Organic Chemistry 5 th Edition L G Wade











- Slides: 55

Organic Chemistry, 5 th Edition L. G. Wade, Jr. Chapter 8 Reactions of Alkenes Jo Blackburn Richland College, Dallas, TX Dallas County Community College District Chapter 8 ã 2003, Prentice Hall

Reactivity of C=C • Electrons in pi bond are loosely held. • Electrophiles are attracted to the pi electrons. • Carbocation intermediate forms. • Nucleophile adds to the carbocation. • Net result is addition to the double bond. => Chapter 8 2

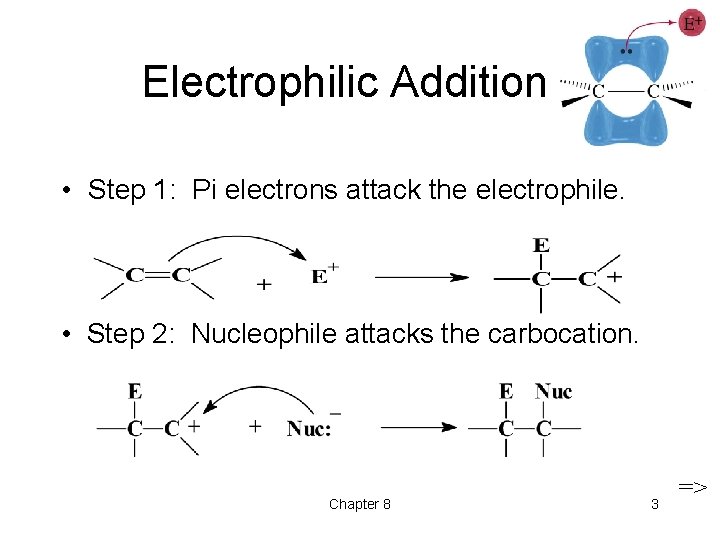
Electrophilic Addition • Step 1: Pi electrons attack the electrophile. • Step 2: Nucleophile attacks the carbocation. Chapter 8 3 =>

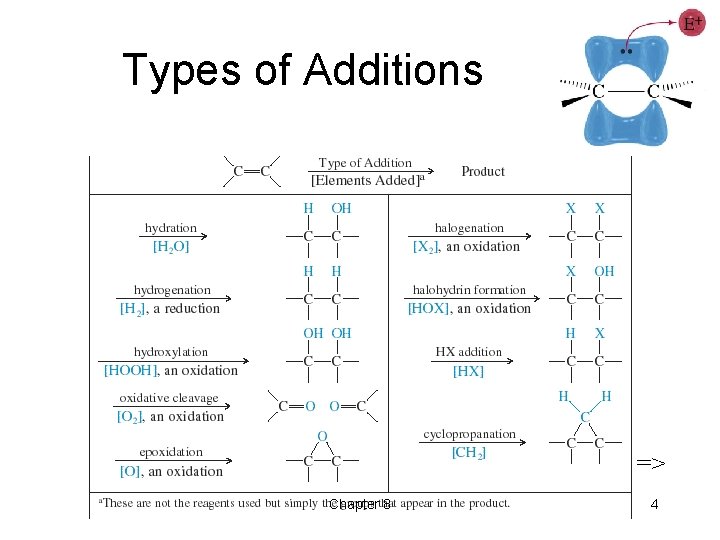
Types of Additions => Chapter 8 4

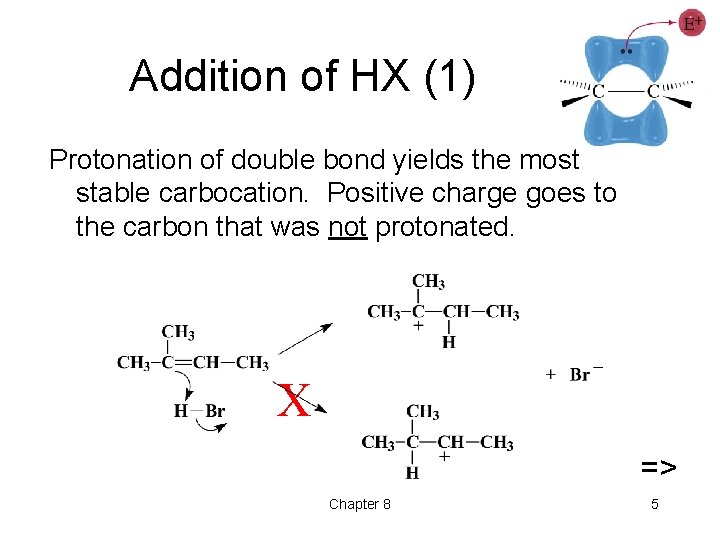
Addition of HX (1) Protonation of double bond yields the most stable carbocation. Positive charge goes to the carbon that was not protonated. X => Chapter 8 5

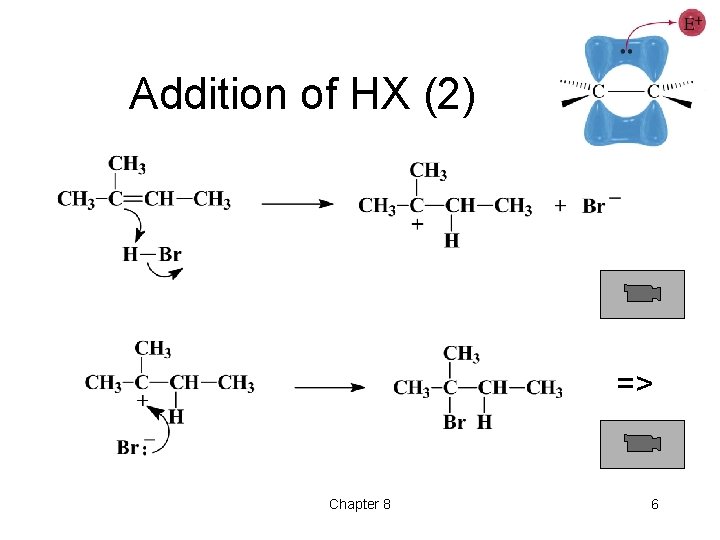
Addition of HX (2) => Chapter 8 6

Regiospecificity • Markovnikov’s Rule: The proton of an acid adds to the carbon in the double bond that already has the most H’s. “Rich get richer. ” • More general Markovnikov’s Rule: In an electrophilic addition to an alkene, the electrophile adds in such a way as to form the most stable intermediate. • HCl, HBr, and HI add to alkenes to form Markovnikov products. => Chapter 8 7

Free-Radical Addition of HBr • In the presence of peroxides, HBr adds to an alkene to form the “anti. Markovnikov” product. • Only HBr has the right bond energy. • HCl bond is too strong. • HI bond tends to break heterolytically to form ions. => Chapter 8 8

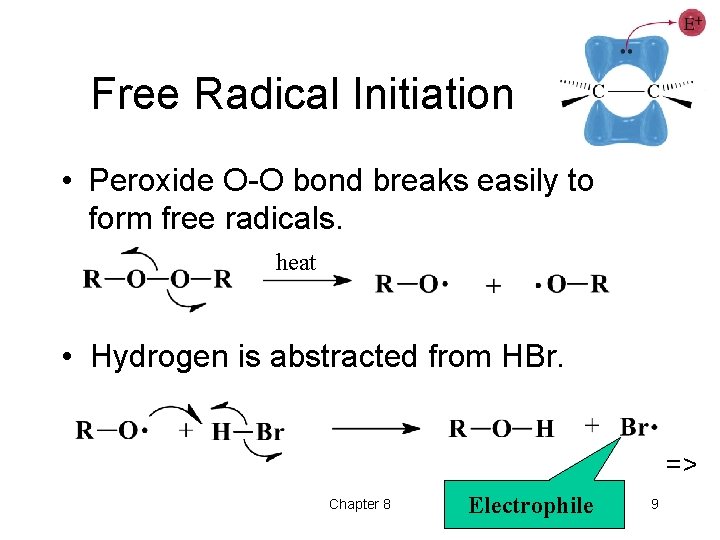
Free Radical Initiation • Peroxide O-O bond breaks easily to form free radicals. heat • Hydrogen is abstracted from HBr. => Chapter 8 Electrophile 9

