Forensic Aspects of Arson and Explosion Investigations Chapter


















































- Slides: 53

Forensic Aspects of Arson and Explosion Investigations Chapter 11

Forensic Aspects of Arson and Explosion Investigations • Complex, difficult, planned • Extensive destruction • Accidental causes: faulty wiring, electrical motors, heating systems, cigarettes • Training, knowledge, experience

Forensic Aspects of Arson and Explosion Investigations • On-site investigation • Motive, modus operandi, suspect • Detect, identify chemicals • Reconstruct, identify ignitors/ detonating mechanisms

The Chemistry of Fire • Oxidation- oxygen combining w/other substances to produce new substances • Energy- potential of a material or system to do work- heat, chemical, electrical, nuclear, light, mechanical • Fire- transformation process, oxidation produces heat and light (flame)

The Chemistry of Fire • Exothermic- chemical rxn that gives off heat, more energy is liberated than what is needed to break bonds • Combustion- oxidation w/ production of heat and light • Heat of combustion- the quantity of heat required to raise the temp of 1 lb of water 1 o. F, Btu- Table 11 -1 • Energy Barrier- energy requirement for initiation of reaction

The Chemistry of Fire • Ignition Temperature- minimum temp. at wh/a fuel will spontaneously ignite, heat energy input exceeds energy barrier, Table 11 -2 ex. Kerosene 410°F, Benzene 928°F • Speed of reaction- physical state and temp of fuel • Flame produced only when fuel in gaseous state (molecules colliding frequently) • Liquid Fuel- Flash point, lowest temp that vaporizes fuel (lower than ignition temp) • Solid Fuel- Pyrolysis, decomposition of solid organic matter by heat • Rate of rxn increases w/ increased temp

The Chemistry of Fire • Ignitors- match, electrical discharges, sparks, chemicals • Flammable Range: gas fuel-air mix, range that fuel concentrations in air are capable of burning • Ex- gasoline 1. 3 -6. 0% • Lean- fuel conc. too low • Rich- fuel conc. too high • Fire is “chain reaction”: fuel + air + energy heat burn heat ( cont. until oxygen or fuel runs out)

The Chemistry of Fire • Glowing Combustion (smoldering)burning at the fuel air interface w/o flames, combustion w/o pyrolization, after flames • Spontaneous Combustion- natural heat-producing process in presence of sufficient air and fuel, poorly ventilated area (bacteria, unsaturated oil soaked rags)

The Chemistry of Fire • Oxidizing Agents- chemicals th/supply oxygen for detonation • Ex- black powder (KNO 3, C, S) nitroglycerin (C, H, N, O)

The Chemistry of Fire • Three Requirements of Combustion: • 1) Fuel • 2) Sufficient oxygen (oxidizing agent) • 3) Initiation heat, heat to sustain rxn

Searching The Fire Scene • • • Immediate examination Safety, health conditions Search warrant not necessary Focus on finding fire’s origin Accelerant residue, containers Ignition device “Streamers” Breaking, entry, theft Eyewitnesses

Searching The Fire Scene • Probable origin- lowest point most intense burning ( fire moves upward) • Drafts, winds, collapsing structures, stairs, elevator shafts, holes • Accelerant flow to lowest point

Searching The Fire Scene • Protect located origin • Notes, sketches, photographs • Detect flammable liquid residues – “sniffer” (portable hydrocarbon detector), trained dogs • Fire Research Laboratory (Maryland)fire origin and cause, fire growth, spread, scene reconstruction

Collection and Preservation of Arson Evidence • Two- three quarts of soot and ash from fire’s point of origin • All porous materials, substances wh/may contain flammable residue (wood, flooring, rugs, rags, upholstery) • Airtight containers- (prevent evaporation), new paint containers, glass jars, leave air space

Collection and Preservation of Arson Evidence • Substrate Control- comparison of uncontaminated control specimens to suspect materials • Ignitors- matches, cigarette, firearms, ammunition, mechanical match striker, sparking device, “Molotav cocktail”

Collection and Preservation of Arson Evidence • Suspect’s clothing • Fluids in open bottles or cans collected and sealed • Soil, vegetation frozen to prevent bacterial degradation

