Forensic Aspects of Arson and Explosion Investigation The
































































































- Slides: 98

Forensic Aspects of Arson and Explosion Investigation

The Chemistry of Fire is a transformation process during which oxygen is united with some other substance to produce noticeable quantities of heat and light.

–The fundamental chemical reaction of fire is oxidation. This is the combination of oxygen with other substances to produce new substances. CH 4 + 2 O 2 CO 2 + 2 H 2 O

–In all oxidation reactions more energy is given off than is required to break the chemical bonds present. The excess energy is given off as heat and often light.

–This excess energy is known as the heat of combustion. These are said to be exothermic reactions (heat is given off).

– Before the reaction can take place an energy barrier must be hurdled. The higher the barrier the more energy is required to start the reaction. Usually this energy source is heat.

n. For substances like gasoline and methane the hurdle is quite high so a high temperature must be applied to begin the oxidation process.

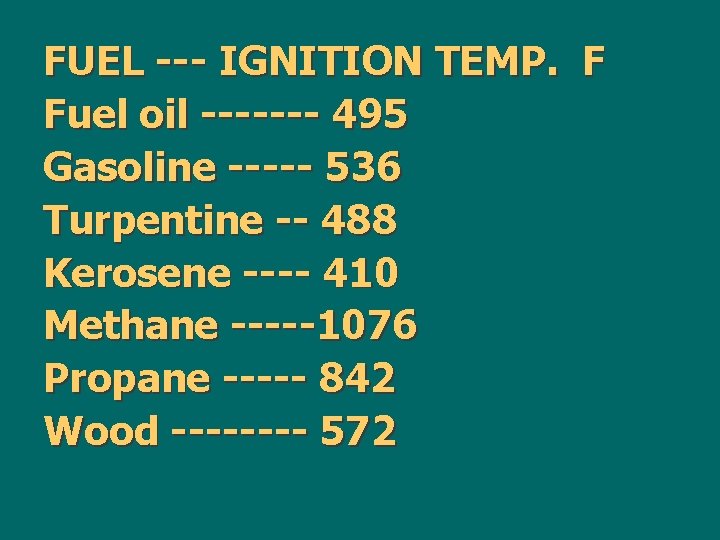
n. Before any fire can result the temperature of these fuels must be raised to a value higher than the energy barrier. This value is known as the ignition temperature.

FUEL --- IGNITION TEMP. F Fuel oil ------- 495 Gasoline ----- 536 Turpentine -- 488 Kerosene ---- 410 Methane -----1076 Propane ----- 842 Wood ---- 572

n. Once combustion begins enough heat is given off to keep the reaction going by itself. The fire will continue to burn until either the supply of oxygen or the supply of fuel is exhausted.

n. Possible sources of ignition include electrical discharge, sparks, chemicals.

n. A flaming fire can only be sustained when fuel is in a gaseous state. So how does a liquid or solid maintain a fire?

n. For a liquid the temp must be high enough to vaporize it. The vapor burns when it mixes with oxygen and combusts as a flame.

n. The flashpoint is the lowest temperature at which a liquid gives off enough vapor to form a mixture with air that will support combustion.

n. Once the flashpoint is reached the fuel can be ignited by an outside source. Gasoline has a flashpoint of – 50 F but an ignition temp of 494 F.

n. With a solid it is harder to generate a vapor. The solid fuel will only burn when it is exposed to heat hot enough to decompose the solid into a gas or vapor.

n. This breakdown is known as pyrolysis. A match or other source of heat initiates the pyrolysis of the solid fuel. The gaseous products react with oxygen in the air to produce heat and light.

n This in turn is used to pyrolyze more solid fuel into gas. n Although a flame will be supported only by a gaseous fuel, in some instances a fuel can burn without the presence of a flame.

n. Ex. Charcoal embers. This is an example of glowing combustion or smoldering.

n. Wood will burn with a flame until all of its pyrolyzable components have been expended. Then the carbonaceous residue will continue to smolder long after the flame has extinguished.

n. The chemical reaction will occur faster when the temperature is increased. For most reactions a 10 C (18 F) increase in temp will double or triple the reaction rate.