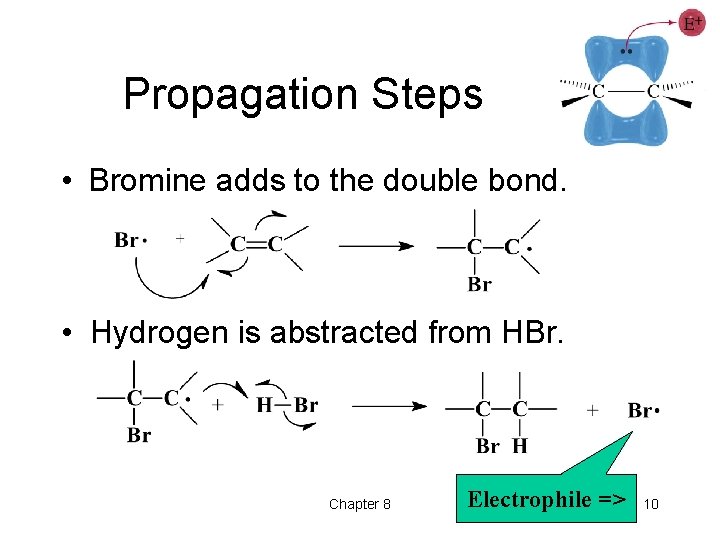
Propagation Steps • Bromine adds to the double bond. • Hydrogen is abstracted from HBr. Chapter 8 Electrophile => 10

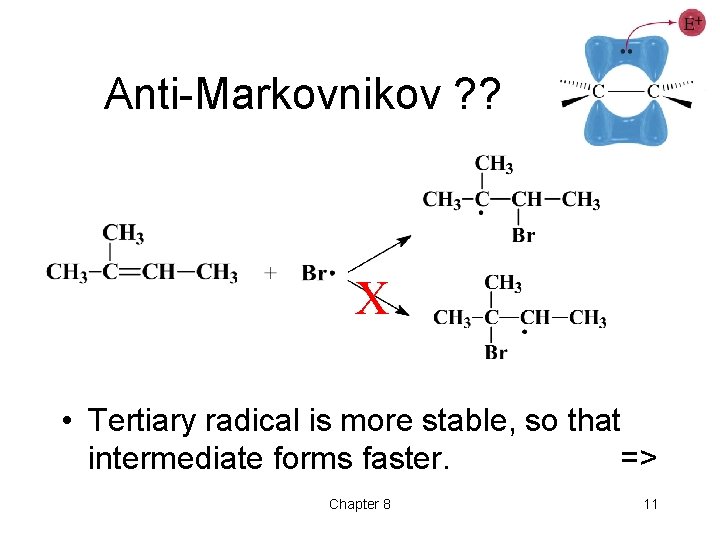
Anti-Markovnikov ? ? X • Tertiary radical is more stable, so that intermediate forms faster. => Chapter 8 11

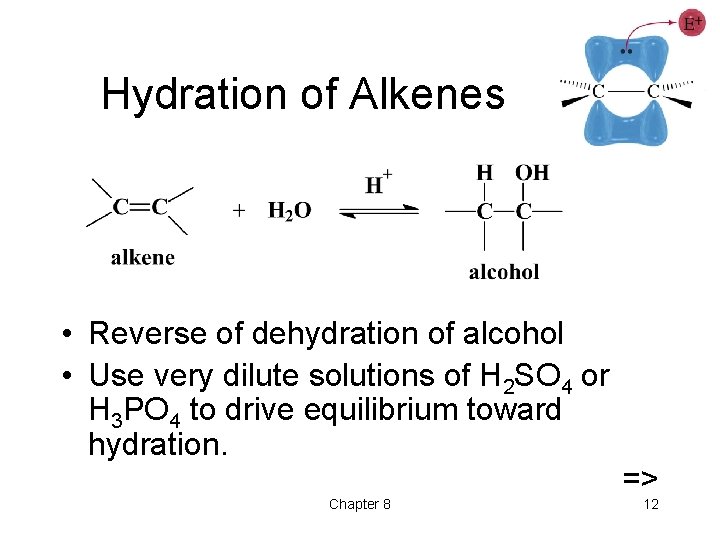
Hydration of Alkenes • Reverse of dehydration of alcohol • Use very dilute solutions of H 2 SO 4 or H 3 PO 4 to drive equilibrium toward hydration. Chapter 8 => 12

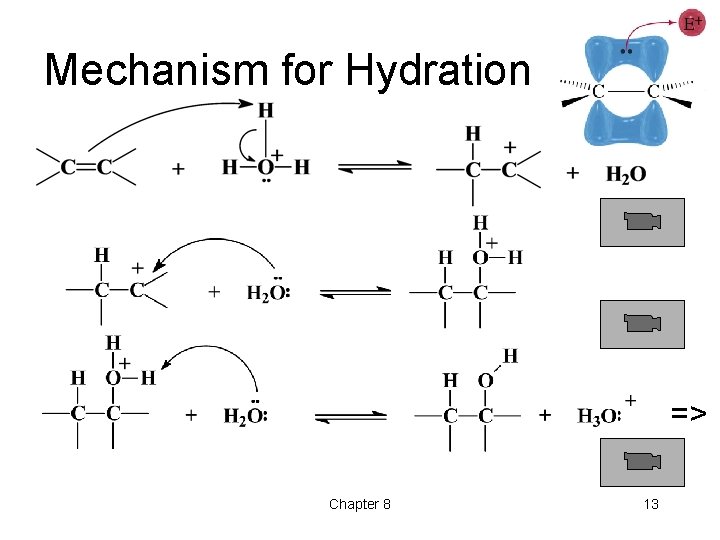
Mechanism for Hydration => Chapter 8 13

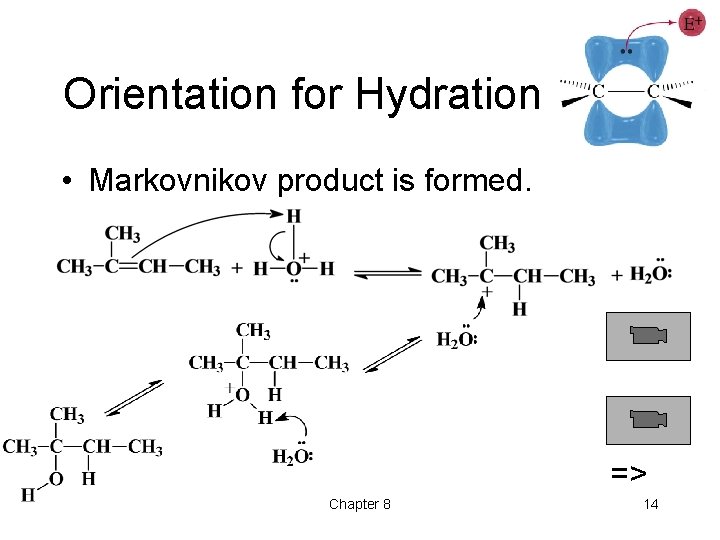
Orientation for Hydration • Markovnikov product is formed. => Chapter 8 14

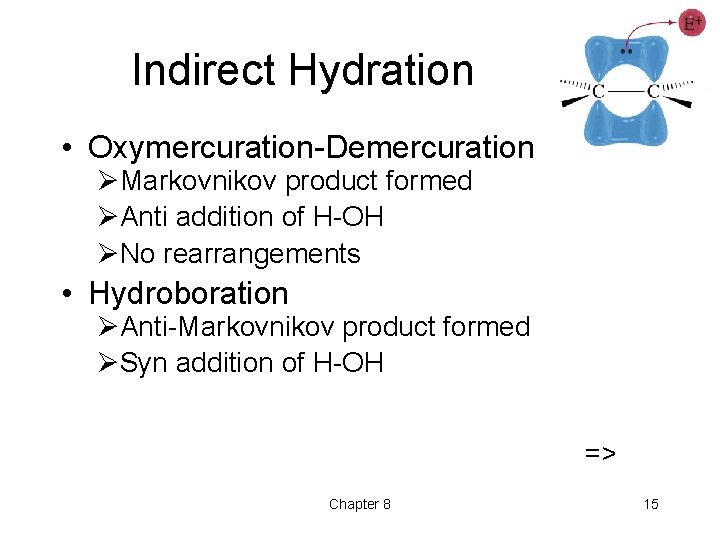
Indirect Hydration • Oxymercuration-Demercuration ØMarkovnikov product formed ØAnti addition of H-OH ØNo rearrangements • Hydroboration ØAnti-Markovnikov product formed ØSyn addition of H-OH => Chapter 8 15

