PHY 110 Introduction to Physics Dr Henry SC



















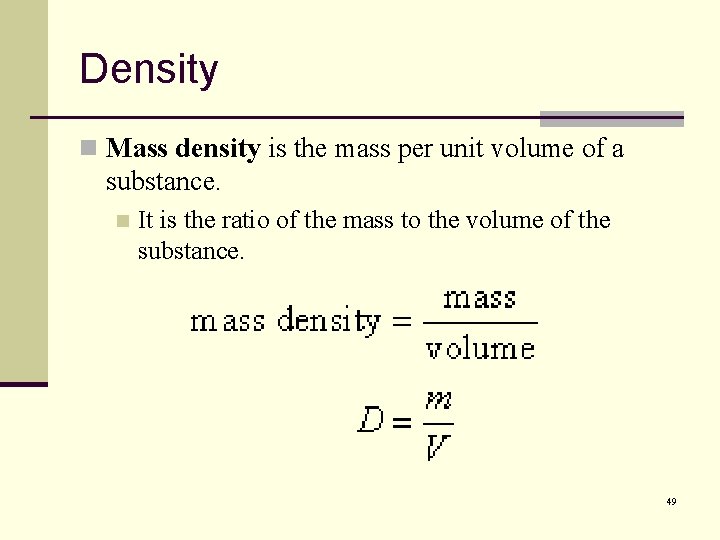
















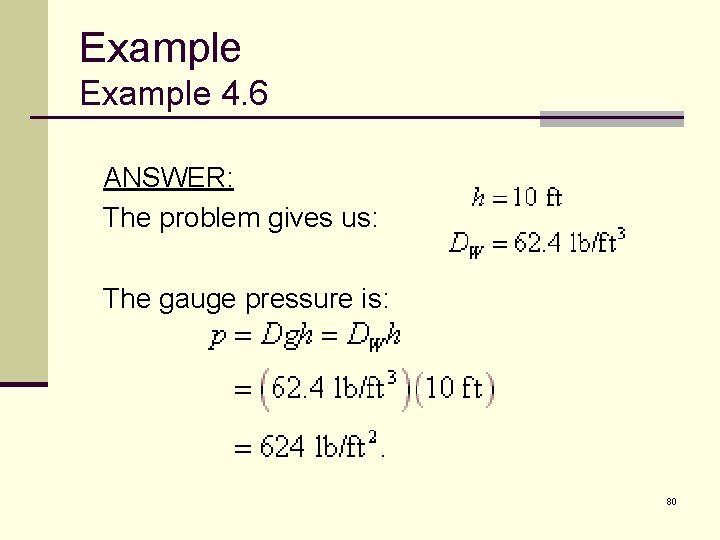















- Slides: 140

PHY 110, Introduction to Physics Dr. Henry SC 453, 572 -6164 (alt 572 -5309) E-Mail: henryh 1@nku. edu Web Site: www. nku. edu/~henryh 1/ © Hugh Henry, 2008

If you are Tardy Initial beside Your Name on the Sheet on the Front Table

Chapter 4 Physics of Matter © Hugh Henry, 2008

4

Matter: Phases, Forms & Forces • Matter is classified into four categories: – Solid: rigid; retain its shape unless distorted by a force. – Liquid: flows readily; conforms to the shape of a container; has a well-defined boundary; has higher densities than gases. – Gas: flows readily; conforms to the shape of a container; does not have a well-defined surface; can be compressed readily. – Plasma: has gaseous properties but also conducts electricity; interacts strongly with magnetic fields; commonly exists at higher temperatures. Common_Phases_of_Matter 5

Matter: Phases, Forms & Forces, cont’d n The chemical elements represent the simplest and purest forms of everyday matter. n n There are currently 114 different elements. 110 of them have accepted names. n Each element is composed of incredibly small objects called atoms. n n n There are 114 different kinds of atoms, one for each of the known elements. Only about 90% of the elements exist naturally on Earth. The others are artificially produced in laboratories. 6

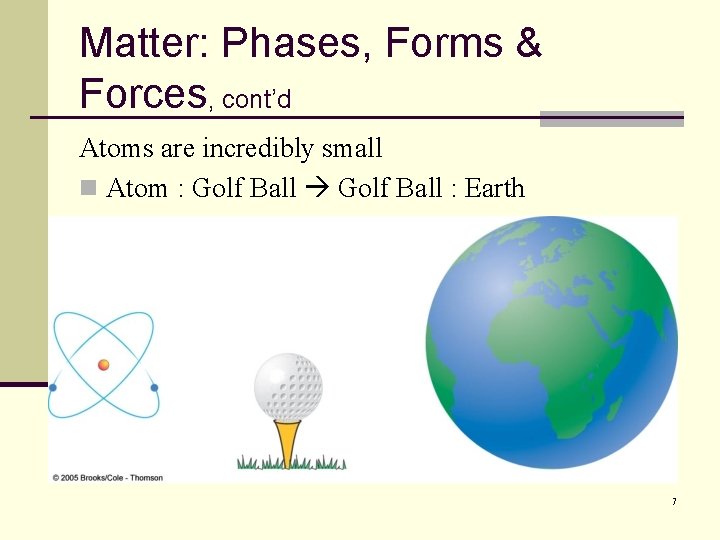
Matter: Phases, Forms & Forces, cont’d Atoms are incredibly small n Atom : Golf Ball : Earth 7

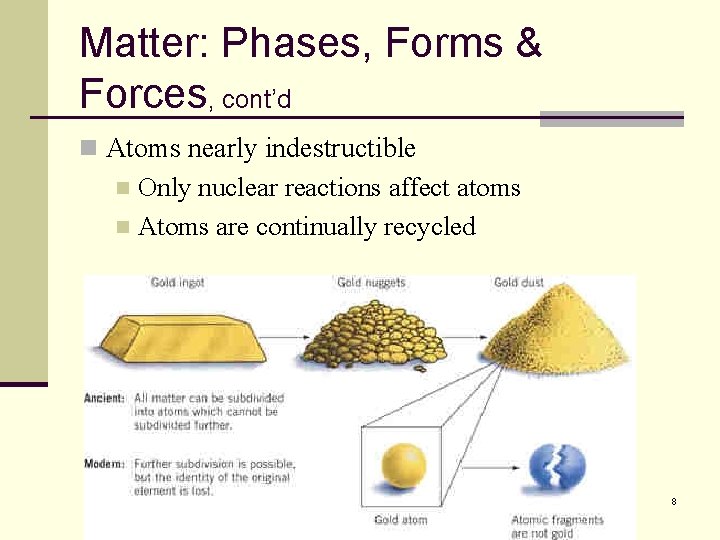
Matter: Phases, Forms & Forces, cont’d n Atoms nearly indestructible Only nuclear reactions affect atoms n Atoms are continually recycled n 8

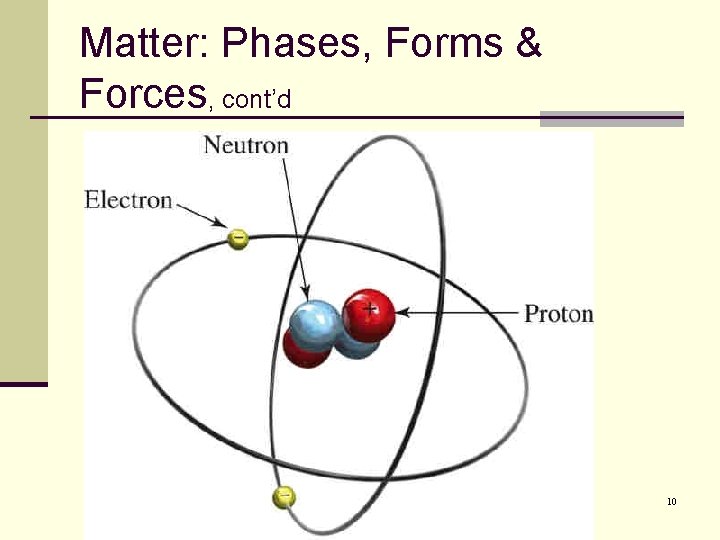
Matter: Phases, Forms & Forces, cont’d n The atom is not indivisible. n It has its own internal structure. n Every atoms has a very dense, compact core called the nucleus. n The nucleus is composed of two kinds of particles: n Protons: have a positive electric charge. Neutrons: have no electric charge. n The nucleus is surrounded by one or more particles called electrons. n Electrons have the same electric charge as protons but are negatively charged. n 9

Matter: Phases, Forms & Forces, cont’d 10

Matter: Phases, Forms & Forces, cont’d n Every atom associated with a particular element has a fixed number of protons. n The number of protons distinguishes the element. n For example, all atoms with two protons are helium. n The atomic number of an element specifies the number of protons. n The atomic number of helium is 2 because it has two protons. n Each element is given its own chemical symbol. n This is a one- or two-letter abbreviation. n The chemical symbol for helium is He 11

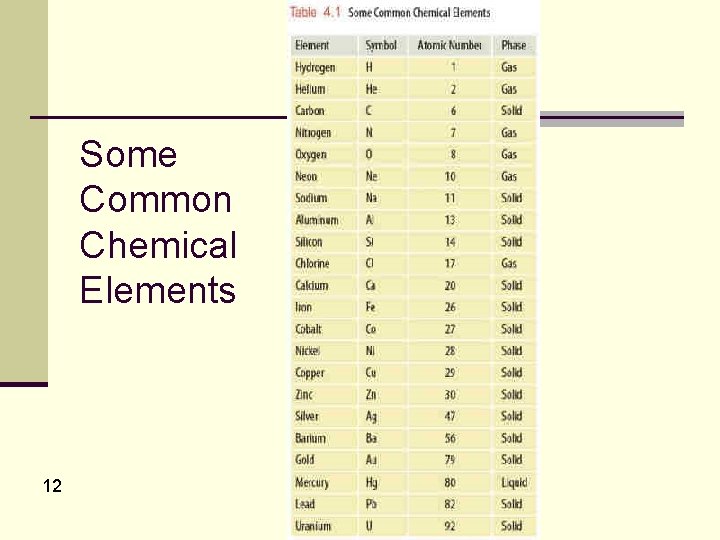
Some Common Chemical Elements 12

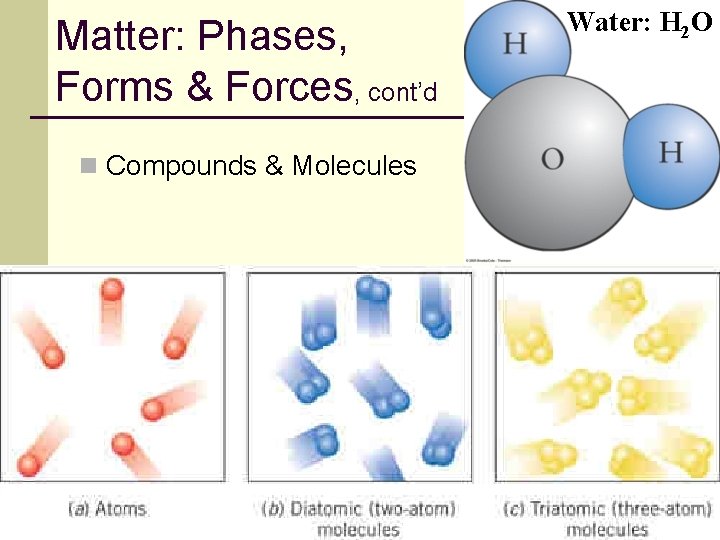
Matter: Phases, Forms & Forces, cont’d n Chemical compounds are the next simplest form of everyday matter. n Examples: water, salt, sugar, etc. n Compounds are made from building blocks called molecules. n Every molecule of a particular compound consists of the same unique combination of two or more atoms. n Each water molecule consists of two hydrogen atoms and one oxygen atom. 13

Matter: Phases, Forms & Forces, cont’d Water: H 2 O n Compounds & Molecules 14

Salt Example of a Compound Properties of a Compound often quite different from properties of constituent elements

Matter: Phases, Forms & Forces, cont’d n Each compound can be represented by a chemical formula. n n n water is H 2 O; salt is Na. Cl; carbon dioxide is CO 2; sugar is C 12 H 22 O 11; ethyl alcohol is C 2 H 5 OH. 16

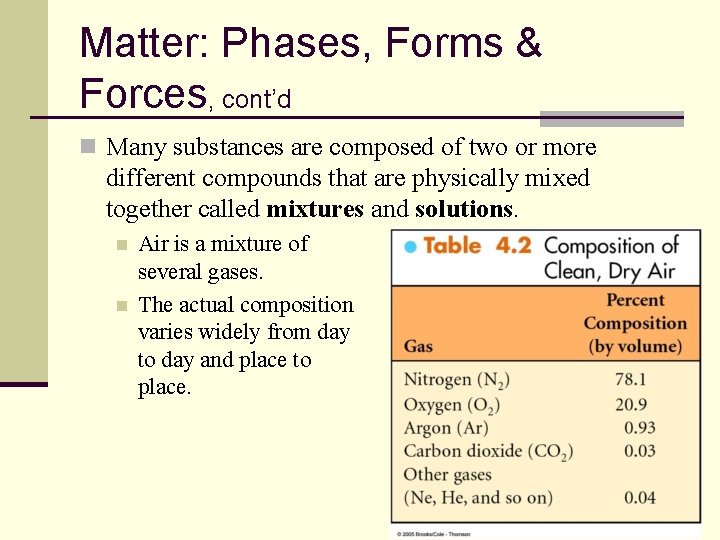
Matter: Phases, Forms & Forces, cont’d n Many substances are composed of two or more different compounds that are physically mixed together called mixtures and solutions. n n Air is a mixture of several gases. The actual composition varies widely from day to day and place to place. 17

Behavior of atoms and molecules n The constituent particles of atoms and molecules exert electrical forces on each other. n “Static cling” is an example of an electrical force. n The forces depend upon the configuration of the atoms in each molecule. 18

Behavior of atoms and molecules, cont’d n Solids: Attractive forces between particles are very strong; the atoms or molecules are rigidly bound to their neighbors and can only vibrate. n Liquids: The particles are bound together, though not rigidly; each atom or molecules move about relative to the others but is always in contact with other atoms or molecules. n Gases: Attractive forces between particles are too weak to bind them together; atoms or molecules move freely with high speed and are widely separated; particles are in contact only when they collide. 19

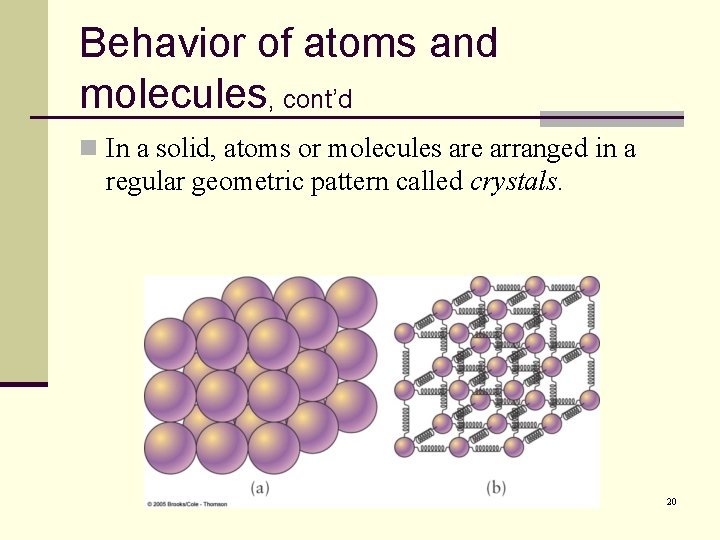
Behavior of atoms and molecules, cont’d n In a solid, atoms or molecules are arranged in a regular geometric pattern called crystals. 20