n This increases the temp even more which increases the reaction rate which increases the temp …

n. Spontaneous Combustion – very rarely accounts for the cause of a fire. n. Spontaneous combustion is the result of a natural heat-producing process in poorly ventilated containers or areas.

n. Examples include hay barns and rags soaked in certain chemicals such as linseed oil.

n. Oxidizing agents – explosives are substances that undergo a rapid exothermic oxidation reaction with the production of large quantities of gases.

n. It is the sudden build up of gas pressure that constitutes the nature of an explosion. Detonation occurs so rapidly that oxygen in the air cannot take part in the reaction.

n. So, many of explosives must have their own source of oxygen. Chemicals that supply oxygen are known as oxidizing agents.

n. Example – black powder – 75% potassium nitrate (KNO 3) – 15% charcoal – 10% sulfur

n. The oxygen in the KNO 3 acts as the oxidizing agent. n. Nitroglycerin, the main ingredient in dynamite combines carbon, hydrogen, nitrogen, and oxygen.

n. When it detonates large quantities of energy are released as the molecule breaks down and oxygen interacts with the other elements.

Remember! 3 requirements must be met for combustion to occur.

n 1. A fuel must be present.

n 2. Oxygen must be present in sufficient amounts to combine with the fuel.

n 3. Heat must be supplied to begin the combustion and sustain it.

n. SEARCHING THE SCENE –Time is of the essence. Because most arsons are started with petroleumbased accelerants such as gasoline or kerosene any remaining residue may evaporate very quickly.

n. The necessity to begin an immediate investigation of the circumstances surrounding a fire even takes precedence over the requirement to obtain a search warrant to enter and search a premises.

n. The search of the fire scene must focus on finding the fire’s origin. This is the area most likely to provide evidence of an accelerant or ignition device.

n. Investigators look for signs such as separate and unconnected fires, the use of streamers to spread the fire from one area to another, the presence of containers capable of holding an accelerant.

n. They also look for evidence such as an ignition device, signs of breaking and entering, theft, and begin to interview witnesses.

n. Once located, the point of origin should be protected. Nothing must be moved or touched before notes, sketches, and photographs are taken.

n. The most common materials used by arsonists to ensure the rapid spread and intensity of a fire are liquid petroleum products such as gasoline and kerosene.

n. Only under ideal circumstances will combustible liquids be entirely consumed during a fire. Some enters cracks in the floor, carpets, plaster, etc.

n. Water used to extinguish the fire can help slow down the evaporation of volatile liquids. Water will not interfere with lab tests used to detect accelerants.

n. Fire investigators look for traces of flammable liquid residue with the aid of highly sensitive portable vapor detectors also called “sniffers”.

n These devices detect volatile residue by inhaling air at the scene. The air is then passed over a heated filament. If a combustible vapor is present in the air it is oxidized and increases the temperature of the filament.

n. These do not provide conclusive identification but are very helpful as screening devices. n. Dogs are also trained to detect the odor of hydrocarbon accelerants.

n. COLLECTION AND PRESERVATION OF ARSON EVIDENCE. n. As a general rule 2 to 3 quarts of ash and soot should be collected at the point of origin.

n. Specimens must be immediately packaged in an airtight container as no loss of residue can occur through evaporation.

n Fluids found in open bottles or cans must also be collected and sealed. n A search must also try to locate an ignitor. These include things such as matches, cigarettes, electrical sparking devices or Molitov cocktails.

n. Another key piece of evidence is the clothing worn by a suspected arsonist if they are arrested within a few hours of the crime.

n. ANALYSIS OF FLAMMABLE LIQUIDS n. The gas chromatograph is considered to be the most sensitive and reliable instrument for detecting flammable residue.

n. This instrument separates the hydrocarbon components and produces a distinctive pattern characteristic of a particular petroleum product.

n. This pattern can then be compared to known patterns to make a conclusive identification.