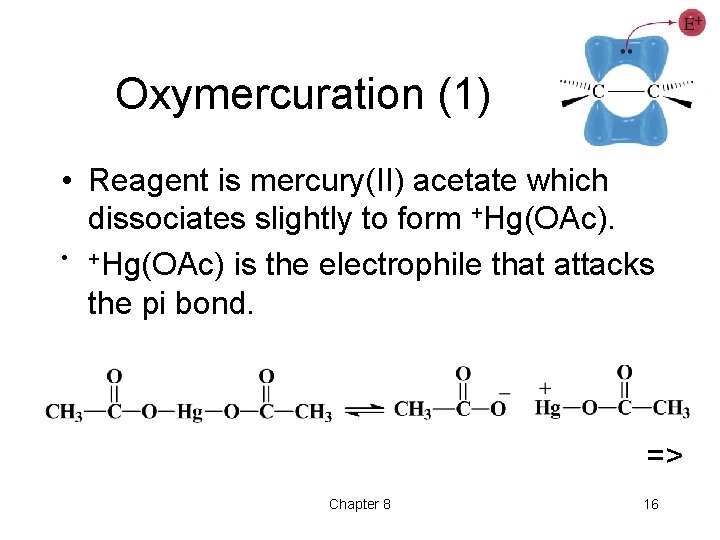
Oxymercuration (1) • Reagent is mercury(II) acetate which dissociates slightly to form +Hg(OAc). • +Hg(OAc) is the electrophile that attacks the pi bond. => Chapter 8 16

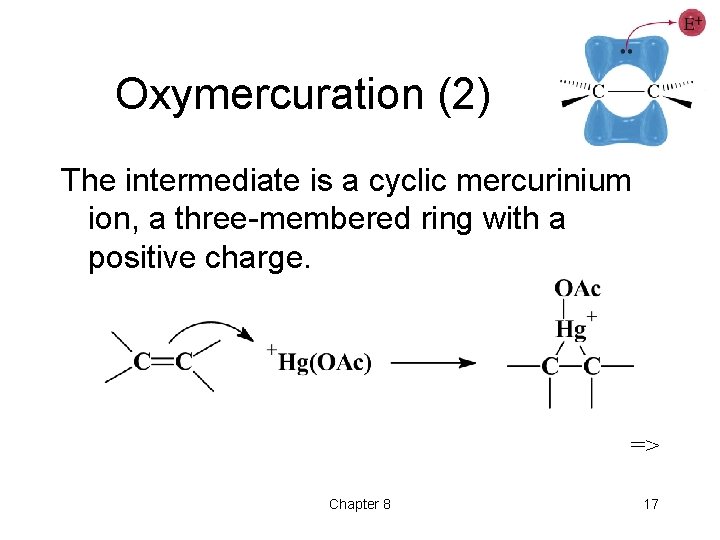
Oxymercuration (2) The intermediate is a cyclic mercurinium ion, a three-membered ring with a positive charge. => Chapter 8 17

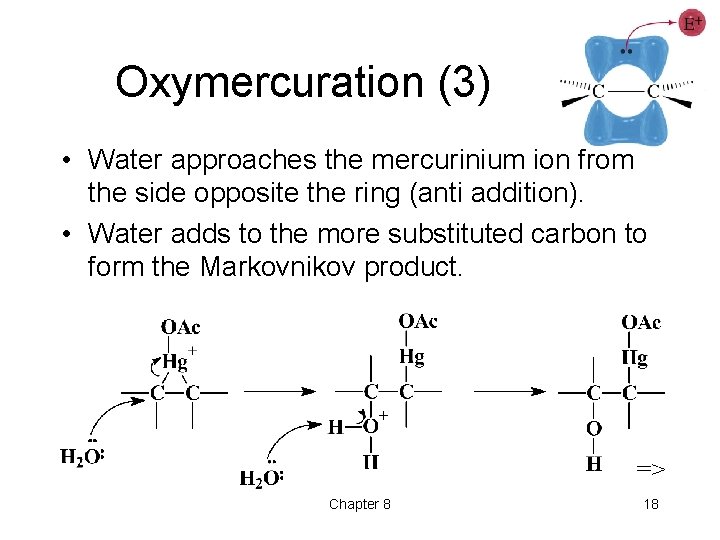
Oxymercuration (3) • Water approaches the mercurinium ion from the side opposite the ring (anti addition). • Water adds to the more substituted carbon to form the Markovnikov product. => Chapter 8 18

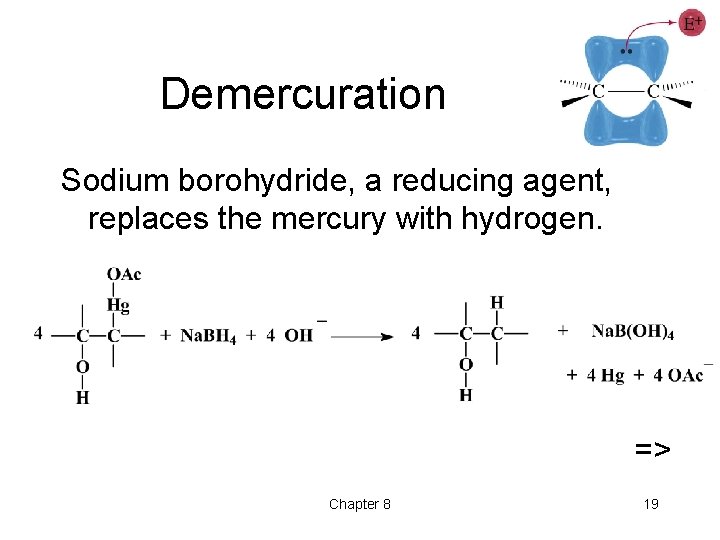
Demercuration Sodium borohydride, a reducing agent, replaces the mercury with hydrogen. => Chapter 8 19

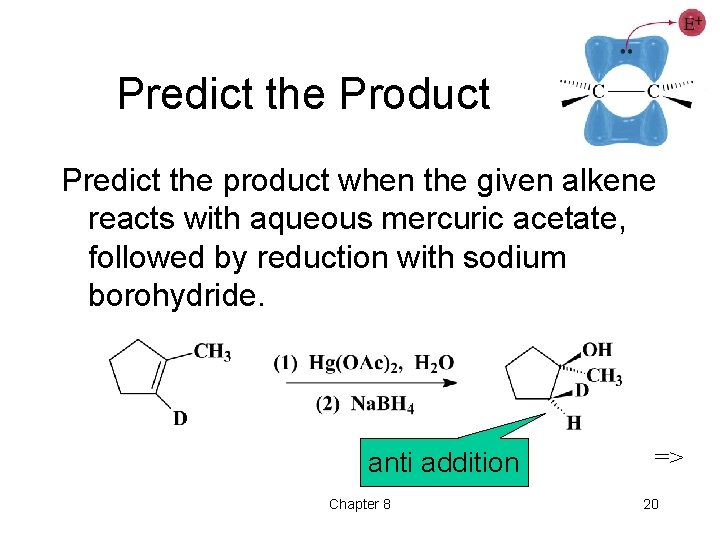
Predict the Product Predict the product when the given alkene reacts with aqueous mercuric acetate, followed by reduction with sodium borohydride. anti addition Chapter 8 => 20