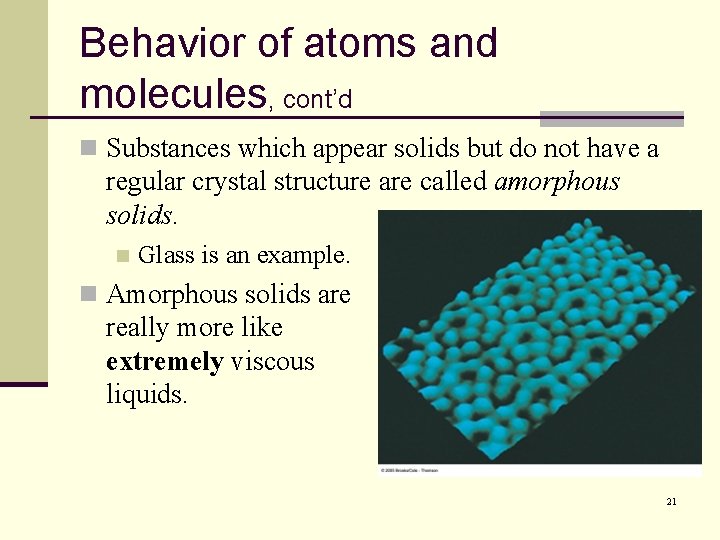
Behavior of atoms and molecules, cont’d n Substances which appear solids but do not have a regular crystal structure are called amorphous solids. n Glass is an example. n Amorphous solids are really more like extremely viscous liquids. 21

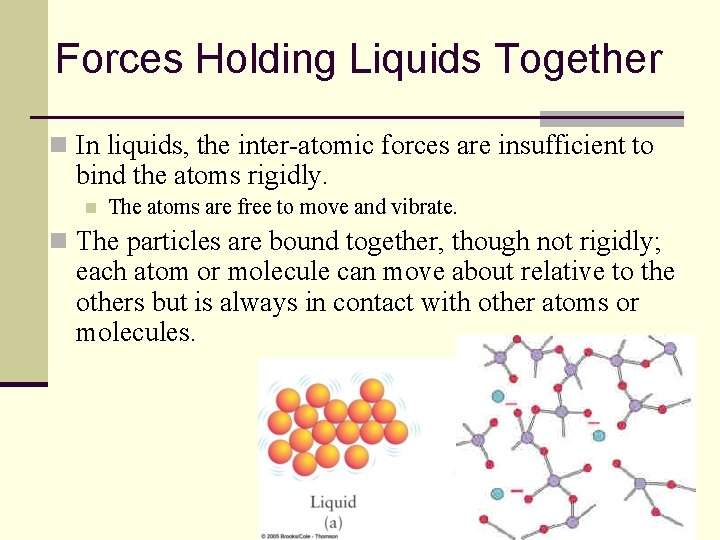
Forces Holding Liquids Together n In liquids, the inter-atomic forces are insufficient to bind the atoms rigidly. n The atoms are free to move and vibrate. n The particles are bound together, though not rigidly; each atom or molecule can move about relative to the others but is always in contact with other atoms or molecules. 22

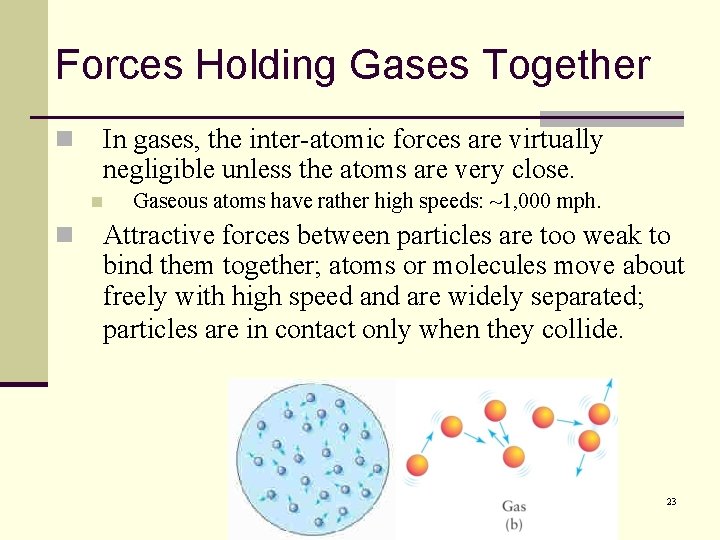
Forces Holding Gases Together n In gases, the inter-atomic forces are virtually negligible unless the atoms are very close. n n Gaseous atoms have rather high speeds: ~1, 000 mph. Attractive forces between particles are too weak to bind them together; atoms or molecules move about freely with high speed and are widely separated; particles are in contact only when they collide. 23

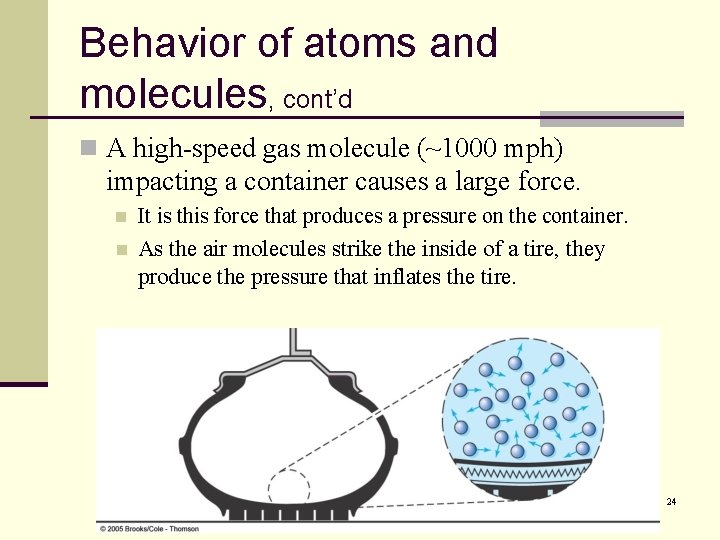
Behavior of atoms and molecules, cont’d n A high-speed gas molecule (~1000 mph) impacting a container causes a large force. It is this force that produces a pressure on the container. n As the air molecules strike the inside of a tire, they produce the pressure that inflates the tire. n 24

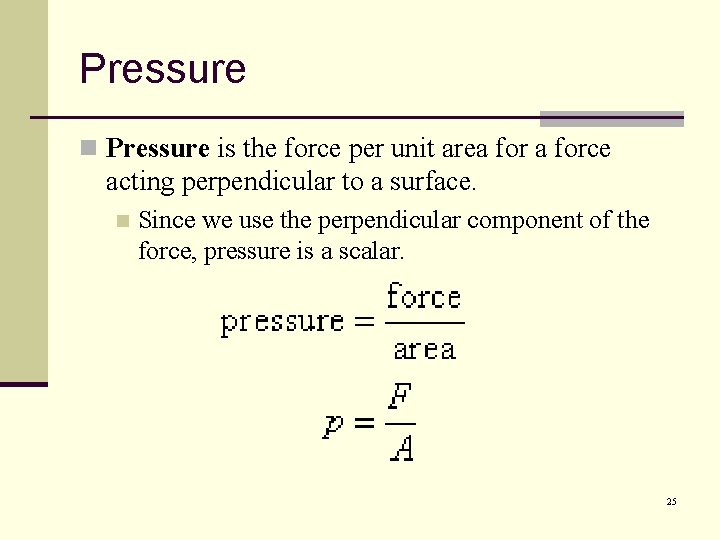
Pressure n Pressure is the force per unit area force acting perpendicular to a surface. n Since we use the perpendicular component of the force, pressure is a scalar. 25

Pressure, cont’d n The units of pressure are: n Metric: n n n Pascal (Pa; 1 Pa = 1 N/m 2) — SI unit; millimeters of mercury (mm Hg or Torr). English: n n n pound per square foot (lb/ft 2); pound per square inch (lb/in 2 or psi); inches of mercury (in Hg). 26

Pressure, cont’d n Some pressure conversions: n 1 psi = 6, 890 Pa. n We also use an atmosphere (atm) as a pressure unit: n One atmosphere is the average pressure exerted by air at sea level: n n 1 atm = 101, 325 Pa = 760 Torr 1 atm = 14. 7 psi. 27

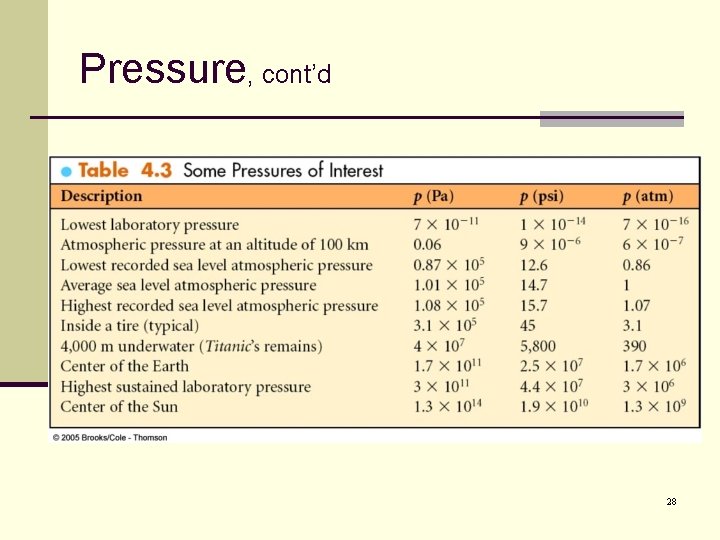
Pressure, cont’d 28

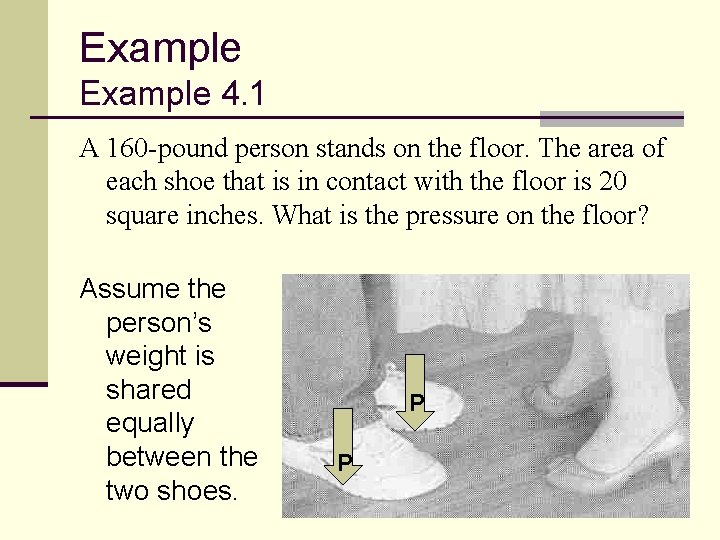
Example 4. 1 A 160 -pound person stands on the floor. The area of each shoe that is in contact with the floor is 20 square inches. What is the pressure on the floor? Assume the person’s weight is shared equally between the two shoes. P P 29

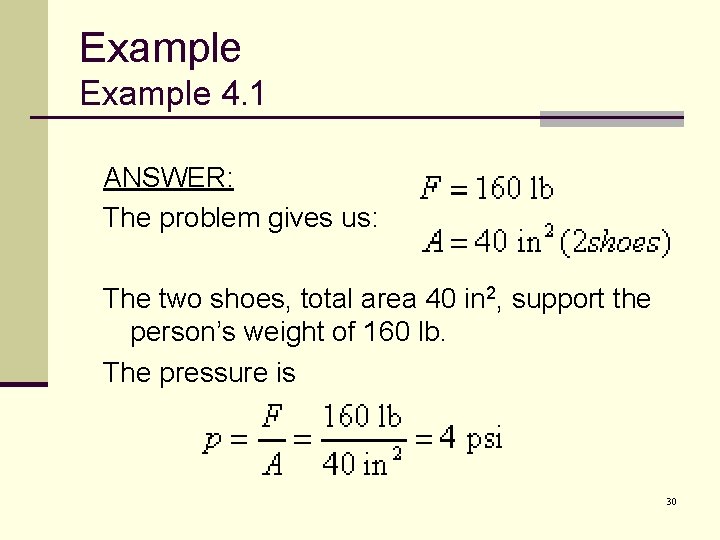
Example 4. 1 ANSWER: The problem gives us: The two shoes, total area 40 in 2, support the person’s weight of 160 lb. The pressure is 30

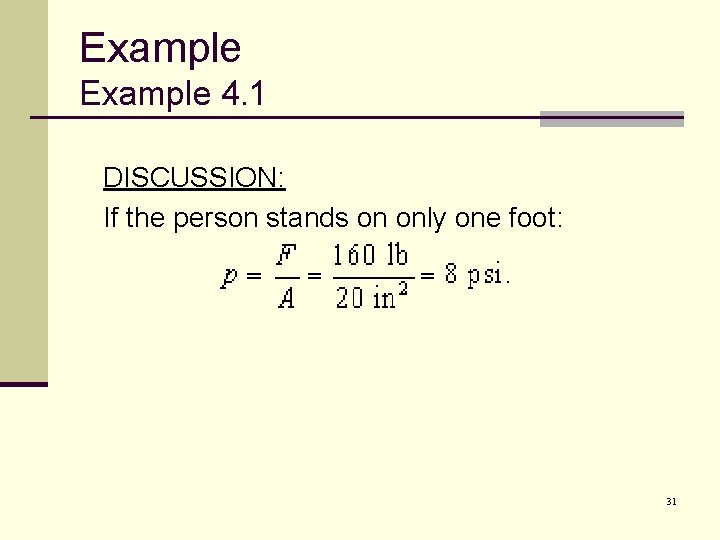
Example 4. 1 DISCUSSION: If the person stands on only one foot: 31

Pressure P = F/A n Skimboards work by distributing a person’s weight across a larger area

Pressure P = F/A P P How to smooth wet concrete: Concrete shoes spread a 200 lb weight across a 600 in 2 area P = F/A = 200 lb / 600 in 2 = 0. 33 psi (lb/in 2) This person’s effective weight is reduced by 93% P With normal shoes, this person would sink into the wet concrete P

Pressure P = F/A Pressure of 125 lb person, standing on one heel of high heeled shoe, 0. 5 in x 0. 5 in P = F/A = 125 lb/(0. 5 x 0. 5)in 2 = 125/0. 25 psi = 500 psi (lb/in 2) P Her effective is 160 what itthis is in flats 34 Carpets must beweight designed totimes withstand pressure Carpets and floors must withstand this much pressure

Pressure P = F/A Force is the same on each end of the tack But the Pressure is greater on the end with the smaller area Ouch!! 35

