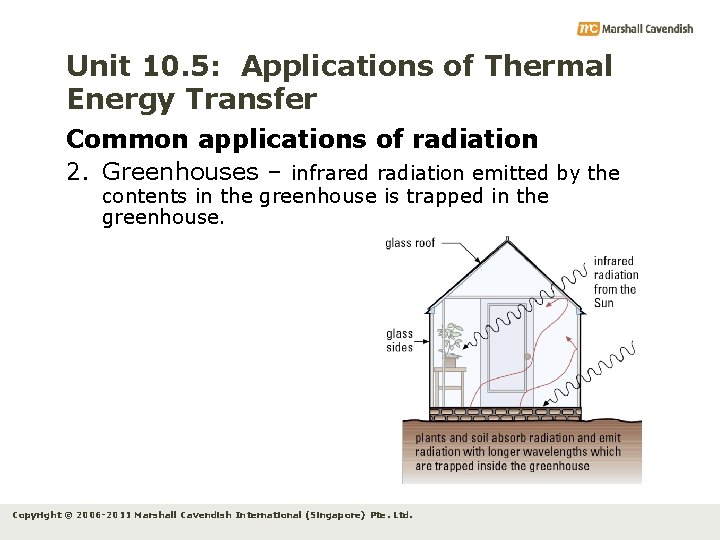
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of radiation 2. Greenhouses – infrared radiation emitted by the contents in the greenhouse is trapped in the greenhouse. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

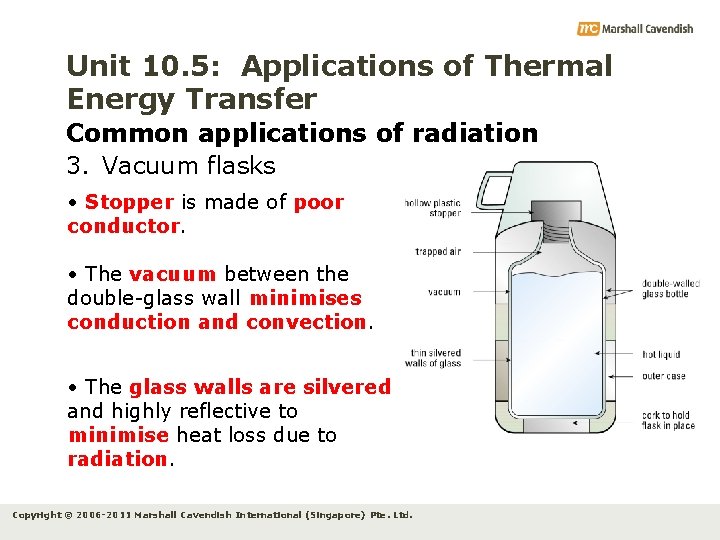
Unit 10. 5: Applications of Thermal Energy Transfer Common applications of radiation 3. Vacuum flasks • Stopper is made of poor conductor. • The vacuum between the double-glass wall minimises conduction and convection. • The glass walls are silvered and highly reflective to minimise heat loss due to radiation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

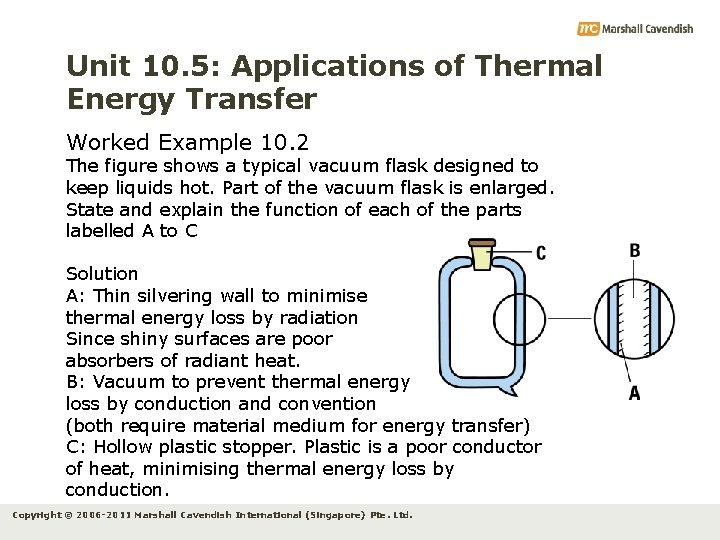
Unit 10. 5: Applications of Thermal Energy Transfer Worked Example 10. 2 The figure shows a typical vacuum flask designed to keep liquids hot. Part of the vacuum flask is enlarged. State and explain the function of each of the parts labelled A to C Solution A: Thin silvering wall to minimise thermal energy loss by radiation Since shiny surfaces are poor absorbers of radiant heat. B: Vacuum to prevent thermal energy loss by conduction and convention (both require material medium for energy transfer) C: Hollow plastic stopper. Plastic is a poor conductor of heat, minimising thermal energy loss by conduction. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Key Ideas 1. Some everyday applications of thermal energy transfer involving conduction include cooking utensils and table mats. 2. Some everyday applications of thermal energy transfer involving convection include household hot water systems and electric kettles. 3. Some everyday applications of thermal energy transfer involving radiation include vacuum flasks and greenhouses. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10. 5: Applications of Thermal Energy Transfer Test Yourself 1. A saucepan with a thick copper base contains water and is placed on a flat electric hot plate. a. State the process by which energy is i. transferred from the hot plate to the water, ii. spread through the water. b. The sides of a saucepan are often polished. How does this reduce energy loss? Answer: a(i) Conduction. (ii) Convection. b. The sides are polished to reduce heat loss due to radiation. Polished and shiny surfaces are poor emitters of radiation Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Unit 10: Transfer of Thermal Energy Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.