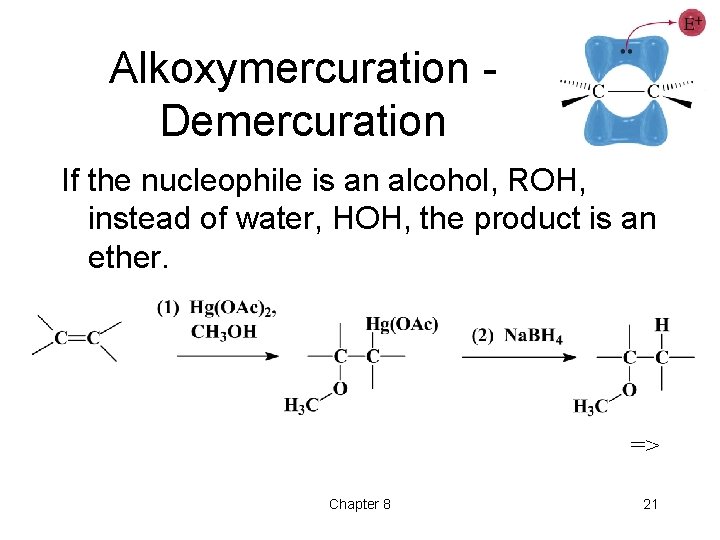
Alkoxymercuration Demercuration If the nucleophile is an alcohol, ROH, instead of water, HOH, the product is an ether. => Chapter 8 21

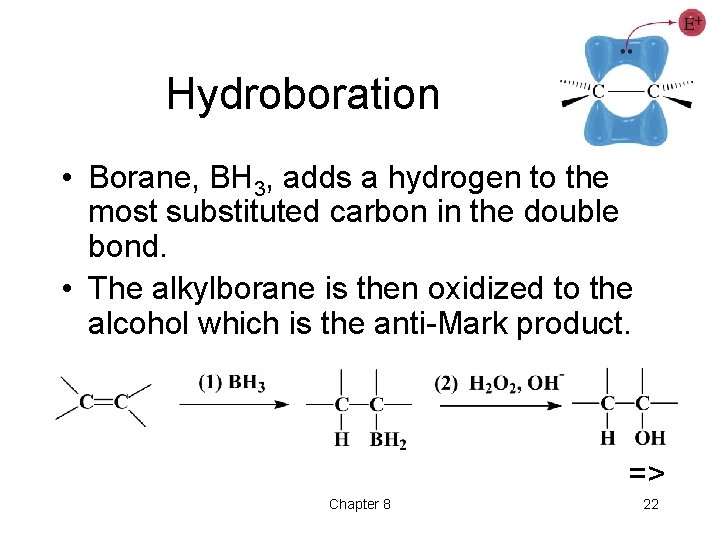
Hydroboration • Borane, BH 3, adds a hydrogen to the most substituted carbon in the double bond. • The alkylborane is then oxidized to the alcohol which is the anti-Mark product. => Chapter 8 22

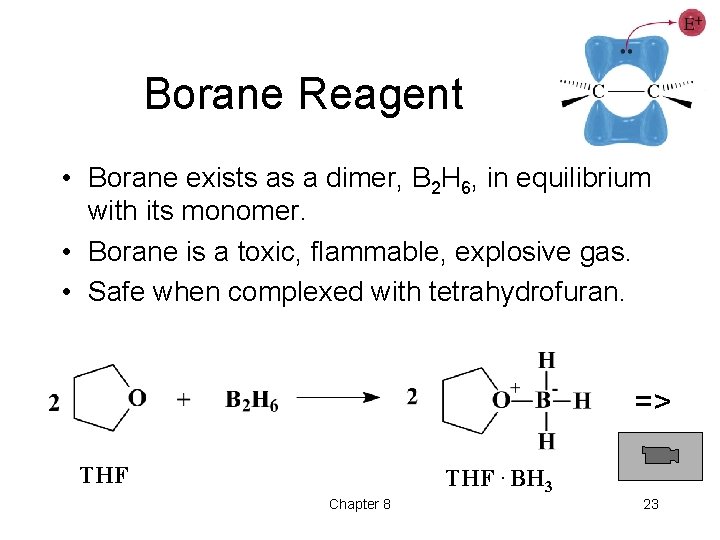
Borane Reagent • Borane exists as a dimer, B 2 H 6, in equilibrium with its monomer. • Borane is a toxic, flammable, explosive gas. • Safe when complexed with tetrahydrofuran. => THF. BH 3 Chapter 8 23

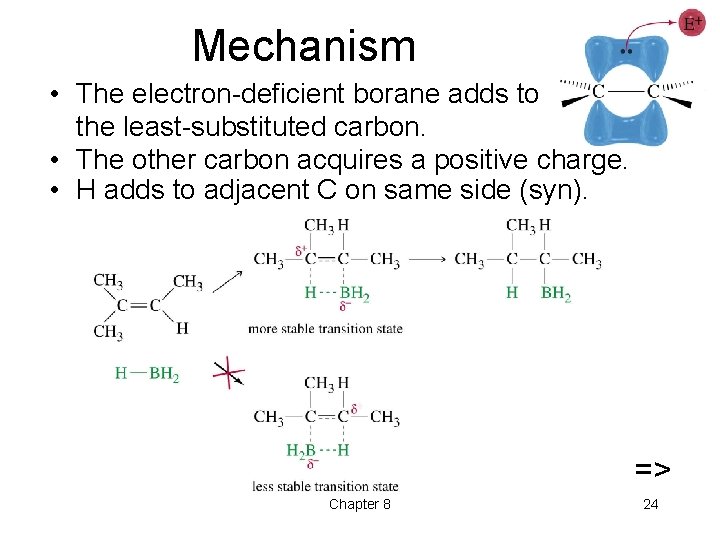
Mechanism • The electron-deficient borane adds to the least-substituted carbon. • The other carbon acquires a positive charge. • H adds to adjacent C on same side (syn). => Chapter 8 24

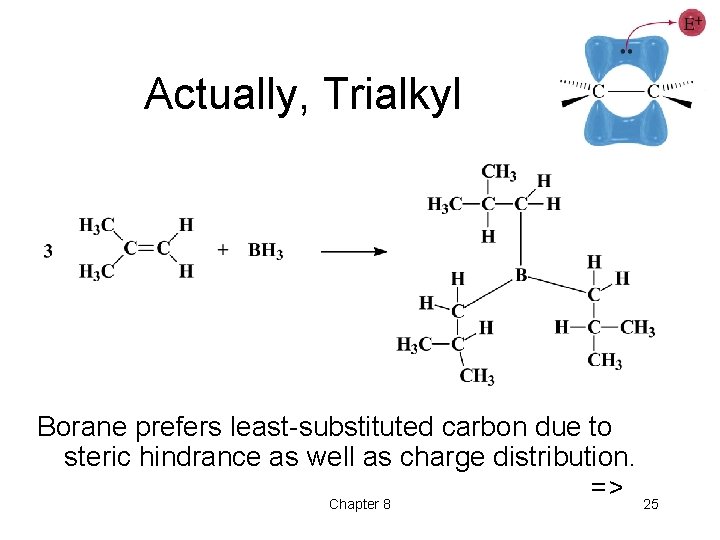
Actually, Trialkyl Borane prefers least-substituted carbon due to steric hindrance as well as charge distribution. => 25 Chapter 8

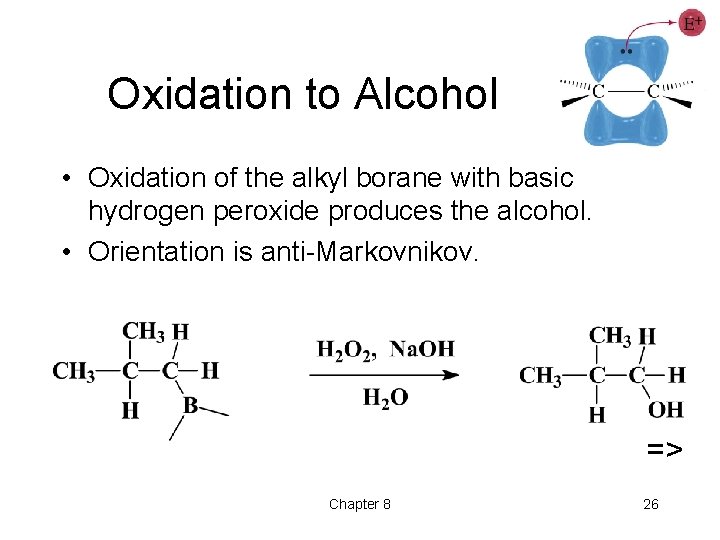
Oxidation to Alcohol • Oxidation of the alkyl borane with basic hydrogen peroxide produces the alcohol. • Orientation is anti-Markovnikov. => Chapter 8 26