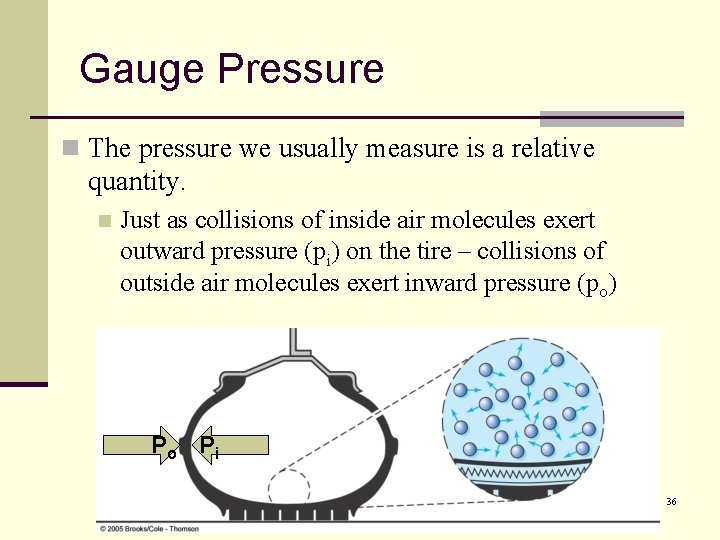
Gauge Pressure n The pressure we usually measure is a relative quantity. n Just as collisions of inside air molecules exert outward pressure (pi) on the tire – collisions of outside air molecules exert inward pressure (po) Po Pi 36

Gauge Pressure, cont’d n Hence we define gauge pressure as pressure relative to the atmospheric pressure. n Gauge pressure plus atmospheric pressure is called absolute pressure. n Absolute pressure is very difficult to measure, but gauge pressure can be measured relatively simply. n Hence we usually focus on gauge pressure. 37

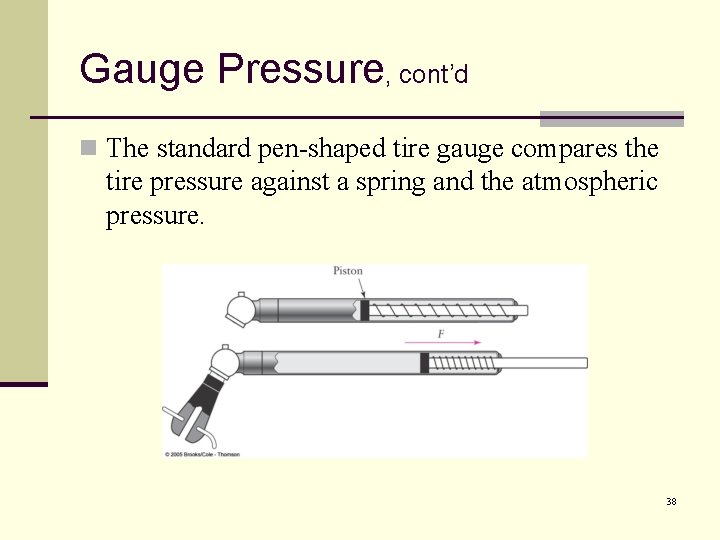
Gauge Pressure, cont’d n The standard pen-shaped tire gauge compares the tire pressure against a spring and the atmospheric pressure. 38

Gauge Pressure, cont’d n Hence the pressure we measure with a tire gauge, is gauge pressure, relative to atmospheric pressure. n It’s the pressure above atmospheric pressure. pgauge = pi - po 39

Gauge Pressure, cont’d n Atmospheric pressure in the upper atmosphere is substantially lower than at sea level. n More on this later n Hence commercial airliners add air pressure inside the cabin so passengers can fly in comfort.

Example 4. 2 In the late 1980’s, there were several spectacular aircraft mishaps involving rapid loss of air pressure in the passenger cabins. The cabin pressure of a passenger jet cruising at high altitude (25, 000 ft) is about 6 psi (0. 41 atm) greater than the pressure outside. What is the outward force on a window measuring 1 foot by 1 foot and on a door measuring 1 meter by 2 meters? 41

Example 4. 2 ANSWER: The force on the window is: The force on the door is 42

Example 4. 2 DISCUSSION: The force on a window is approximately the weight of four adults. The force on the door is nearly the weight of ten pickups. Feel safe? ? 43

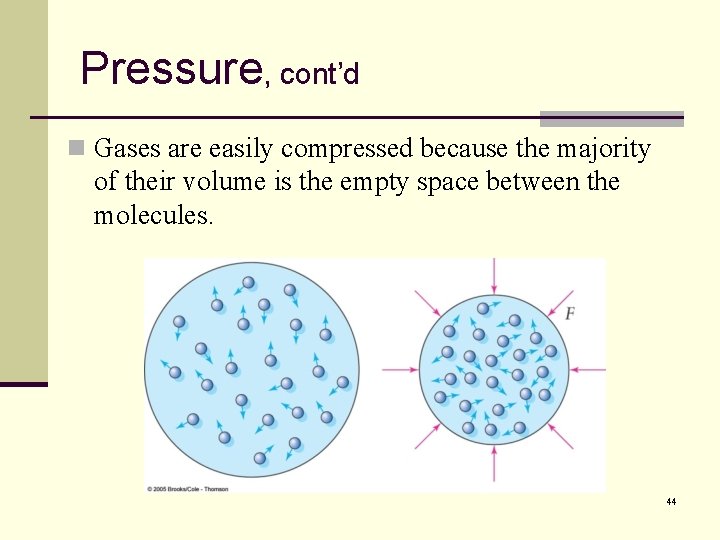
Pressure, cont’d n Gases are easily compressed because the majority of their volume is the empty space between the molecules. 44

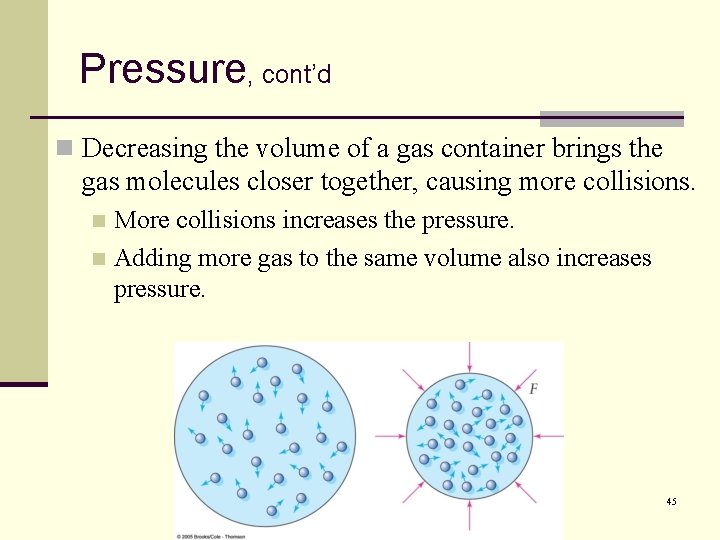
Pressure, cont’d n Decreasing the volume of a gas container brings the gas molecules closer together, causing more collisions. More collisions increases the pressure. n Adding more gas to the same volume also increases pressure. n 45

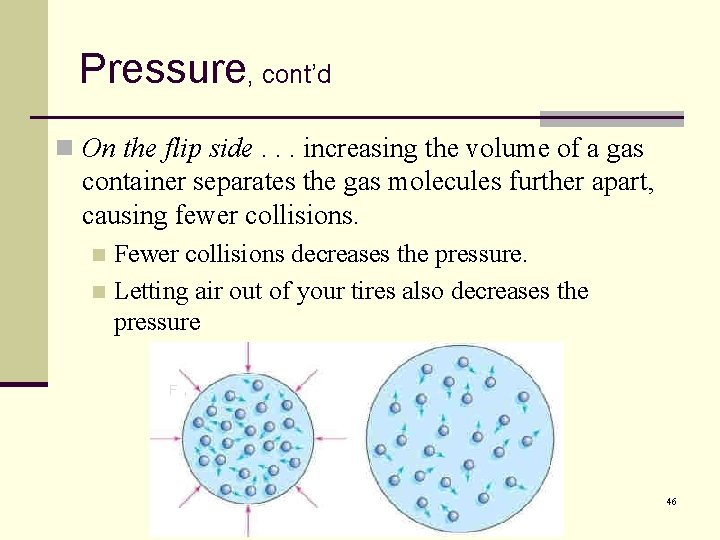
Pressure, cont’d n On the flip side. . . increasing the volume of a gas container separates the gas molecules further apart, causing fewer collisions. Fewer collisions decreases the pressure. n Letting air out of your tires also decreases the pressure n F 46

Pressure, cont’d n An important statement about gases. n Consider a gas held at constant temperature. n Since decreasing the volume increases the pressure – and vice versa – there is a relationship between volume and pressure: 47

Pressure, cont’d n We know gas pressure is caused by collisions of high-speed gas molecules (~1000 mph). n We will learn in chapter 5 that increasing the temperature of a gas increases the kinetic energy (and hence velocity) of the gas molecules. n Since pressure is the result of gas molecules colliding with the container, increasing the temperature (and hence molecular velocity) of a gas increases the pressure 48
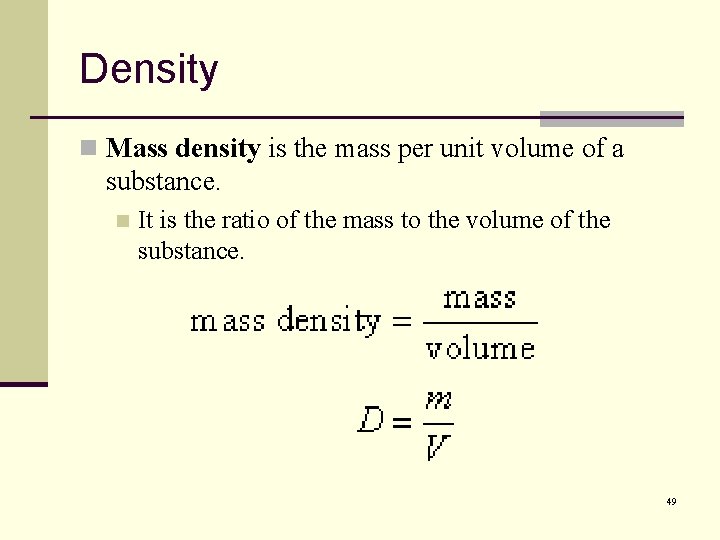
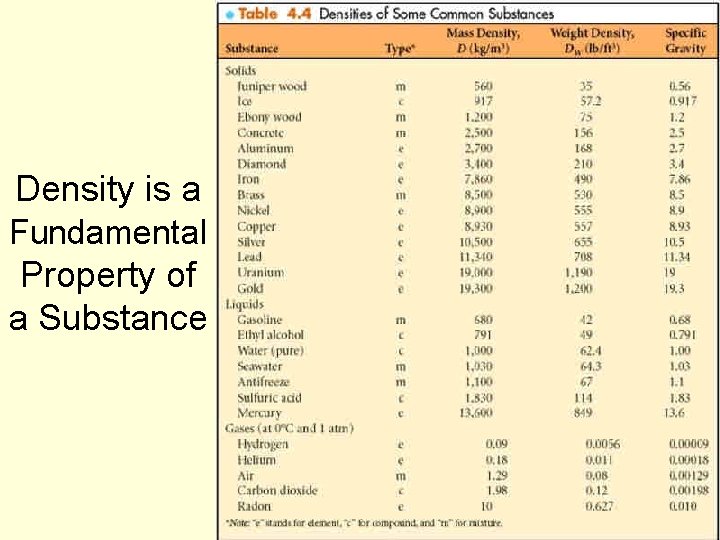
Density n Mass density is the mass per unit volume of a substance. n It is the ratio of the mass to the volume of the substance. 49

Density, cont’d n Units of mass density: n Metric: n n n kilogram per cubic meter (kg/m 3); gram per cubic centimeter (g/cm 3). English: n slug per cubic foot (slug/ft 3). 50

Density, cont’d n Measure density of a sample by finding its mass and dividing by its volume. n The actual size of the sample is irrelevant. n If you use a sample with twice the volume it will have twice the mass. 51

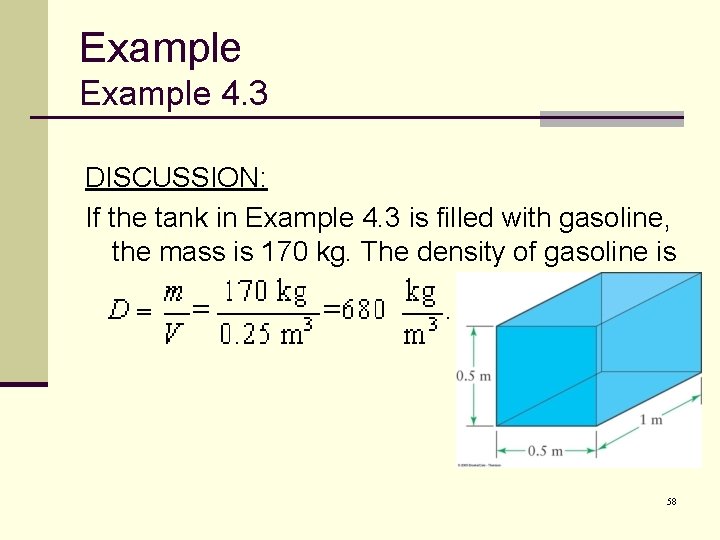
Example 4. 3 The dimensions of a rectangular aquarium are 0. 5 meters by 1 meter by 0. 5 meters. The mass of the aquarium is 250 kilograms greater when full of water than when empty. What is the density of the water? 52

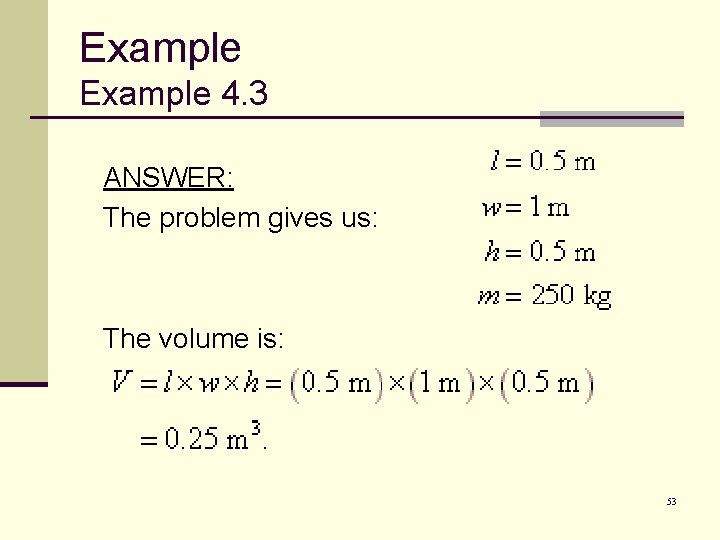
Example 4. 3 ANSWER: The problem gives us: The volume is: 53

Example 4. 3 ANSWER: The mass density is then This illustrates that the water is used to define metric volume and mass: 1 cc (1 ml) water ≡ 1 gram 1 m 3 water = 1000 kg 54

Example 4. 3 DISCUSSION: If the width of the tank was doubled, the amount of water would be doubled — the density would remain the same. 55

Density is a Fundamental Property of a Substance 56

For solids and liquids, mass density is essentially constant n Mass and volume calculations can be made with great reliability based on mass density tables – and such tables can be used to identify a substance. n Changes in temperature and pressure generally cause insignificant changes in mass density of solids and liquids n NOTE: This is not true with gases n Gas density varies substantially with temperature and pressure changes 57