TYPES OF EXPLOSIONS Most bombing incidents that confront police agencies today involve the use of homemade explosives and incendiary devices.

n. The forensic scientists job is to detect and identify explosive chemicals recovered from the crime scene as well as identifying the detonating mechanisms.

n. Like fire, an explosion is the product of combustion accompanied by the creation of gases and heat.

n The distinguishing characteristic of an explosion is the rapid rate at which the reaction proceeds. It is the sudden buildup of expanding gas pressure at the origin that produces the violent physical disruption of the environment.

n. Ex. Pipe bombs n. Upon release from confinement the gaseous products of the explosion expand compress layers of surrounding air as they move outward from the origin.

n. This blast effect at a rate as high as 7, 000 mph creates an artificial gale that can overthrow walls, collapse roofs, and disturb objects in its path.

n. The speed at which explosives decompose varies greatly from one to another and permits their classification as high or low explosives.

n. In the low explosive, this speed is called the speed of deflagration. It is characterized by very rapid oxidation that produces heat, light, and a subsonic pressure wave.

n. In high explosives it is called the speed of detonation. Detonation refers to the creation of a supersonic shock wave within the explosive charge.

n. This shock wave causes the chemical bonds of the explosive charge to break apart, leading to the new instantaneous buildup of heat and gases.

Low explosives, such as black and smokeless powder decomposes relatively slowly at rates that vary up to 1, 000 meters per second.

n. Because of their slow burning rates they produce a propelling or throwing action that makes them suitable as propellants for ammunition or sky rockets.

n High explosives include items such as dynamite. They detonate almost instantaneously at rates from 1, 000 to 8, 500 meters per second producing a smashing or shattering effect on their target.

n. LOW EXPLOSIVES –The most widely used explosives are black and smokeless powders. Why? –Black powder is a mixture of what?

n. Unconfined it merely burns. It is used as an ingredient in safety fuses in fireworks. It only becomes lethal when it is confined.

n. HIGH EXLOSIVES –High explosives can be classified into two groups. –Primary explosives are ultra-sensitive to heat, shock, or friction.

n. Under normal circumstances they will detonate violently instead of burning. This is why they are used to detonate other explosives through a chain reaction and are referred to as primers.

n. They are the major ingredient of a blasting caps. n. Because of their extreme sensitivity they are rarely used as the main charge of a homemade bomb.

n. Secondary explosives are relatively insensitive to heat, shock, or friction.

n. They will burn rather than detonate if they are ignited in small quantities in open air. This group comprises the majority of high explosives used for commercial and military blasting.

n. Dynamite, invented by Alfred Nobel, is classified by the amount of nitroglycerin it contains. n 40% straight dynamite contains 40 percent nitroglycerin.

n. In recent years nitro-based dynamite has almost disappeared from the market. It has been replaced by ammonium nitrate – based explosives.

n. These explosives mix oxygen-rich ammonium nitrate with a fuel to form a low cost, stable mixture. n. It usually comes in three forms- water gels, emulsions, and ANFO explosives.

n. Water gels – consistency of tooth paste, water resistant, used to blast under wet conditions.

n. Emulsions – consists of two distinct phases, an oil phase and a water phase. They often contain micronsized glass resin, or ceramic spheres known as microspheres or micro balloons.

n. The size of these spheres control the explosives sensitivity and detonation velocity.

n. ANFO is an explosive composed of ammonium nitrate soaked in fuel oil. They are inexpensive and safe to handle. Generally used in the mining industry.

n. The availability of ammonium nitrate in the form of fertilizer makes a readily obtainable as an ingredient for homemade explosives.

n. TATP is a homemade explosive that has been used as an improvised explosive by terrorist organization in the Middle East.

n. It is made with acetone, hydrogen peroxide, and an acid catalyst. It is a very sensitive explosive that is explosive and potent when confined in a container such as a pipe.

n. Military High Explosives –The most popular and powerful military explosive is RDX. It is often encountered in the form of a pliable plastic with the consistency of dough. Composition C-4

n. COLLECTION and ANALYSIS of EXPLOSIVES –The single most important step in the detection and analysis of explosive residues is the collection of appropriate samples from the scene.

n Undetonated residue of the explosive can often be found at the site of the explosion. The detection and identification of explosives depends on the investigators ability to recognize and sample the areas most likely containing the materials.

n. The most obvious characteristic of a high or a contained low explosive is the presence of a crater at the origin of the blast. Loose soil and debris must be collected from this site.

n. Other good sources of explosive residue are objects located near the origin of detonation. Wood, insulation, rubber, or other soft materials that are readily penetrated often collect traces of explosives.