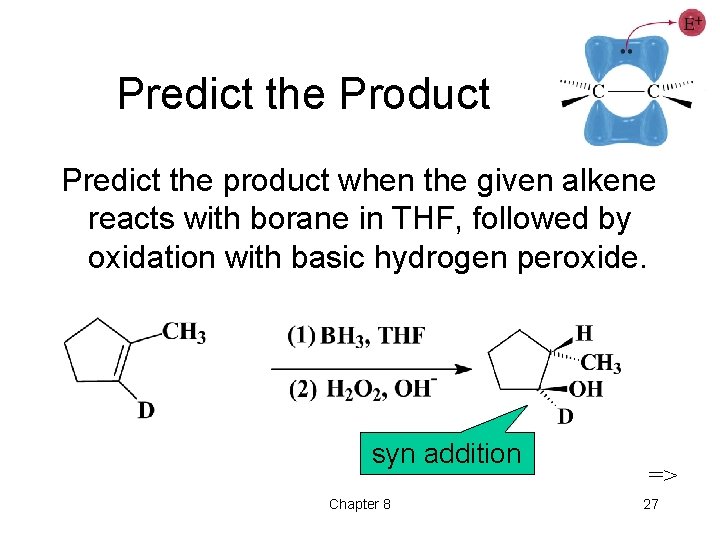
Predict the Product Predict the product when the given alkene reacts with borane in THF, followed by oxidation with basic hydrogen peroxide. syn addition Chapter 8 => 27

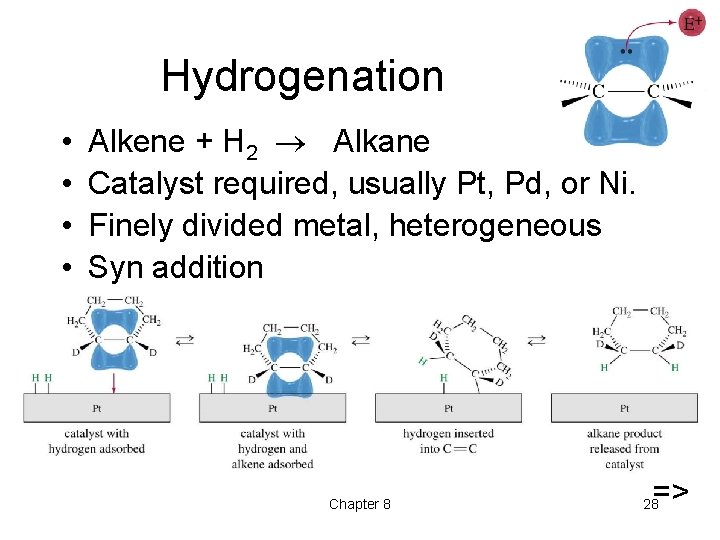
Hydrogenation • • Alkene + H 2 Alkane Catalyst required, usually Pt, Pd, or Ni. Finely divided metal, heterogeneous Syn addition Chapter 8 => 28

Addition of Carbenes • Insertion of -CH 2 group into a double bond produces a cyclopropane ring. • Three methods: ØDiazomethane ØSimmons-Smith: methylene iodide and Zn(Cu) ØAlpha elimination, haloform => Chapter 8 29

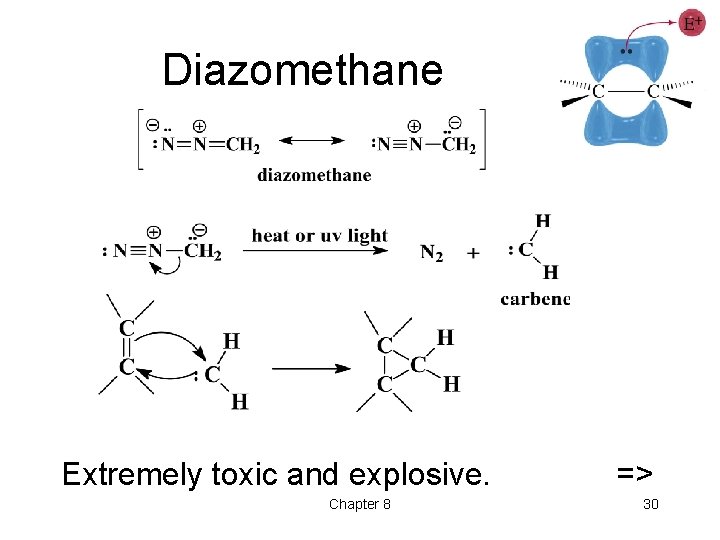
Diazomethane Extremely toxic and explosive. Chapter 8 => 30

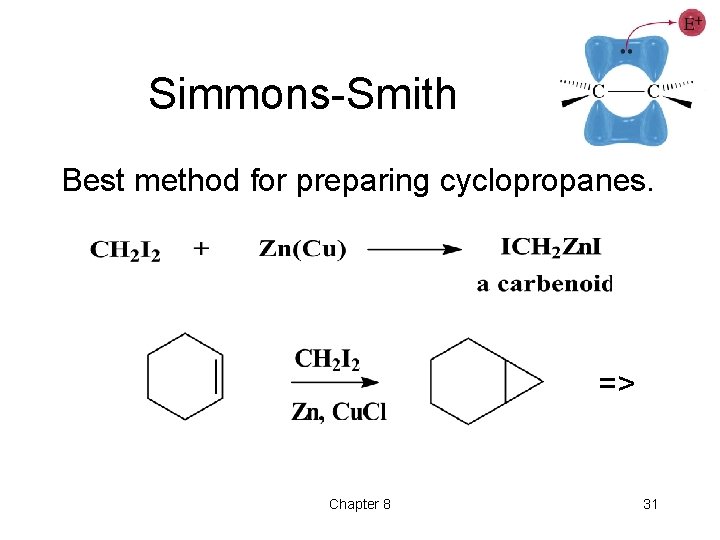
Simmons-Smith Best method for preparing cyclopropanes. => Chapter 8 31

Alpha Elimination • Haloform reacts with base. • H and X taken from same carbon => Chapter 8 32

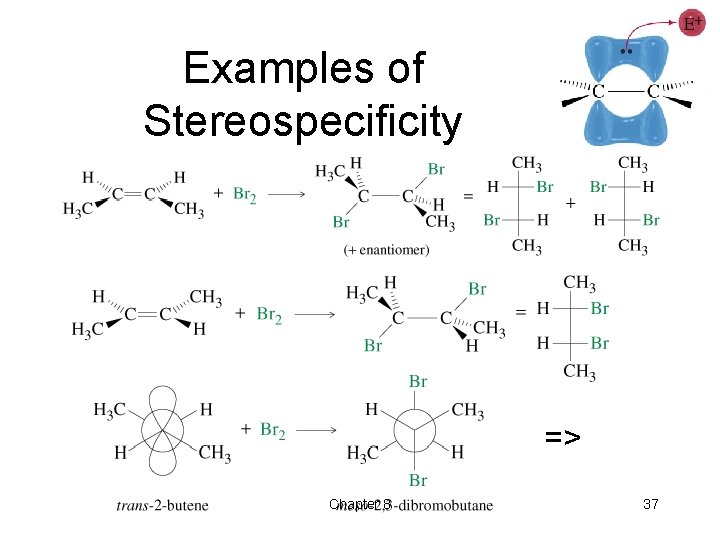
Stereospecificity Cis-trans isomerism maintained around carbons that were in the double bond. => Chapter 8 33

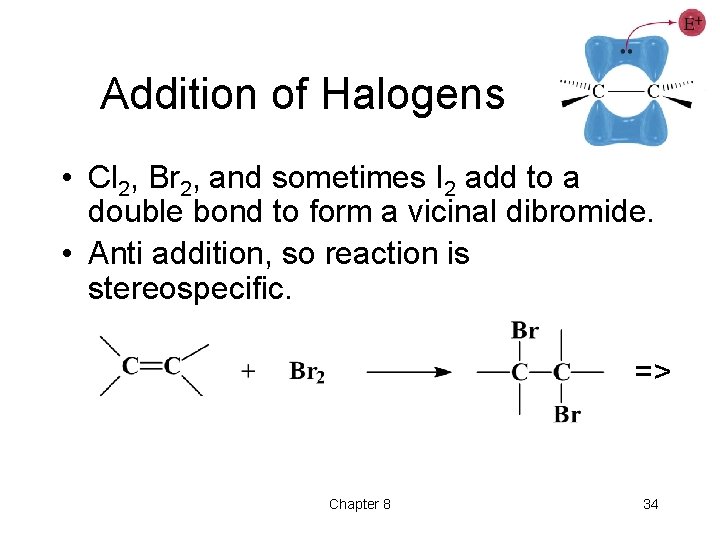
Addition of Halogens • Cl 2, Br 2, and sometimes I 2 add to a double bond to form a vicinal dibromide. • Anti addition, so reaction is stereospecific. => Chapter 8 34