Example 4. 3 DISCUSSION: If the tank in Example 4. 3 is filled with gasoline, the mass is 170 kg. The density of gasoline is 58

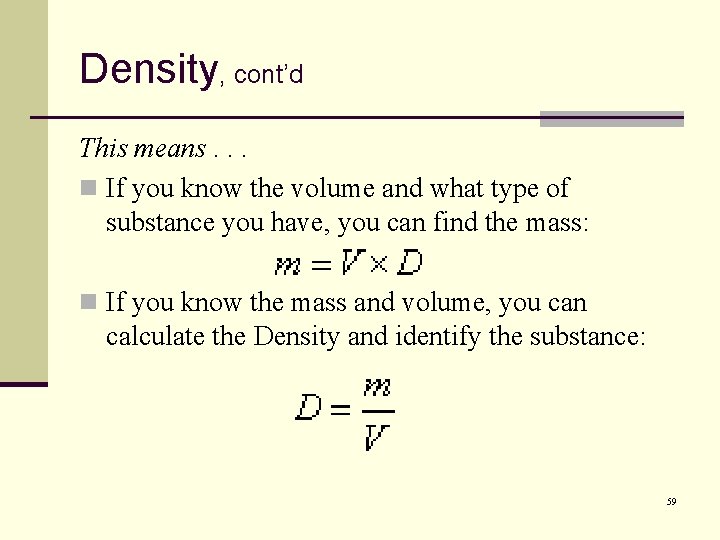
Density, cont’d This means. . . n If you know the volume and what type of substance you have, you can find the mass: n If you know the mass and volume, you can calculate the Density and identify the substance: 59

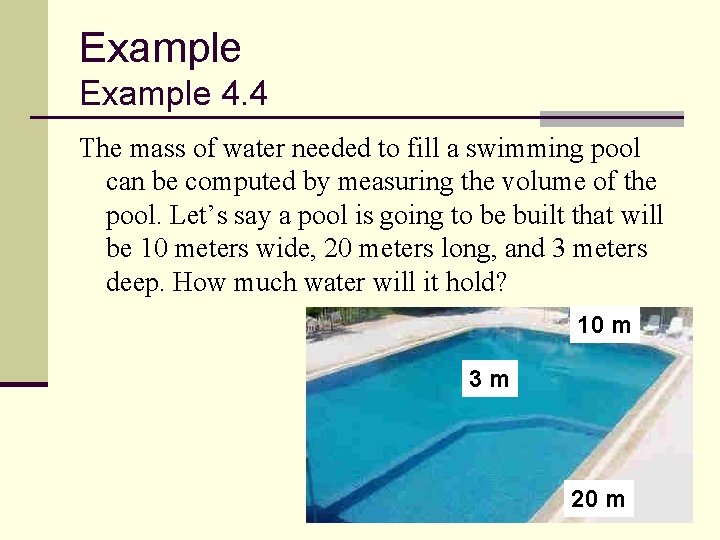
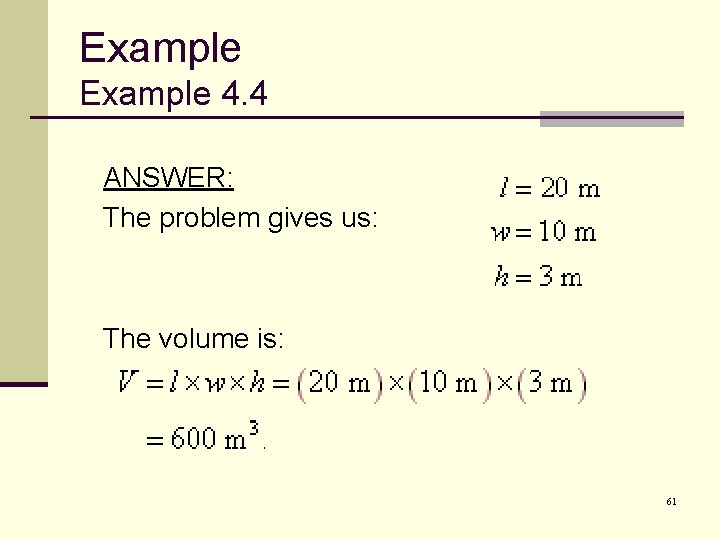
Example 4. 4 The mass of water needed to fill a swimming pool can be computed by measuring the volume of the pool. Let’s say a pool is going to be built that will be 10 meters wide, 20 meters long, and 3 meters deep. How much water will it hold? 10 m 3 m 20 m 60

Example 4. 4 ANSWER: The problem gives us: The volume is: 61

Example 4. 4 ANSWER: Since the density of water is 1, 000 kg/m 3, the amount of water is: 62

Example 4. 4 DISCUSSION: n This is over 1, 300, 000 lbs; the structure of the swimming pool must be strong enough to support this weight 63

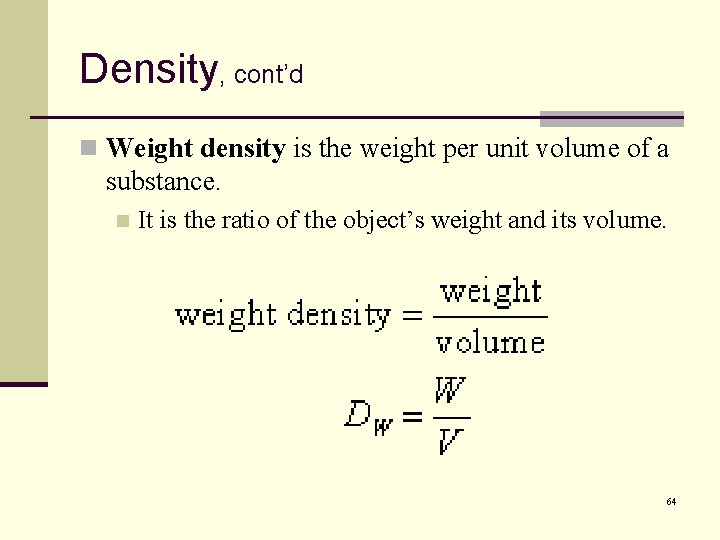
Density, cont’d n Weight density is the weight per unit volume of a substance. n It is the ratio of the object’s weight and its volume. 64

Density, cont’d n Units or weight density: n Metric: n n newton per cubic meter (N/m 3). English: n n pound per cubic foot (lb/ft 3); pound per cubic inch (lb/in 3). 65

Example 4. 5 A college dormitory room measures 12 feet wide by 16 feet long by 8 feet high. What is the weight of the air in it under normal conditions? 16 ft 12 ft 8 ft 66

Example 4. 5 ANSWER: The problem gives us: The volume is: V = l * w * h = (16 ft) * (12 ft) * (8 ft) V = 1536 ft 3 67

Example 4. 5 ANSWER: The weight of the air in the room is 68

1, 000 troy ounces of gold weighs 68, 600 lbs. What is its volume? DW = W/V V = W/DW V = 57. 17 ft 3 (1. 6 m 3 ) This is the total output of 11 years’ mining at a major Australian gold mine, No wonder gold is so expensive! 69

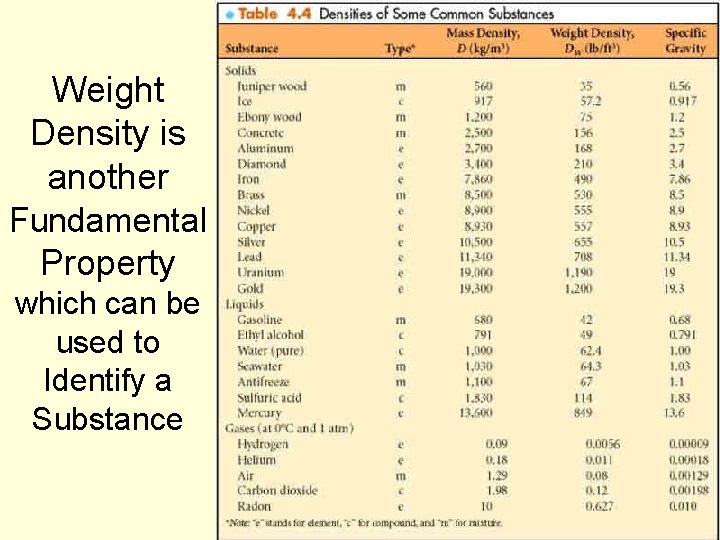
Weight Density is another Fundamental Property which can be used to Identify a Substance 70

Weight Density vs Mass Density Weight Density is the mass density times the acceleration of gravity W = mg DW = Dg Weight Density is typically used with English units since weight is used more generally than mass in English units

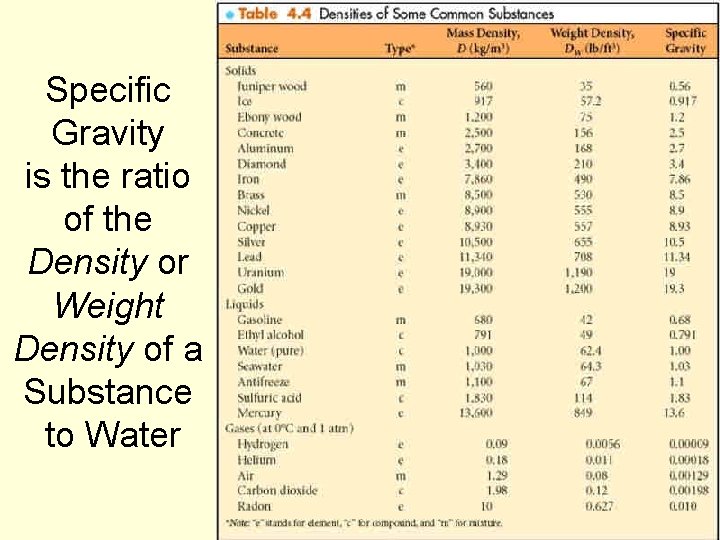
Specific Gravity is the ratio of the Density or Weight Density of a Substance to Water

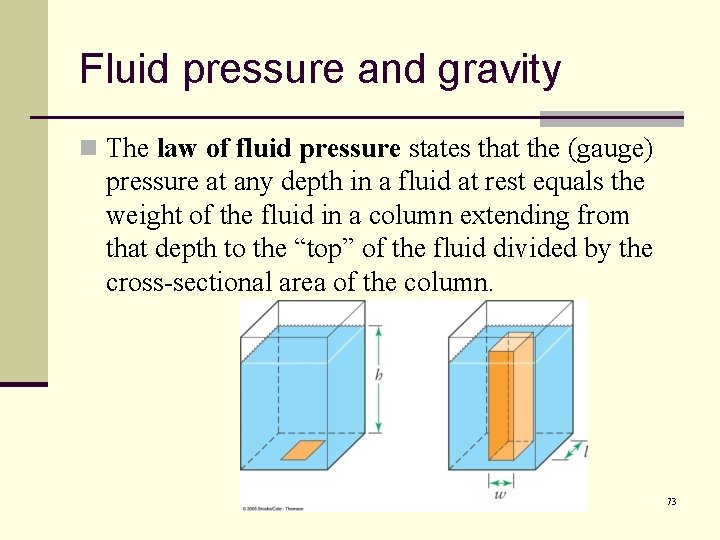
Fluid pressure and gravity n The law of fluid pressure states that the (gauge) pressure at any depth in a fluid at rest equals the weight of the fluid in a column extending from that depth to the “top” of the fluid divided by the cross-sectional area of the column. 73

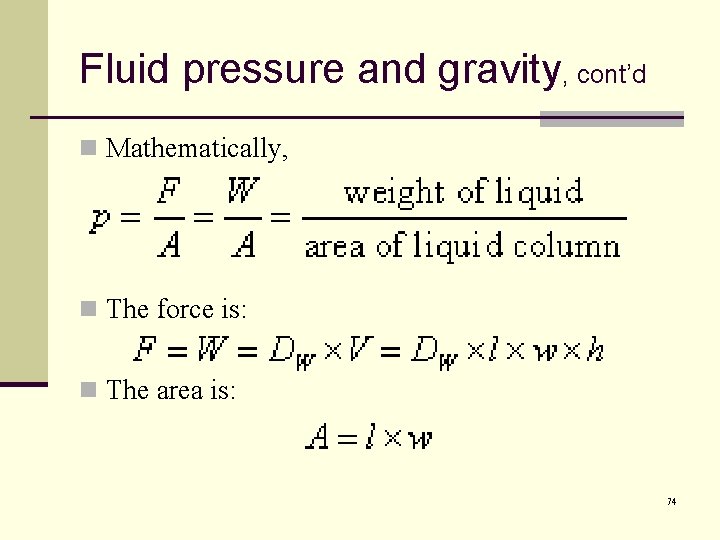
Fluid pressure and gravity, cont’d n Mathematically, n The force is: n The area is: 74

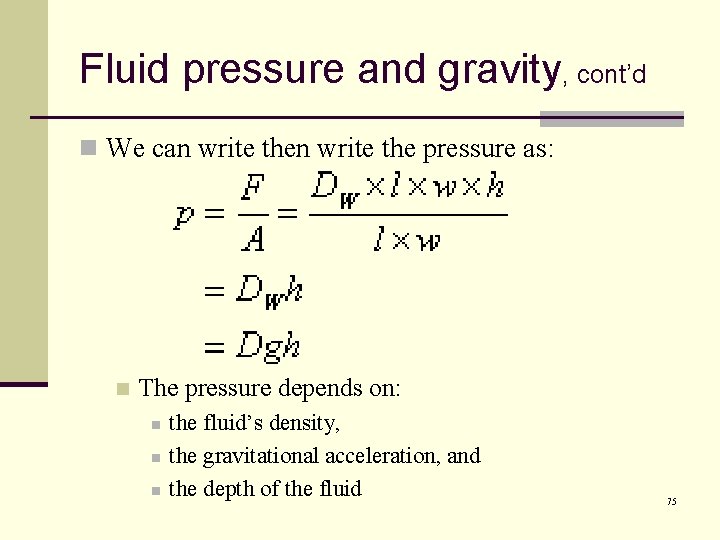
Fluid pressure and gravity, cont’d n We can write the pressure as: n The pressure depends on: n n n the fluid’s density, the gravitational acceleration, and the depth of the fluid 75

Fluid pressure and gravity, cont’d n Note the relative pressure depends only on the depth. n This is why water comes out faster at the bottom of the boot than at the top. p = DWh = Dgh 76

p = DWh = Dgh This is also why more concrete is needed at the bottom of the dam than at the top. 77

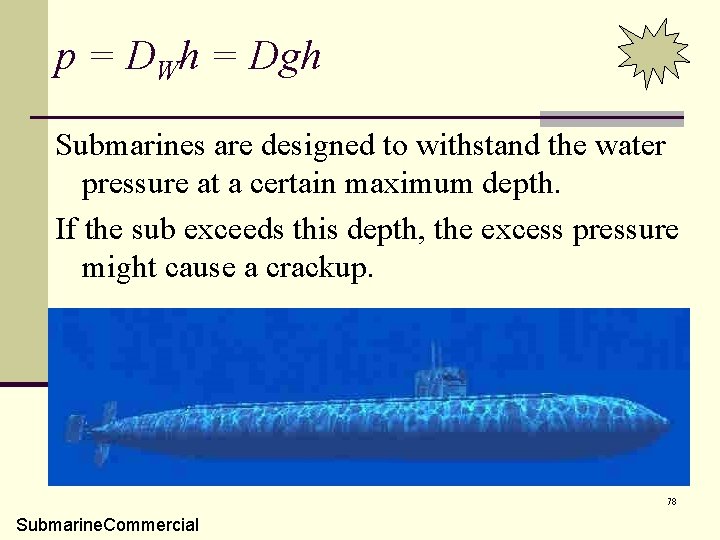
p = DWh = Dgh Submarines are designed to withstand the water pressure at a certain maximum depth. If the sub exceeds this depth, the excess pressure might cause a crackup. 78 Submarine. Commercial