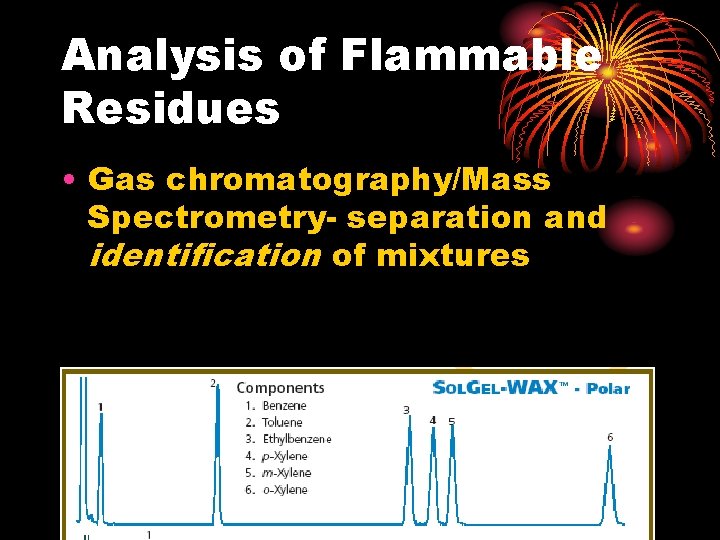
Analysis of Flammable Residues • Gas Chromatograph most sensitive and reliable for detection and characterization of flammable residues • Hydrocarbons (gasoline, kerosene) produce characteristic chromatographic pattern (retention times) • Headspace (air space)- syringe • Vapor concentration (charcoal strip)inc concentration and sensitivity • Debris compared to standard

Analysis of Flammable Residues • Gas chromatography/Mass Spectrometry- separation and identification of mixtures

Science casts doubt on arson cases • Science Casts Doubt on Arson Convictions. doc

Types of Explosives • Homemade explosives and incendiary devices • Investigation by trained and experienced personnel • Bomb disposal, bomb-site investigation, forensic analysis, criminal investigation • Detect and identify explosive chemicals and detonating mechanisms

Types of Explosives • Explosion- combustion reaction proceeding at very rapid rate • Build-up of pressure from expanding gases • Blast effect (outward rush of gases) • Damage due to fragmentation debris and artificial gale

Types of Explosives • Classified according to speed at which explosives decompose • High Explosives(1000 - 8500 mps) • Low Explosives (< 1000 mps)

Low Explosives • Speed of Deflagration- very rapid oxidation, produces heat, light, low intensity subsonic pressure wave, slow burning rate • Propellant for ammunition or skyrockets • Fuel + oxidizing agent

Low Explosives • Black Powder- Potassium (sodium) nitrate (75%), carbon (charcoal) (15%), sulfur (10%) • Safety Fuse- black powder wrapped in casing • Confinement causes explosion

Low Explosives • Smokeless Powder- safest, most powerful low explosive • Single-base: nitrocellulose (nitrated cotton) • Double-base: nitrocellulose + nitroglycerin • Natural Gas + Air

High Explosives • Speed of detonation- extremely rapid oxidation reaction, supersonic shock wave, explosive charge, extremely sensitive

High Explosives- Primary Explosives • Ultrasensitive to heat, shock, friction, detonate violently w/o burning • Primers- detonate other explosives through chain rxn, explosive train • Blasting caps- lead azide, lead styphnate, diazodinitrophenol

High Explosives. Secondary Explosives • Insensitive to heat, shock, friction, burn when ignited, detonated by primary explosive • Dynamite, TNT (trinitrotoluene), PETN (pentaerythritol tetranitrate), RDX (cyclotrimethylenetrinitramine) and tetryl (2, 4, 6 trinitrophenylmethylnitramine)

High Explosives • Ammonium Nitrate-based explosives : • Water gels- water-resistant • Emulsions- oil and water phases • ANFO- aluminum nitrate soaked in fuel oil (Fertilizer + fuel oil)