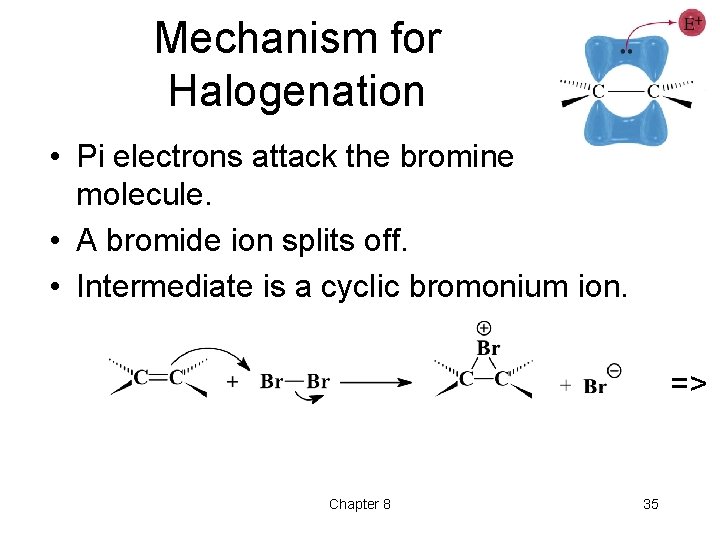
Mechanism for Halogenation • Pi electrons attack the bromine molecule. • A bromide ion splits off. • Intermediate is a cyclic bromonium ion. => Chapter 8 35

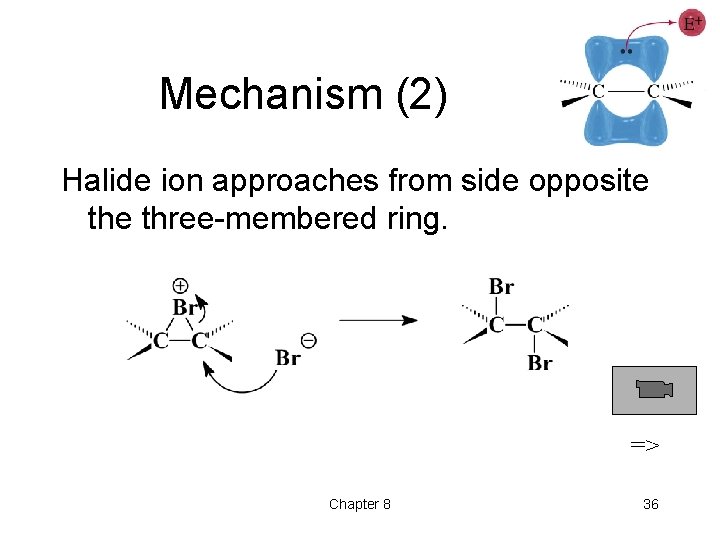
Mechanism (2) Halide ion approaches from side opposite three-membered ring. => Chapter 8 36

Examples of Stereospecificity => Chapter 8 37

Test for Unsaturation • Add Br 2 in CCl 4 (dark, red-brown color) to an alkene in the presence of light. • The color quickly disappears as the bromine adds to the double bond. • “Decolorizing bromine” is the chemical test for the presence of a double bond. => Chapter 8 38

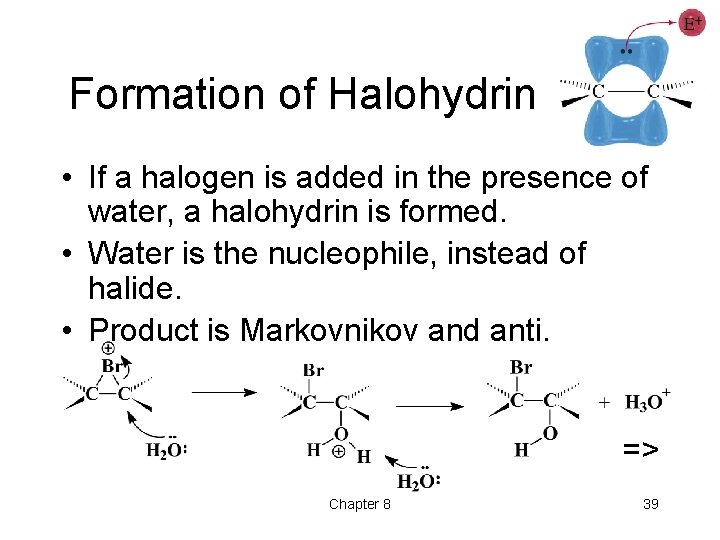
Formation of Halohydrin • If a halogen is added in the presence of water, a halohydrin is formed. • Water is the nucleophile, instead of halide. • Product is Markovnikov and anti. => Chapter 8 39

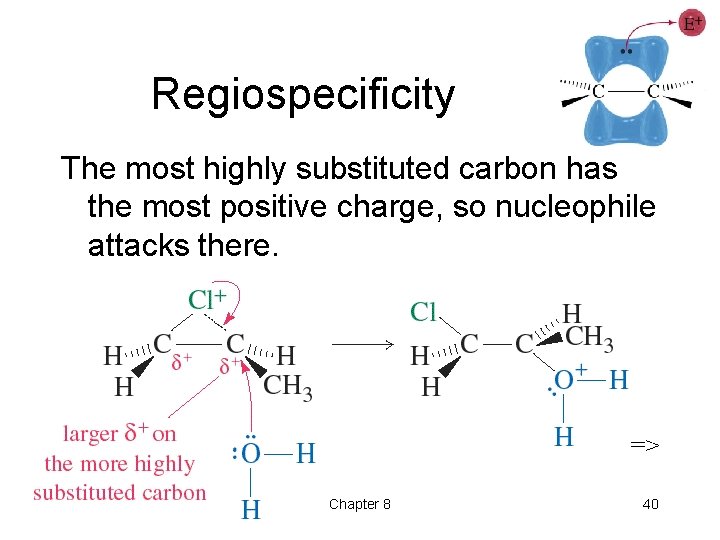
Regiospecificity The most highly substituted carbon has the most positive charge, so nucleophile attacks there. => Chapter 8 40

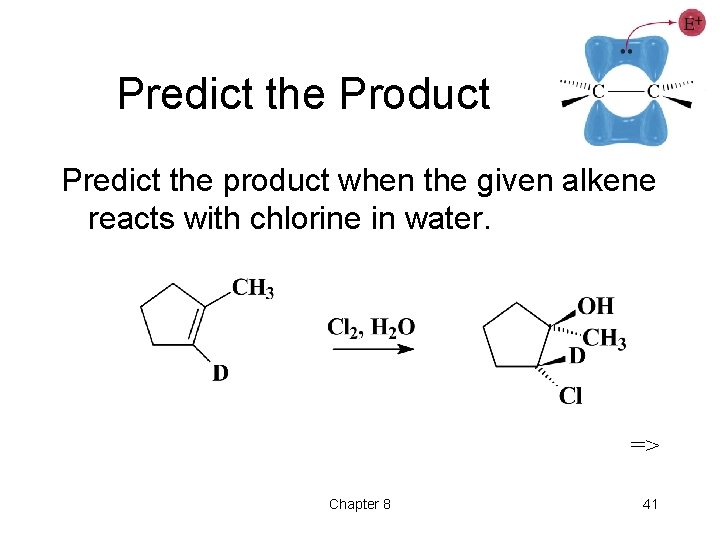
Predict the Product Predict the product when the given alkene reacts with chlorine in water. => Chapter 8 41