Example 4. 6 Let’s calculate the gauge pressure at the bottom of a typical swimming pool — one that is 10 feet (3. 05 meters) deep. 10 m 3 m 20 m 79
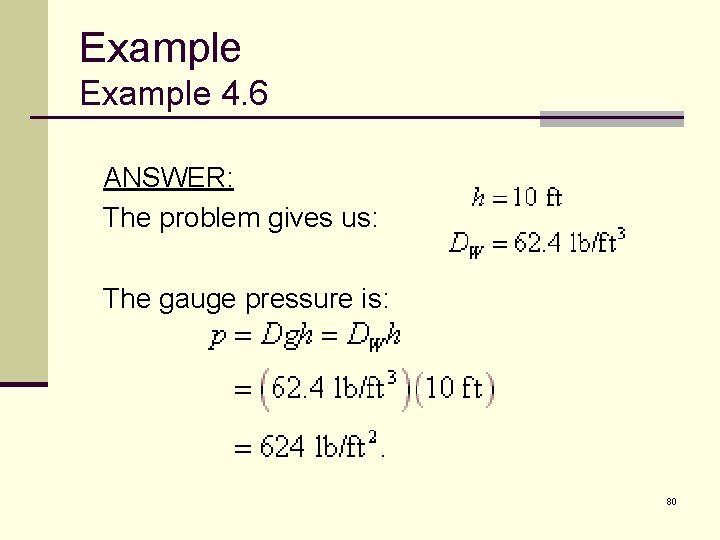
Example 4. 6 ANSWER: The problem gives us: The gauge pressure is: 80

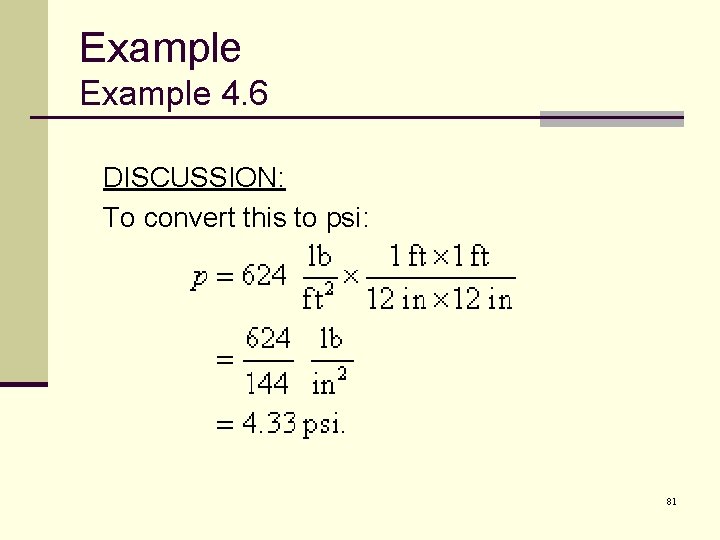
Example 4. 6 DISCUSSION: To convert this to psi: 81

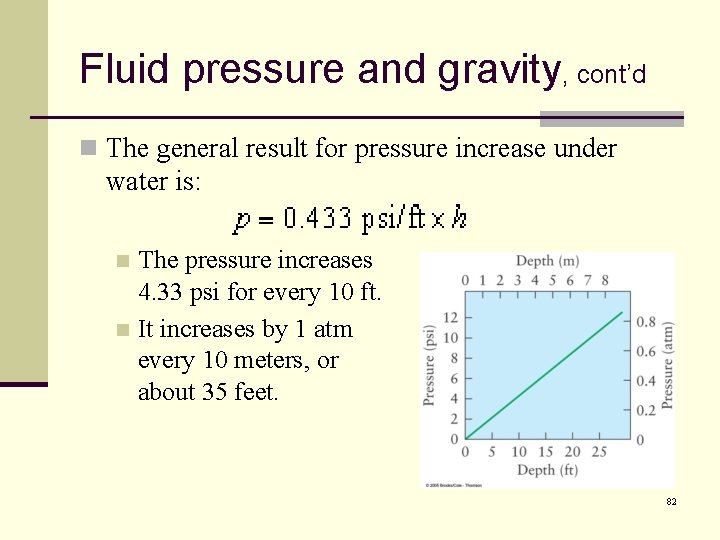
Fluid pressure and gravity, cont’d n The general result for pressure increase under water is: The pressure increases 4. 33 psi for every 10 ft. n It increases by 1 atm every 10 meters, or about 35 feet. n 82

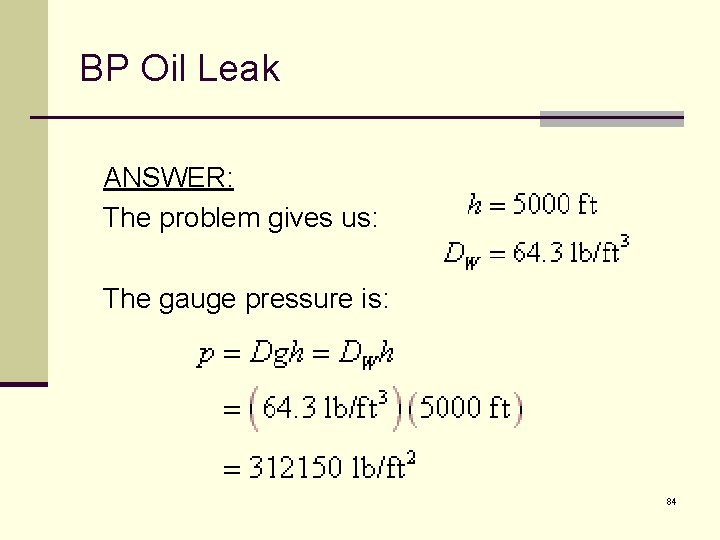
BP Oil Leak n Why was it so hard to “Plug the damn hole” (Pres Obama) at the BP Deep Water Horizons Oil leak? n The wellhead is at a depth of 5000 feet. n US Navy’s DSRV Mystic submarine rescue vehicle only goes to 2000 ft – 40% of this depth. n What is the pressure in 5000 feet of seawater?

BP Oil Leak ANSWER: The problem gives us: The gauge pressure is: 84

BP Oil Leak n Why was it so hard to “Plug the damn hole” (Pres Obama) at the BP Deep Water Horizons Oil leak? n This means. . . if the area of the hole is 1 ft 2, there was 312, 150 lbs of pressure on the cap as robots positioned it. If it was 10 ft 2, there was 3, 121, 500 lbs of pressure!

Fluid pressure and gravity, cont’d n Recall. . . this is the gauge pressure at the depth h. n To get the absolute pressure, we need to add the atmospheric pressure pushing down at the top of the fluid column. n To measure this, we must make a vacuum (zero gas molecules zero pressure) at the top of a fluid column 86

Fluid pressure and gravity, cont’d Measuring absolute pressure: n Top of column has zero pressure n Surface of fluid experiences pressure from atmosphere. n Below surface of fluid, the pressure must be the same – whether or not below the column. (Otherwise, fluid would move until pressure equalizes. ) n Hence pressure from the atmosphere is equivalent to the weight of the fluid column 87

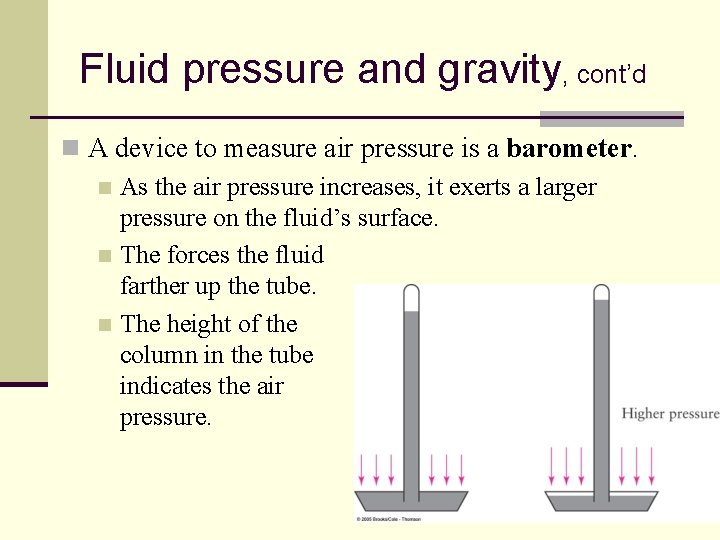
Fluid pressure and gravity, cont’d n A device to measure air pressure is a barometer. n As the air pressure increases, it exerts a larger pressure on the fluid’s surface. n The forces the fluid farther up the tube. n The height of the column in the tube indicates the air pressure. 88

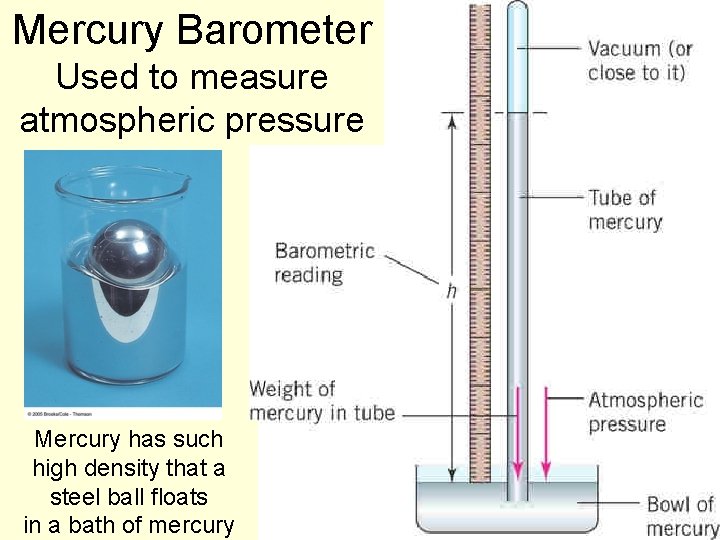
Mercury Barometer Used to measure atmospheric pressure Mercury has such high density that a steel ball floats in a bath of mercury

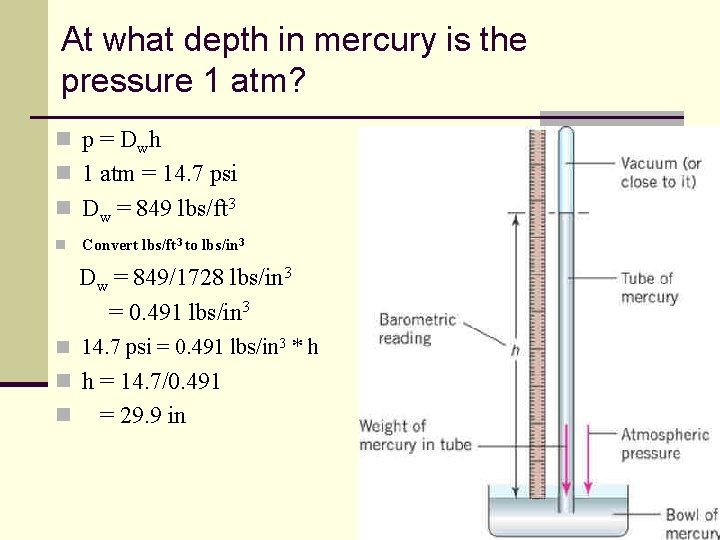
At what depth in mercury is the pressure 1 atm? n p = D wh n 1 atm = 14. 7 psi n Dw = 849 lbs/ft 3 n Convert lbs/ft 3 to lbs/in 3 Dw = 849/1728 lbs/in 3 = 0. 491 lbs/in 3 n 14. 7 psi = 0. 491 lbs/in 3 * h n h = 14. 7/0. 491 n = 29. 9 in 90

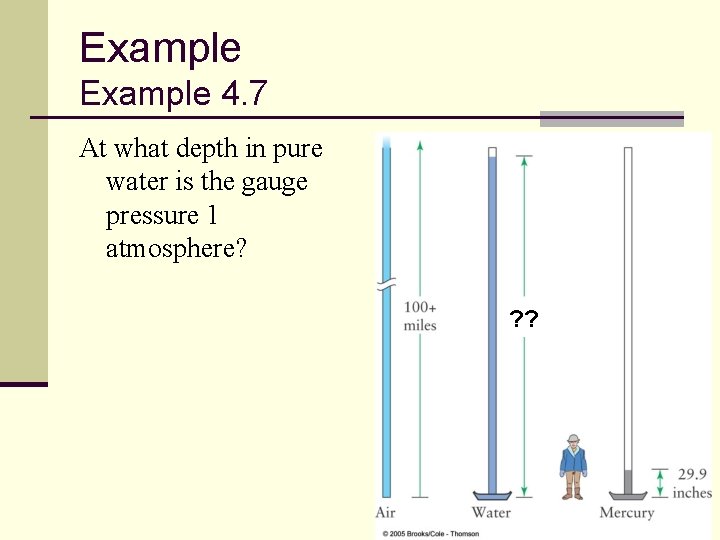
Example 4. 7 At what depth in pure water is the gauge pressure 1 atmosphere? ? ? 91

Example 4. 7 ANSWER: The problem gives us: The pressure depends on depth according to: Rearranging for the depth: 92

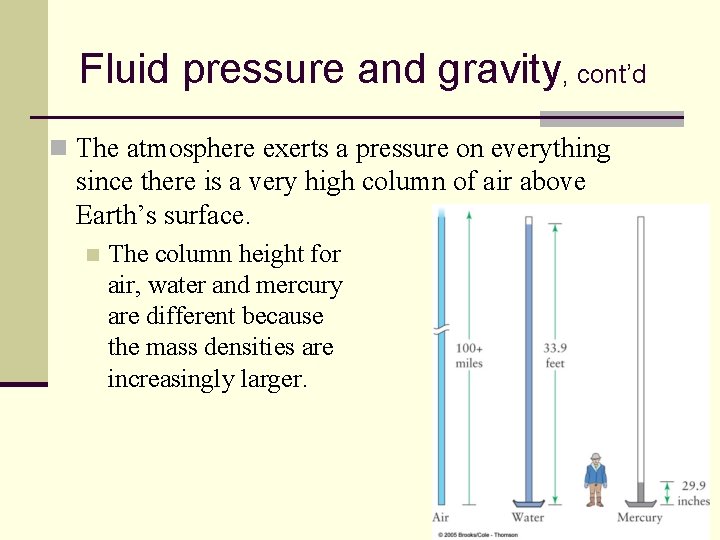
Fluid pressure and gravity, cont’d n The atmosphere exerts a pressure on everything since there is a very high column of air above Earth’s surface. n The column height for air, water and mercury are different because the mass densities are increasingly larger. 93