n Materials blown away from the blast’s origin should be recovered because it may retain explosive residues. n All personnel involved in searching the bomb scene must take appropriate measures to avoid contaminating the scene.

n. This includes wearing disposable gloves, shoe covers, and overalls.

n. Wire mesh screens are best utilized for sifting through debris. In pipebomb explosions, particles of the explosive are frequently found adhering to the pipe cap, pipe threads.

n. This may be the result of being impacted into the metal by the force of the explosion or being deposited in the threads during construction.

n. One approach for screening objects for the presence of explosive residues in the field or lab is the ion mobility spectrometer. (IMS)

n. It uses a vacuum to collect explosive residues from a suspected surface. n. It can rapidly detect a full range of explosives even at low levels.

n. All materials collected for examination by the lab must be placed in sealed containers and labeled.

n. Typically, in the lab debris collected at explosion scenes will be examined microscopically for unconsumed explosives particles.

n. Recovered debris may also be thoroughly rinsed with organic solvents and analyzed by testing procedures that include color spot tests, chromatography, and mass spectrometry.

 Fire and arson investigation ppt
Fire and arson investigation ppt Forensic science arson activity
Forensic science arson activity Forensic science foodborne outbreak investigation answers
Forensic science foodborne outbreak investigation answers Pathologist and anthropologist
Pathologist and anthropologist Who is this
Who is this Line of demarcation fire
Line of demarcation fire Burned fire scientist questions arson
Burned fire scientist questions arson The boundary between charred and uncharred material.
The boundary between charred and uncharred material. Arson pour patterns
Arson pour patterns Ion detector
Ion detector Incendio
Incendio David berkowitz arson
David berkowitz arson A news item text
A news item text Why is arson considered a low priority crime
Why is arson considered a low priority crime Fire and explosion index
Fire and explosion index Philip larkin the explosion
Philip larkin the explosion Famous author candy
Famous author candy Evangelism explosion outline
Evangelism explosion outline Population explosion refers to the-
Population explosion refers to the- A freight train is being assembled in a switching yard
A freight train is being assembled in a switching yard Torpedo 48 shots
Torpedo 48 shots Thinking routine step inside
Thinking routine step inside Dmc explosion welding
Dmc explosion welding Mass explosion hazard placard
Mass explosion hazard placard Georg grosz explosion
Georg grosz explosion Factors affecting population explosion
Factors affecting population explosion Evangelism skills
Evangelism skills Dfd biblioteca
Dfd biblioteca Minor explosion hazard sign
Minor explosion hazard sign Kendall truitt
Kendall truitt San juanico mexico city
San juanico mexico city Population explosion
Population explosion Mexico city explosion 1984
Mexico city explosion 1984 Ttu chemistry department
Ttu chemistry department Language explosion
Language explosion Population explosion
Population explosion A 6000 kg railroad car moving at 5m/s
A 6000 kg railroad car moving at 5m/s Dinamics
Dinamics Oil rig explosion
Oil rig explosion Explosion gulf of mexico
Explosion gulf of mexico Oil rig explosion
Oil rig explosion Explosion hazard
Explosion hazard Explosion proof electrical junction boxes
Explosion proof electrical junction boxes John gave a bar of chocolate to jill.
John gave a bar of chocolate to jill. Oil spill
Oil spill Gulf of mexico pipeline rupture
Gulf of mexico pipeline rupture Explosion tredi hombourg
Explosion tredi hombourg Cambrian explosion
Cambrian explosion The population explosion
The population explosion Population explosion example
Population explosion example Dow fei
Dow fei Oil rig explosion
Oil rig explosion Name
Name Hash oil explosion
Hash oil explosion Population explosion in india
Population explosion in india Ophiuchus cluster explosion
Ophiuchus cluster explosion Rwset
Rwset Snowball sampling
Snowball sampling Net material requirements plan
Net material requirements plan Population explosion industrial revolution
Population explosion industrial revolution Spriggna
Spriggna Halifax explosion definition
Halifax explosion definition Data explosion
Data explosion Cleaver brooks scotch marine boiler
Cleaver brooks scotch marine boiler Data explosion
Data explosion Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên là gì
Số nguyên là gì Tư thế ngồi viết
Tư thế ngồi viết