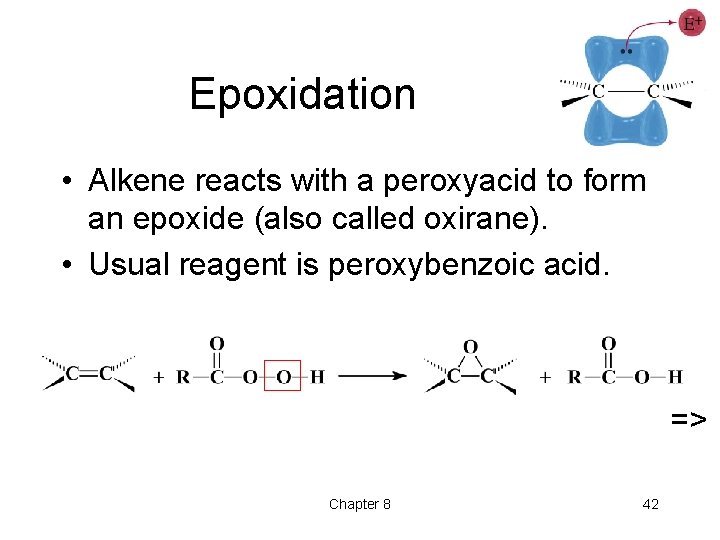
Epoxidation • Alkene reacts with a peroxyacid to form an epoxide (also called oxirane). • Usual reagent is peroxybenzoic acid. => Chapter 8 42

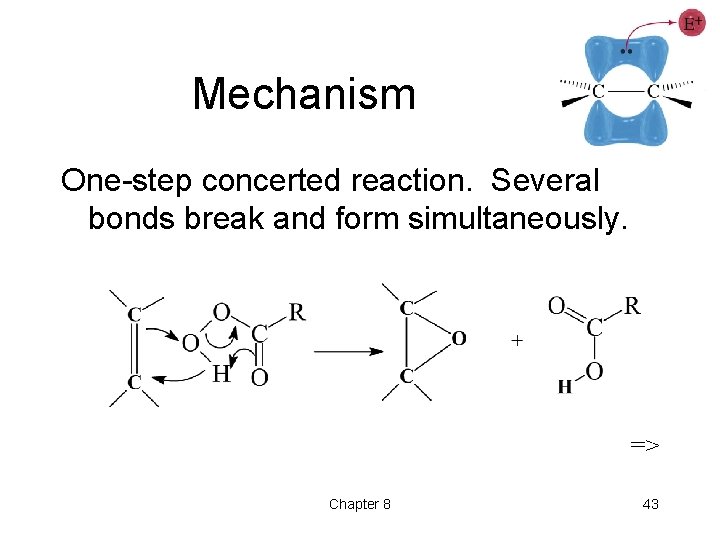
Mechanism One-step concerted reaction. Several bonds break and form simultaneously. => Chapter 8 43

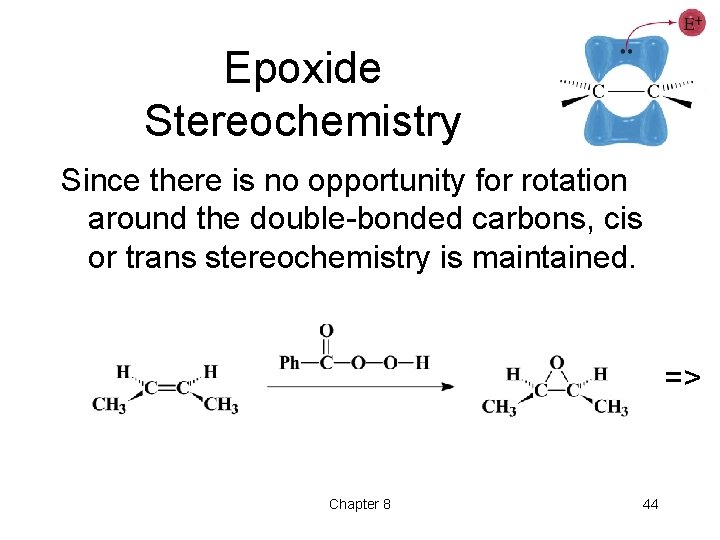
Epoxide Stereochemistry Since there is no opportunity for rotation around the double-bonded carbons, cis or trans stereochemistry is maintained. => Chapter 8 44

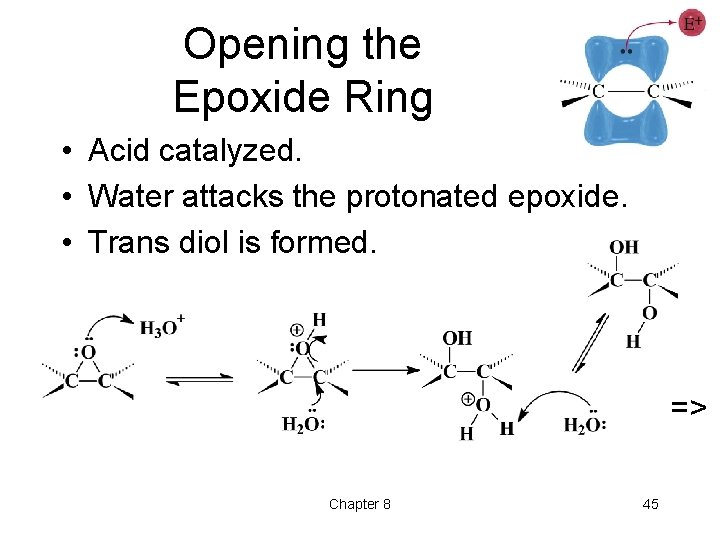
Opening the Epoxide Ring • Acid catalyzed. • Water attacks the protonated epoxide. • Trans diol is formed. => Chapter 8 45

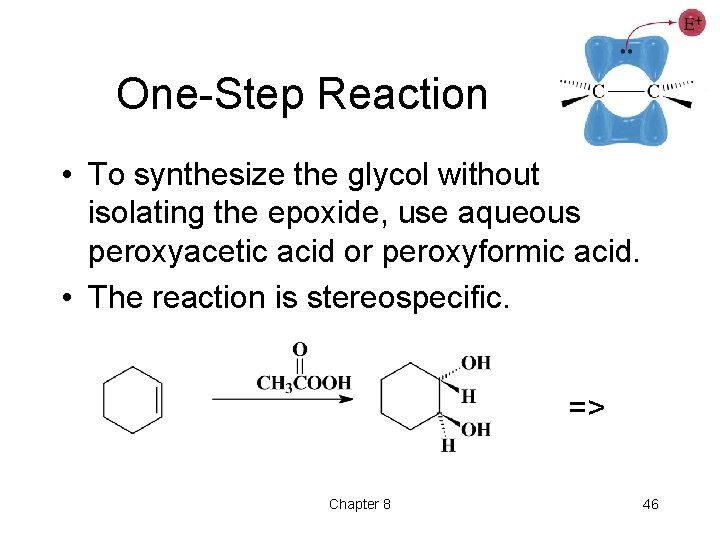
One-Step Reaction • To synthesize the glycol without isolating the epoxide, use aqueous peroxyacetic acid or peroxyformic acid. • The reaction is stereospecific. => Chapter 8 46

Syn Hydroxylation of Alkenes • Alkene is converted to a cis-1, 2 -diol, • Two reagents: ØOsmium tetroxide (expensive!), followed by hydrogen peroxide or ØCold, dilute aqueous potassium permanganate, followed by hydrolysis with base Chapter 8 => 47

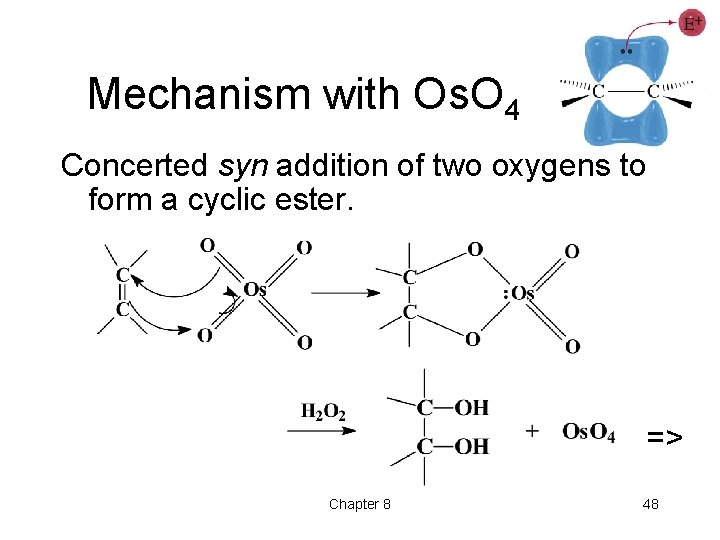
Mechanism with Os. O 4 Concerted syn addition of two oxygens to form a cyclic ester. => Chapter 8 48