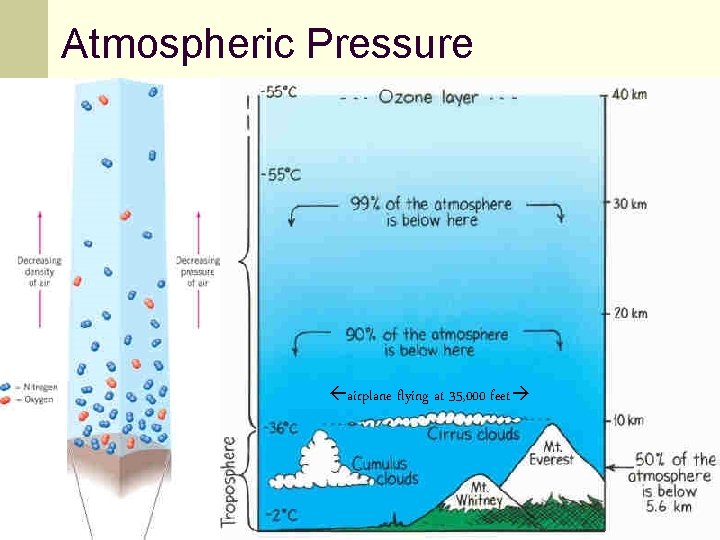
Atmospheric Pressure airplane flying at 35, 000 feet 94

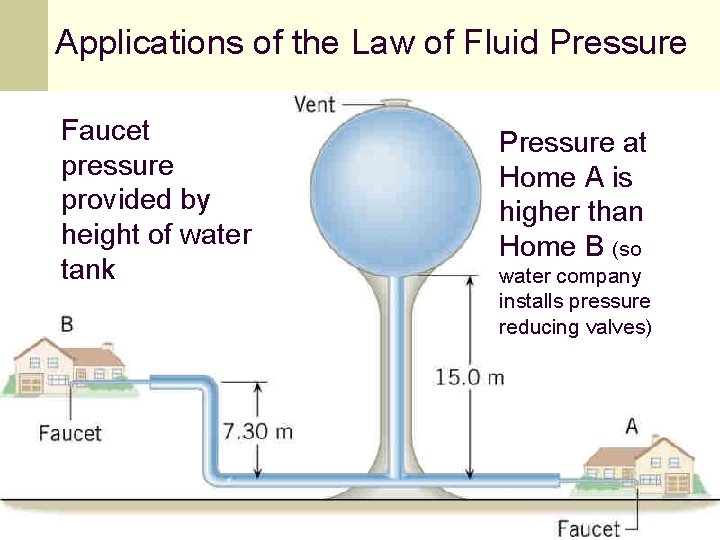
Applications of the Law of Fluid Pressure Faucet pressure provided by height of water tank Pressure at Home A is higher than Home B (so water company installs pressure reducing valves) 95

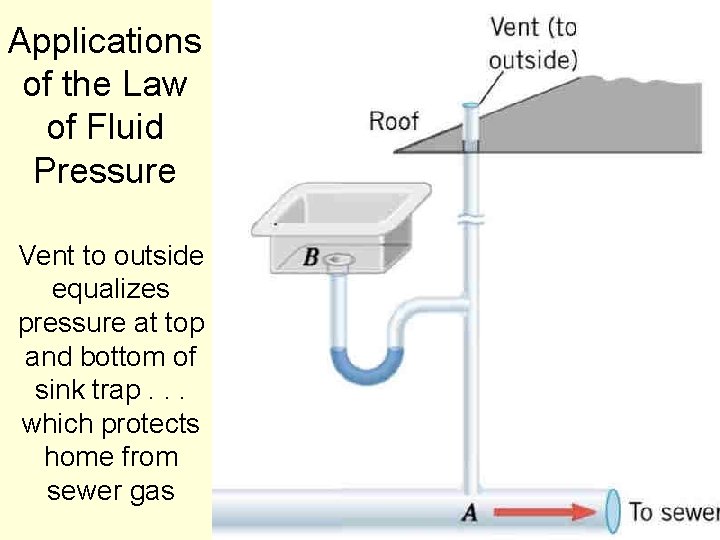
Applications of the Law of Fluid Pressure Vent to outside equalizes pressure at top and bottom of sink trap. . . which protects home from sewer gas 96

Aneroid Barometer More “portable” than mercury barometer Operates on gauge pressure – calibrated to standard atmospheric pressure 97

Archimedes’ Principle n It’s about 250 BC. The king of Syracuse – a brutal tyrant – thinks he has been cheated: he suspects his new crown is not pure gold. n He asks Archimedes to prove or disprove this – without taking any metal off the crown Archimedes 98

Archimedes’ Principle Eureka!! n Stepping into the public bath, Archimedes notices that the water rises higher, and that the water exerts an upward force on him! n He realizes this is the key to answering the king’s question. According to legend. . . he was so excited by this discovery that he ran home naked! 99

Archimedes’ principle, cont’d n Archimedes discovered the buoyant force: an upward force exerted by a fluid on a substance partly or completely immersed in the fluid. n The buoyant force depends on the density of the fluid and on the substance. 100

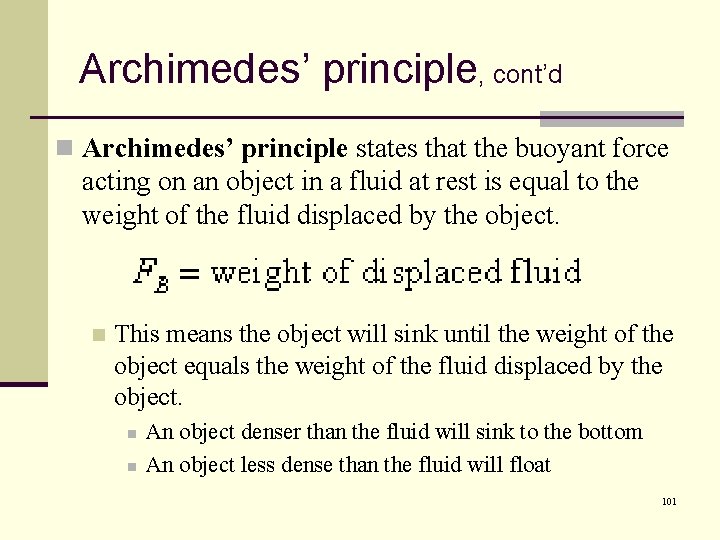
Archimedes’ principle, cont’d n Archimedes’ principle states that the buoyant force acting on an object in a fluid at rest is equal to the weight of the fluid displaced by the object. n This means the object will sink until the weight of the object equals the weight of the fluid displaced by the object. n n An object denser than the fluid will sink to the bottom An object less dense than the fluid will float 101

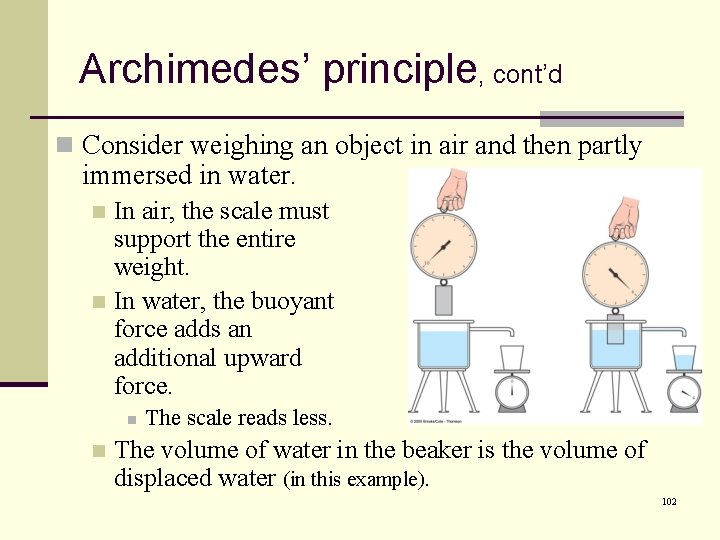
Archimedes’ principle, cont’d n Consider weighing an object in air and then partly immersed in water. In air, the scale must support the entire weight. n In water, the buoyant force adds an additional upward force. n n n The scale reads less. The volume of water in the beaker is the volume of displaced water (in this example). 102

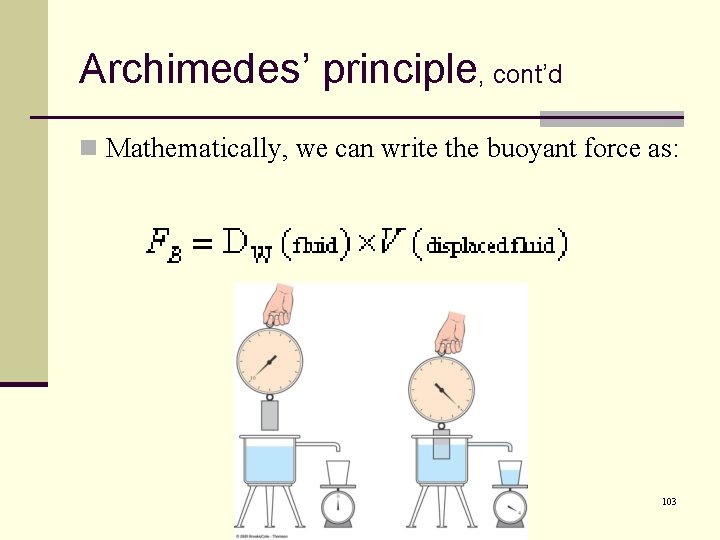
Archimedes’ principle, cont’d n Mathematically, we can write the buoyant force as: 103

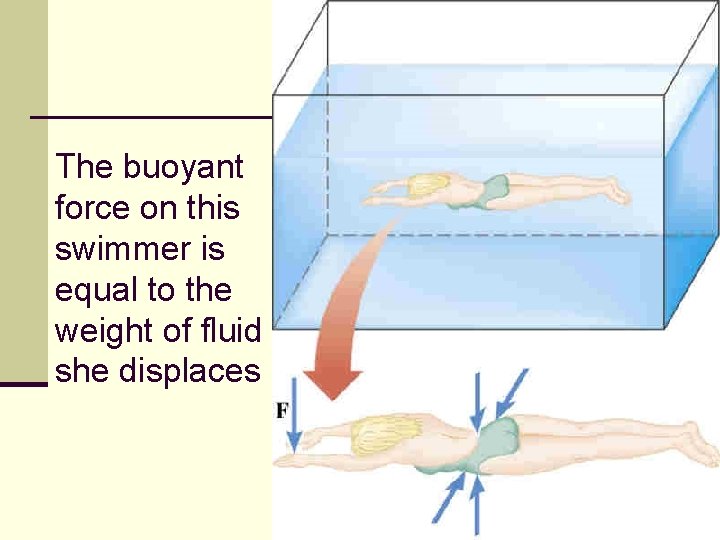
The buoyant force on this swimmer is equal to the weight of fluid she displaces 104

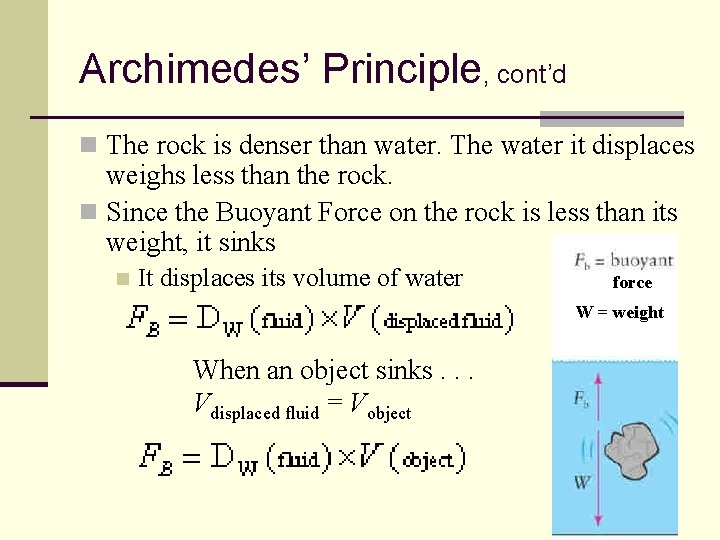
Archimedes’ Principle, cont’d n The rock is denser than water. The water it displaces weighs less than the rock. n Since the Buoyant Force on the rock is less than its weight, it sinks n It displaces its volume of water force W = weight When an object sinks. . . Vdisplaced fluid = Vobject 105

Archimedes’ Principle, cont’d In case you wondered. . . n Archimedes weighed the crown n He put it in a water bath and determined its volume from the volume of water displaced n He deduced the material from the formula: DW = W/V n The crown was not pure gold, and the goldsmith who cheated the king was tortured so horribly that death became a welcome relief! 106

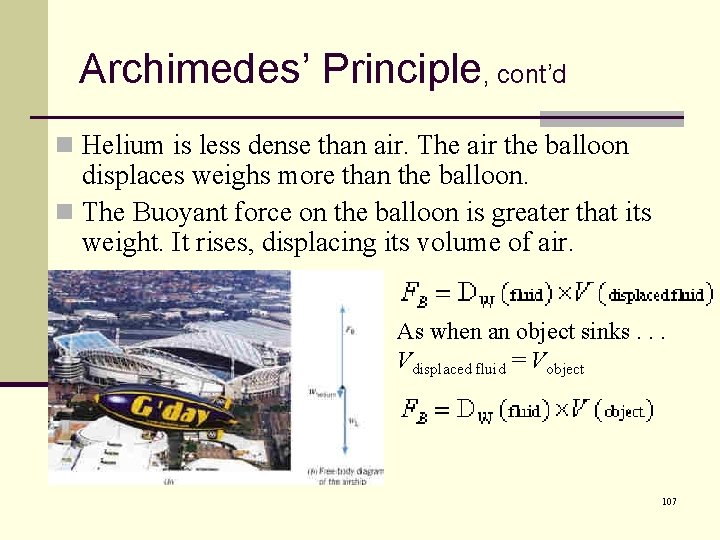
Archimedes’ Principle, cont’d n Helium is less dense than air. The air the balloon displaces weighs more than the balloon. n The Buoyant force on the balloon is greater that its weight. It rises, displacing its volume of air. As when an object sinks. . . Vdisplaced fluid = Vobject 107

Archimedes’ Principle, cont’d Archimedes Principle and Floating Objects n Careful!!. . . There is a subtle difference if the substance is less dense than water (and floats). When an object floats. . . Vdisplaced fluid ≠ Vobject 108

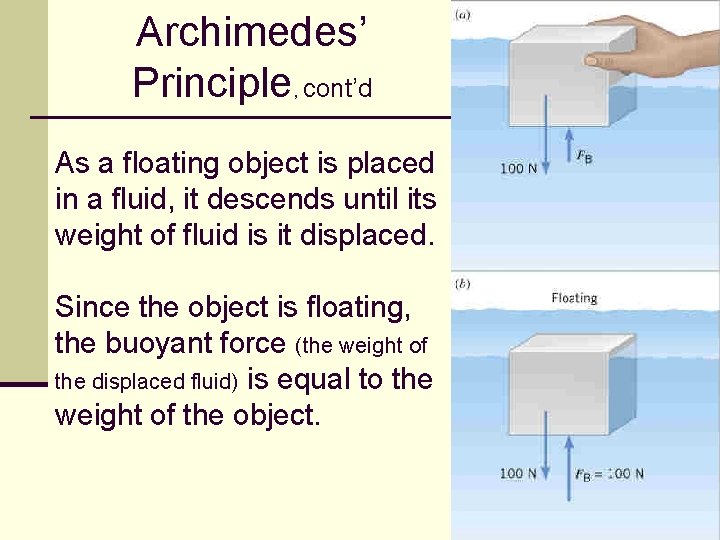
Archimedes’ Principle, cont’d As a floating object is placed in a fluid, it descends until its weight of fluid is it displaced. Since the object is floating, the buoyant force (the weight of the displaced fluid) is equal to the weight of the object. 109

Archimedes’ Principle, cont’d 106

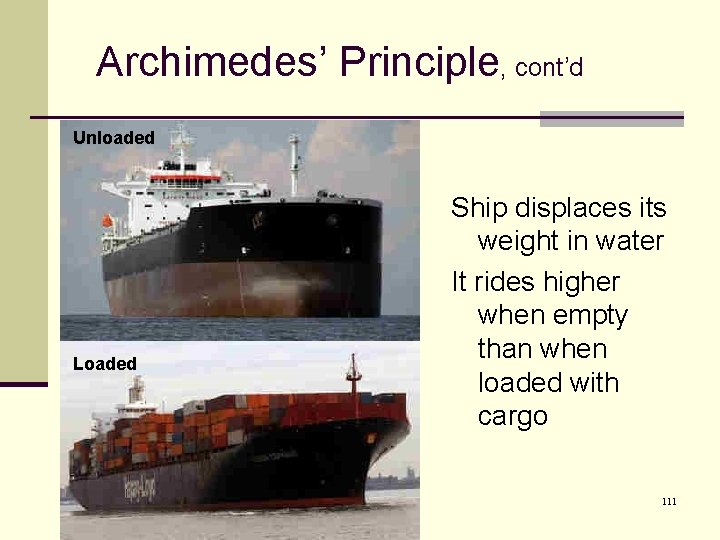
Archimedes’ Principle, cont’d Unloaded Loaded Ship displaces its weight in water It rides higher when empty than when loaded with cargo 111

Archimedes’ Principle, cont’d USS Ronald Reagan passing USS Arizona Memorial, Pearl Harbor USS Reagan floats because its density is lower than the water Ship displaces its weight in water 112

Archimedes’ Principle with an Iceberg floats because its density is lower than the water Iceberg displaces its weight in water Since density of ice is very close to density of water, most of iceberg is below the surface 113

Example 4. 8 A modern Huckleberry Finn wants to construct a raft by attaching empty, plastic, 1 -gallon milk jugs to the bottom of a sheet of plywood. The raft and passengers will have a total weight of 300 pounds. How many jugs are required to keep the raft afloat on water? 114

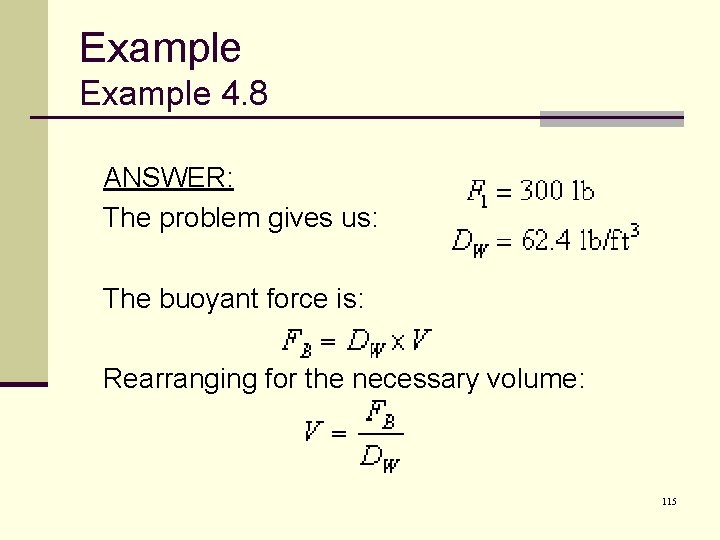
Example 4. 8 ANSWER: The problem gives us: The buoyant force is: Rearranging for the necessary volume: 115

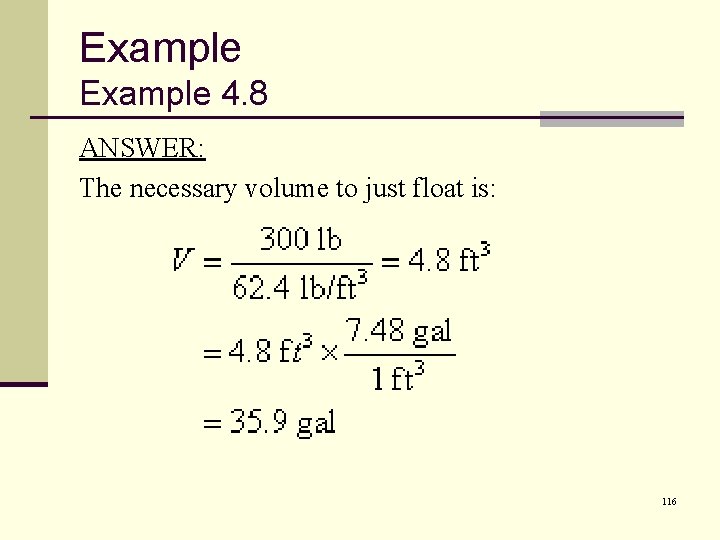
Example 4. 8 ANSWER: The necessary volume to just float is: 116

Example 4. 8 DISCUSSION: So Huck needs at least 36 jugs to just float with a small (0. 1 gal) margin of error. For a safety margin, he should probably have more than just 36 jugs — just in case a wave rolls through or he wants to take a friend along. 117

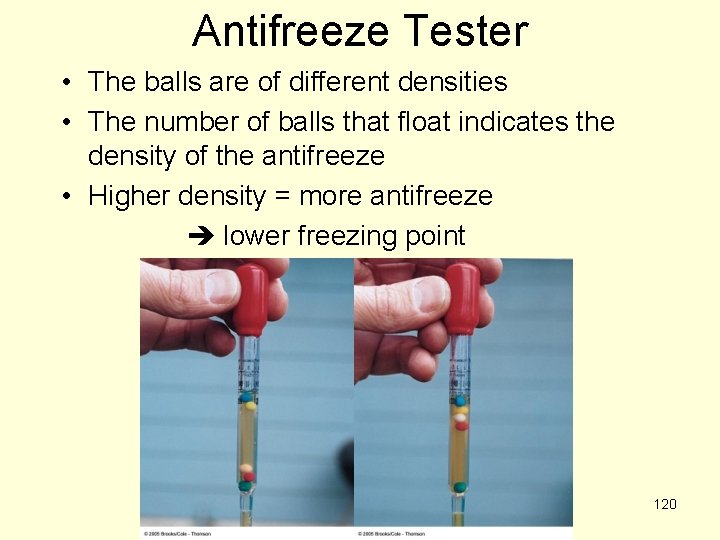
Archimedes’ Principle with an Antifreeze Tester 118

Archimedes’ Principle with an Antifreeze Tester, cont’d n An antifreeze tested uses Archimedes’ Principle to determine the freezing point of your car’s coolant by measuring its density. n The density of water and antifreeze are different. n n n Water: 1, 000 kg/m 3. Antifreeze: 1, 100 kg/m 3. The overall density depends on the ratio of the amount of water and amount of antifreeze. n A 50 -50 mixture has a density of 1, 050 kg/m 3 119

Antifreeze Tester • The balls are of different densities • The number of balls that float indicates the density of the antifreeze • Higher density = more antifreeze lower freezing point 120

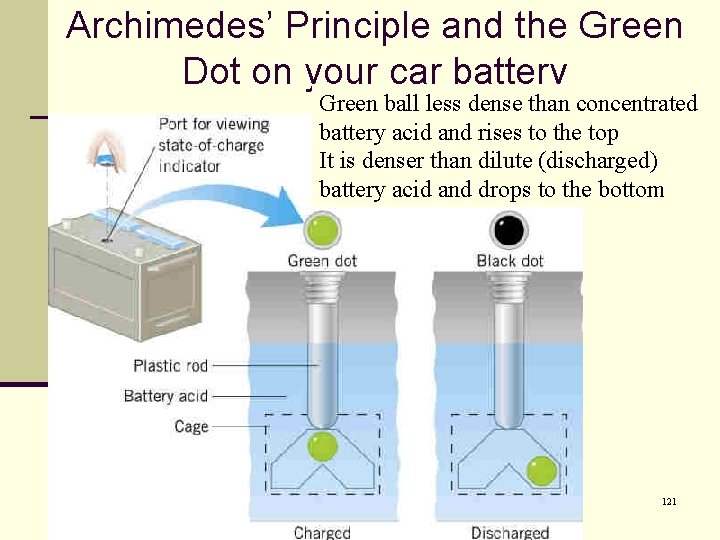
Archimedes’ Principle and the Green Dot on your car battery Green ball less dense than concentrated battery acid and rises to the top It is denser than dilute (discharged) battery acid and drops to the bottom 121

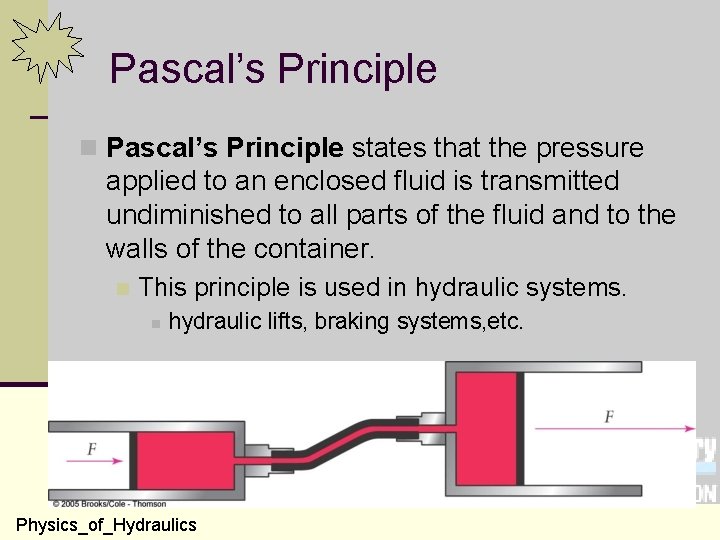
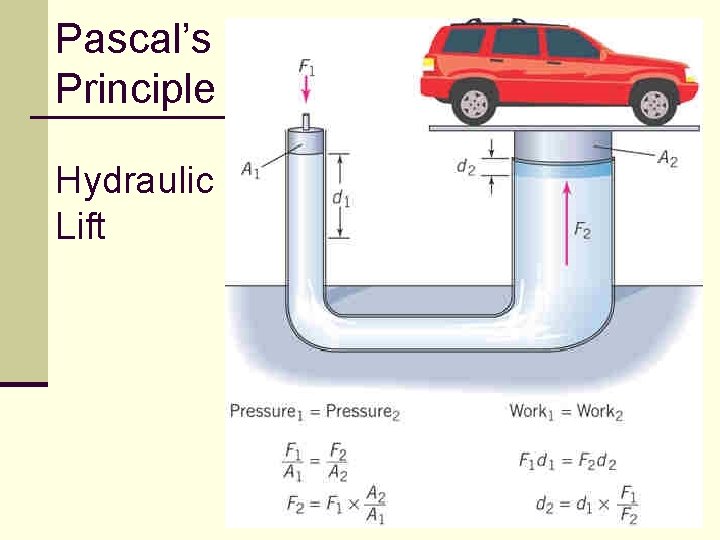
Pascal’s Principle n Pascal’s Principle states that the pressure applied to an enclosed fluid is transmitted undiminished to all parts of the fluid and to the walls of the container. n This principle is used in hydraulic systems. n hydraulic lifts, braking systems, etc. 122 Physics_of_Hydraulics

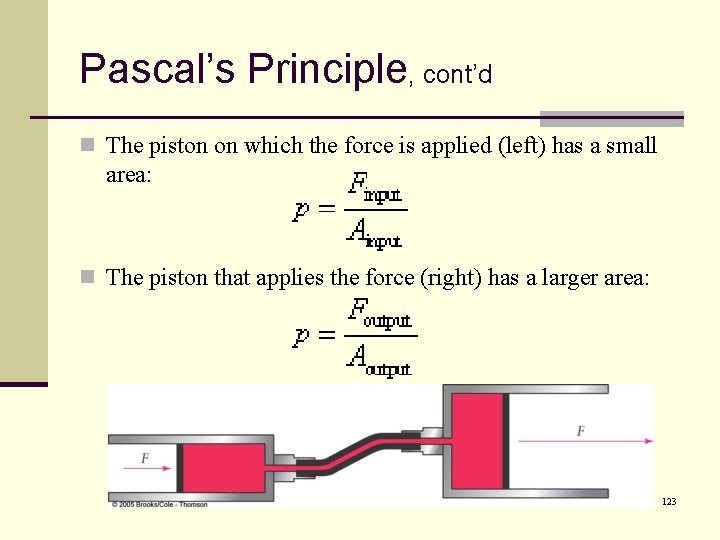
Pascal’s Principle, cont’d n The piston on which the force is applied (left) has a small area: n The piston that applies the force (right) has a larger area: 123

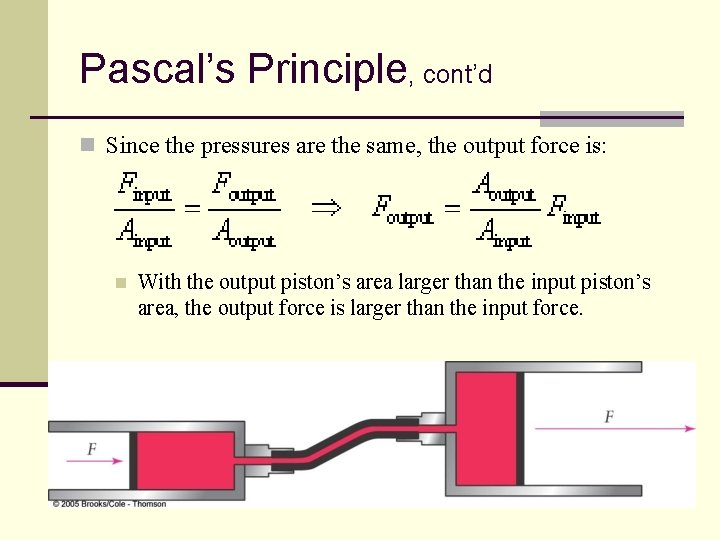
Pascal’s Principle, cont’d n Since the pressures are the same, the output force is: n With the output piston’s area larger than the input piston’s area, the output force is larger than the input force. 124