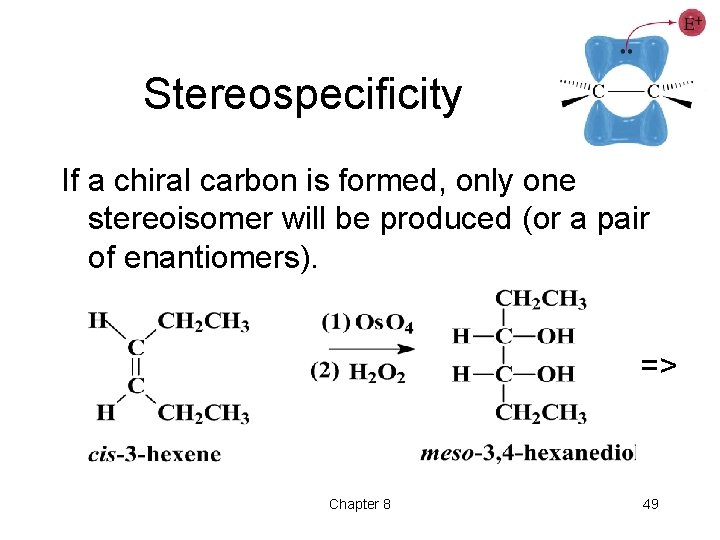
Stereospecificity If a chiral carbon is formed, only one stereoisomer will be produced (or a pair of enantiomers). => Chapter 8 49

Oxidative Cleavage • Both the pi and sigma bonds break. • C=C becomes C=O. • Two methods: ØWarm or concentrated or acidic KMn. O 4. ØOzonolysis • Used to determine the position of a double bond in an unknown. Chapter 8 => 50

Cleavage with Mn. O 4 • Permanganate is a strong oxidizing agent. • Glycol initially formed is further oxidized. • Disubstituted carbons become ketones. • Monosubstituted carbons become carboxylic acids. • Terminal =CH 2 becomes CO 2. => Chapter 8 51

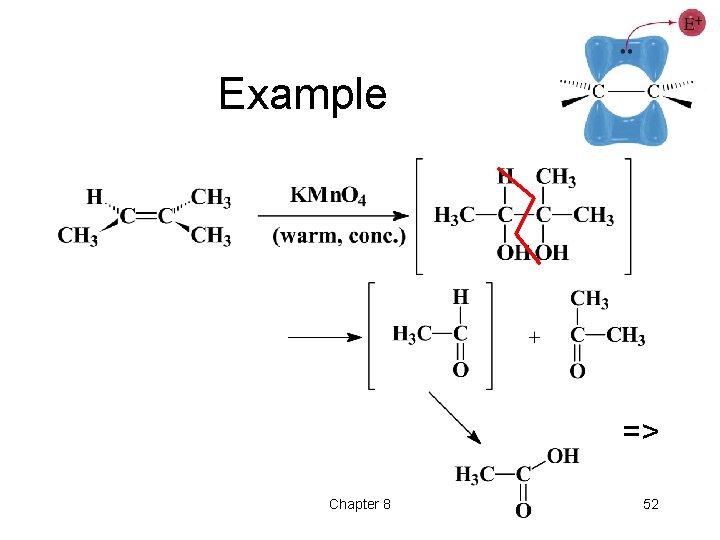
Example => Chapter 8 52

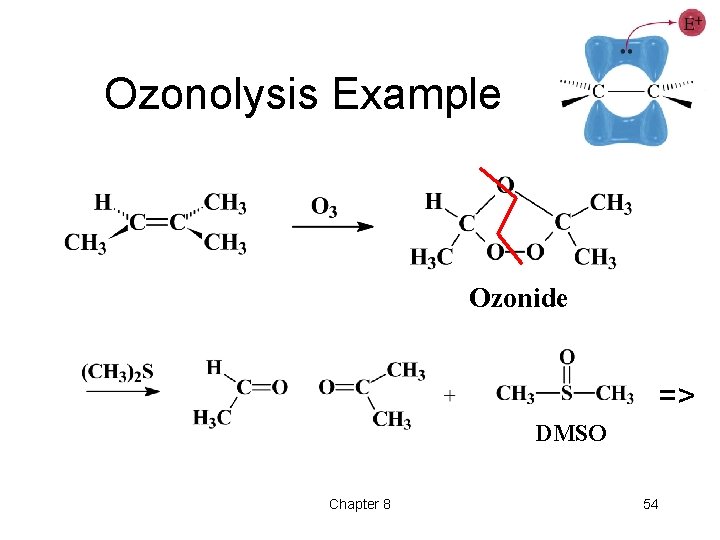
Ozonolysis • Reaction with ozone forms an ozonide. • Ozonides are not isolated, but are treated with a mild reducing agent like Zn or dimethyl sulfide. • Milder oxidation than permanganate. • Products formed are ketones or aldehydes. => Chapter 8 53

Ozonolysis Example Ozonide => DMSO Chapter 8 54

End of Chapter 8 55
 Organic chemistry wade
Organic chemistry wade Organic chemistry
Organic chemistry Transition state energy diagram
Transition state energy diagram David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Organic chemistry second edition david klein
Organic chemistry second edition david klein Pericyclic
Pericyclic Organic chemistry third edition david klein
Organic chemistry third edition david klein Is alkane an organic compound
Is alkane an organic compound Organic chemistry (3rd) edition chapter 2 problem 17s
Organic chemistry (3rd) edition chapter 2 problem 17s Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry M+2 mass spec
M+2 mass spec Organic chemistry
Organic chemistry Organic and biochemistry
Organic and biochemistry Organic chemistry
Organic chemistry Chemistry mind map
Chemistry mind map Organic chemistry lab report format
Organic chemistry lab report format Chapter 3 organic chemistry
Chapter 3 organic chemistry Organic chemistry william h brown
Organic chemistry william h brown Brooklyn college organic chemistry
Brooklyn college organic chemistry Danswer
Danswer A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Nbs chemistry
Nbs chemistry Father of organic chemistry
Father of organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry
Organic chemistry Organic chemistry chapter 1
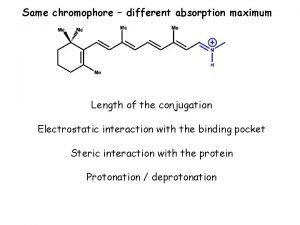
Organic chemistry chapter 1 Conjugation organic chemistry
Conjugation organic chemistry Ario organic chemistry
Ario organic chemistry Carbohydrates organic chemistry
Carbohydrates organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry
Organic chemistry Neon organic or inorganic
Neon organic or inorganic Organic chemistry class 11 notes
Organic chemistry class 11 notes Hono organic chemistry
Hono organic chemistry Isomers of pentane
Isomers of pentane Organic chemistry myanmar
Organic chemistry myanmar Organic chemistry
Organic chemistry Organic chemistry stuart warren
Organic chemistry stuart warren Britannica.com
Britannica.com Analytical chemistry chapter 1
Analytical chemistry chapter 1 Polarimetry organic chemistry
Polarimetry organic chemistry Conjugation organic chemistry
Conjugation organic chemistry Rancidity meaning
Rancidity meaning Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Canola oil
Canola oil Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry
Organic chemistry Hept oct non dec
Hept oct non dec Leveling effect organic chemistry
Leveling effect organic chemistry Resonance in benzyl carbocation
Resonance in benzyl carbocation Meth eth prop but pent hex
Meth eth prop but pent hex Enols and enolates organic chemistry
Enols and enolates organic chemistry Organic chemistry case studies
Organic chemistry case studies