Pascal’s Principle Hydraulic Lift 125

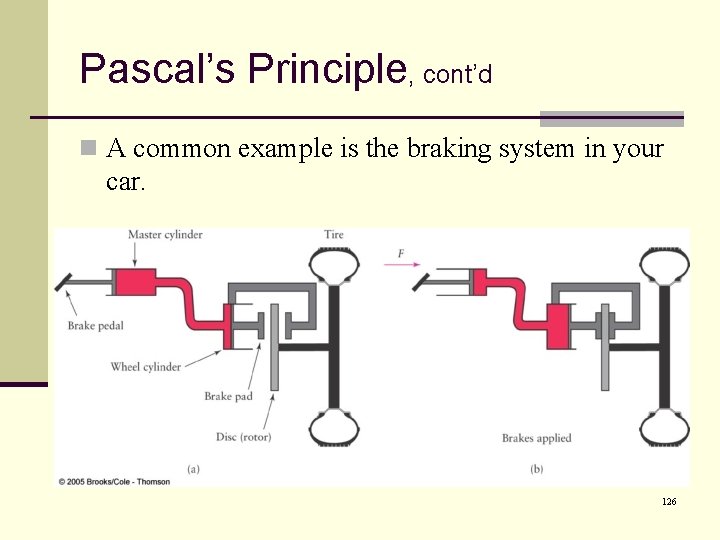
Pascal’s Principle, cont’d n A common example is the braking system in your car. 126

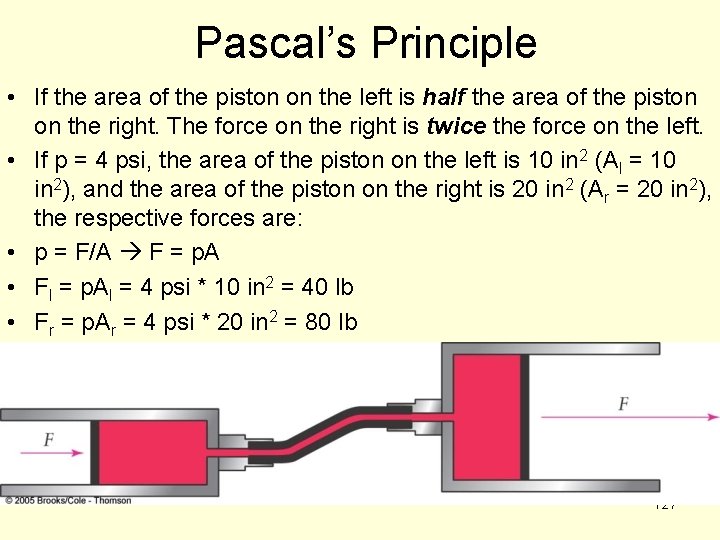
Pascal’s Principle • If the area of the piston on the left is half the area of the piston on the right. The force on the right is twice the force on the left. • If p = 4 psi, the area of the piston on the left is 10 in 2 (Al = 10 in 2), and the area of the piston on the right is 20 in 2 (Ar = 20 in 2), the respective forces are: • p = F/A F = p. A • Fl = p. Al = 4 psi * 10 in 2 = 40 lb • Fr = p. Ar = 4 psi * 20 in 2 = 80 lb 127

Bernoulli’s principle n So far we have spoken only of static fluids. n Now we talk about moving fluids. n Bernoulli’s principle states that for a fluid undergoing steady flow, the pressure is lower where the fluid is flowing faster. n Steady flow means the fluid flow is smooth. n No random swirling, eddies, or “white water. ” n Bernoulli’s principle reflects Mass Conservation and Energy Conservation 128

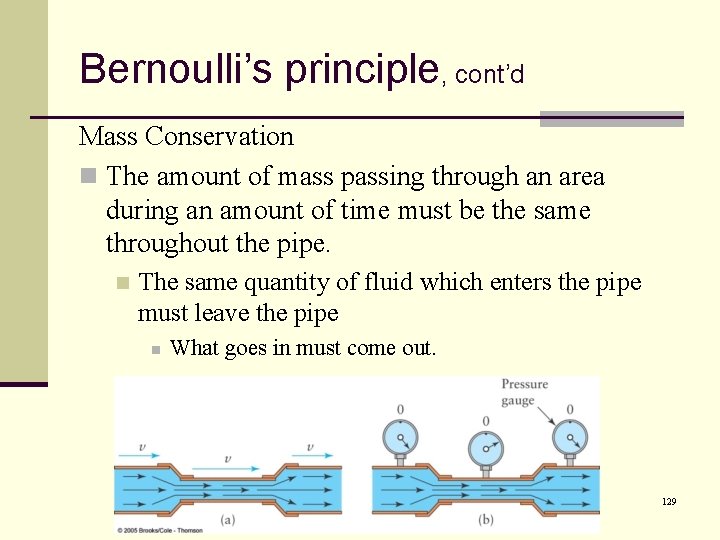
Bernoulli’s principle, cont’d Mass Conservation n The amount of mass passing through an area during an amount of time must be the same throughout the pipe. n The same quantity of fluid which enters the pipe must leave the pipe n What goes in must come out. 129

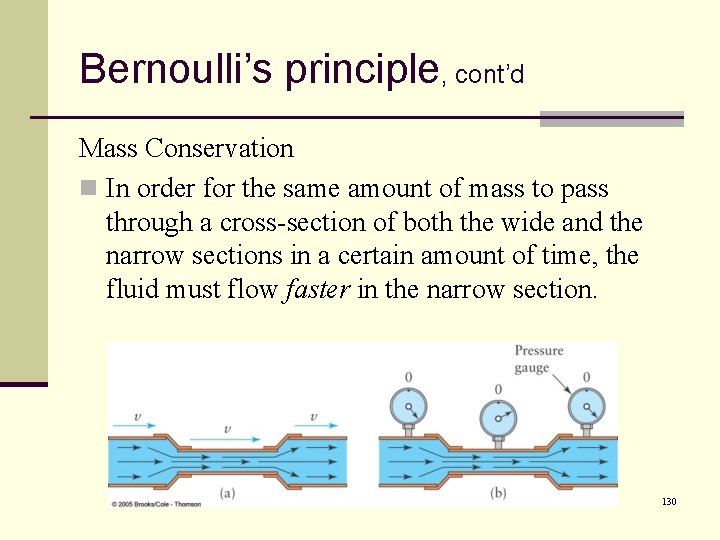
Bernoulli’s principle, cont’d Mass Conservation n In order for the same amount of mass to pass through a cross-section of both the wide and the narrow sections in a certain amount of time, the fluid must flow faster in the narrow section. 130

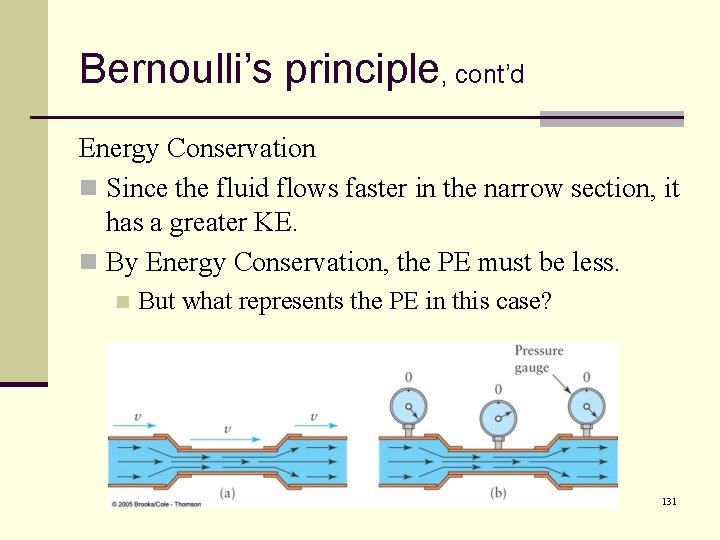
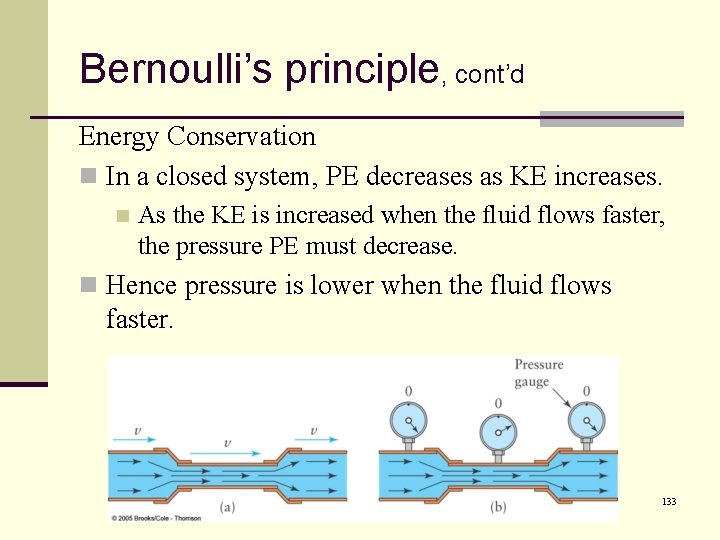
Bernoulli’s principle, cont’d Energy Conservation n Since the fluid flows faster in the narrow section, it has a greater KE. n By Energy Conservation, the PE must be less. n But what represents the PE in this case? 131

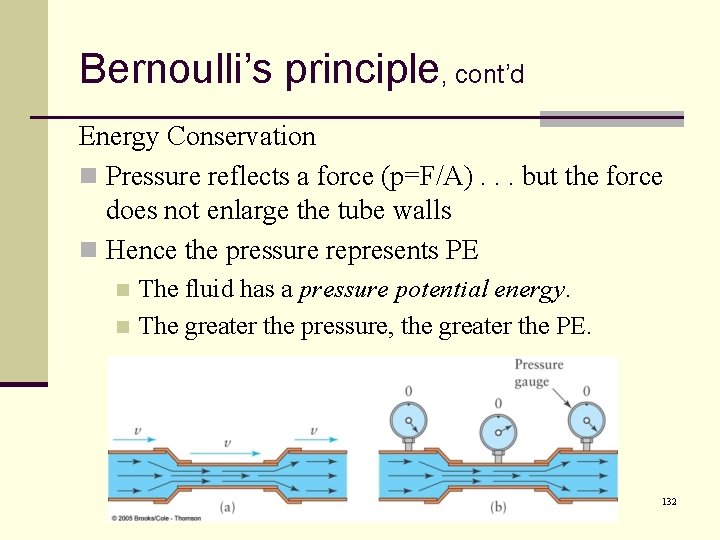
Bernoulli’s principle, cont’d Energy Conservation n Pressure reflects a force (p=F/A). . . but the force does not enlarge the tube walls n Hence the pressure represents PE The fluid has a pressure potential energy. n The greater the pressure, the greater the PE. n 132

Bernoulli’s principle, cont’d Energy Conservation n In a closed system, PE decreases as KE increases. n As the KE is increased when the fluid flows faster, the pressure PE must decrease. n Hence pressure is lower when the fluid flows faster. 133

Bernouli’s Principle 134

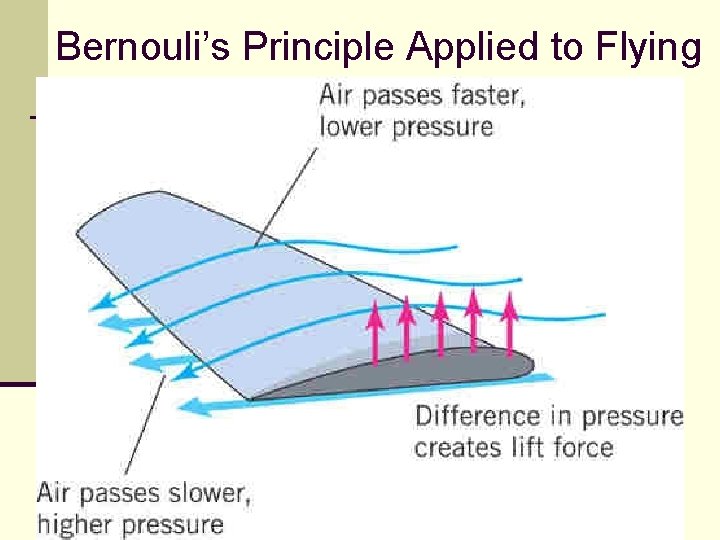
Bernouli’s Principle Applied to Flying 135

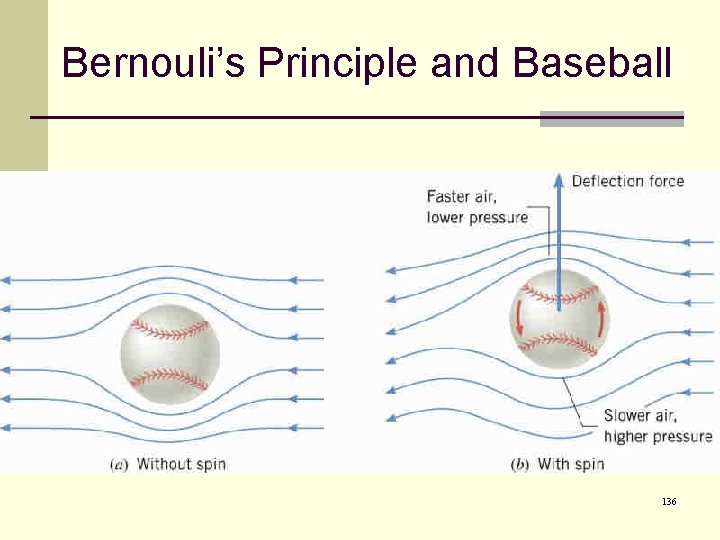
Bernouli’s Principle and Baseball 136

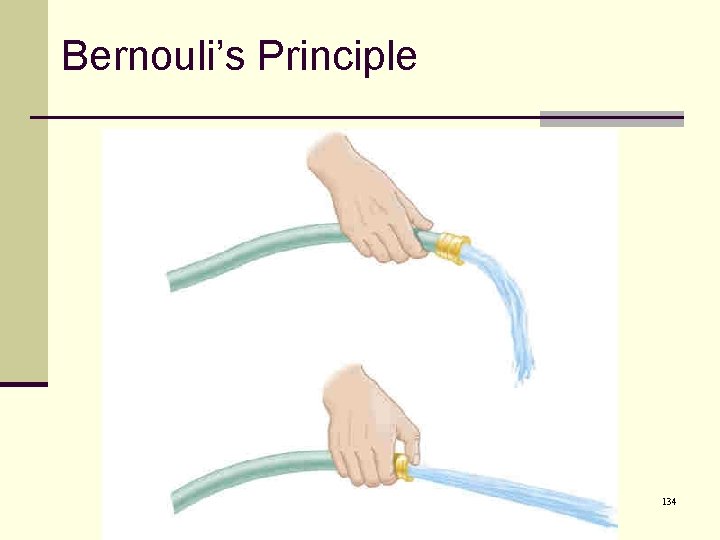
Bernoulli’s principle, cont’d n Another example is an atomizer. n When you squeeze the bulb, you increase the pressure in the nozzle. n This lowers the pressure in the nozzle. n The higher pressure in the bottle draws the fluid up the tube. n The moving air carries the fluid out of the nozzle. 137

Bernouli’s Principle and the peregrine falcon n n An “ideal” peregrine falcon dives at an angle of 45 o, and its velocity accelerates from 15 m/s to 80 m/s in 20 s. According to Bernouli’s Principle, the 80 m/s air passing over the falcon’s beak must have substantially lower pressure than the air in the falcon’s lungs – so the air would be sucked out of its lungs when it breathes during the dive. But a unique baffle system in the falcon’s beak allow it to breathe at this high speed. No other bird – or bird fossil – has anything like it. 138

Important Equations 139

END