High Explosives • TATP (triacetone triperoxide)homemade explosive used by terrorist organizations, friction and impact sensitive • Acetone, hydrogen peroxide, HCl acid

High Explosives- Military Explosives • RDX- most popular and powerful, composition C-4 • TNT- shells, bombs, grenades, demolition explosives, propellant • Military “dynamite”- TNT + RDX (does not contain NTG) • PETN- TNT mixtures for small caliber projectiles and grenades, commercially- explosive core of detonating cord (primacord) for simultaneous detonation

Collection and Analysis of Explosives • Collection of appropriate samples from scene • Crater at origin, material blown away • Undetonated residue of explosive, detonating mechanism • Collect soil, debris, porous and nonporous materials (wood, insulation, rubber, metal, etc. ) • Avoid contaminating scene (proper attire)

Collection of Explosives • Systematic search • Wire mesh sifters • IMS (ion mobility spectrometer)portable detection machinepreliminary ID of residues based on travel time due to size and structure

Collection of Explosives • Materials collected, packaged, labeled • Metal containers, plastic bags? • Separately

Analysis of Explosives 1 - Microscopic examination for unconsumed explosive (low explosives) 2 - Debris rinsed w/acetone (or water) to remove explosives 3 - Acetone extract concentrated analyzed

Analysis of Explosives. Acetone Extract • Color spot tests- Griess, Diphenylamine, Alcoholic KOH (Table 11 -3) • TLC • HPLC • GC/MS

Analysis of Explosives. Confirmatory Tests • IR Spectrophotometry-organic explosives (Ex- RDX) • X-ray diffraction -inorganic components of explosives (Ex- potassium nitrate, potassium sulfate)

Collection and Analysis of Explosives- Taggants • Proposed program • Tiny color-coded chips added to commercial explosives • Fluorescent and magnetic • Survive and recover from explosion • Trace and locate legal possessor • Switzerland

The World Trade Center Bombing (1993) • Urea nitrate bomb put into truck and driven into underground WTC garage and parked at 4 th level down • Subsequent explosion did extensive damage to several levels of the garage and less damage to other levels • Although goal was to topple WTC, little structural damage was done • Some loss of life

Goals of Investigation • Identify victims • Identify explosive • Recover bomb and timing device • Determine method of delivery

Evidence Sought • Investigators had to remove large quantities of concrete, steel and cars to get to bomb seat • Bomb seat contained most of the important evidence • Bomb parts; timer, casing, etc. • Explosive residue • Parts of truck that contained explosive

Areas of Forensic Science • • • Explosives Engineering Questioned documents Fingerprints Pathology DNA

The Murrah Building, Oklahoma City (1995) • ANFO explosive and timer packed into a rented truck, which was then parked outside Murrah building • Explosive confined to closed space such as truck is much more powerful • Resulting explosion resulted in severe damage to building and loss of more than 100 lives

Goals of Investigation • Identify victims • Identify explosive • Find timer and bomb parts • Determine method of delivery

Evidence Sought • Easier to find than in WTC because bomb seat outside building • Explosive residues • Bomb parts • Bodies and body parts; cadaver dogs, flies • Personal effects; helps in identification of human remains

Areas of Forensic Science • • • Anthropology DNA and serology Pathology Entomology Explosives Trace evidence Engineering Questioned documents Fingerprints

WTC Destruction (2001) • Large airplanes, loaded with fuel, crash into WTC buildings • Raging fires ignite everything in building above crash sites. • Metal supports melt from heat • Building collapses due to inability to support its own weight after structural damage • Thousands of people killed

Goals of Investigation • Cause known, no need to determine how destruction occurred • Recover and identify bodies, parts of bodies and charred remains • Recover personal effects that might help identify victims or perpetrators • Evidence that might determine how hijackings occurred.

Evidence Sought • Bodies and body parts; cadaver dogs, flies • Charred remains • Personal effects • Trace evidence such as charred papers • Weapons such as knives

Areas of Forensic Science • • • Anthropology DNA and serology Odontology Pathology Entomology Trace evidence Questioned documents Fingerprints Tools and toolmarks

TWA Flight 800 • • July 17, 1996 230 killed Calverton Explosive residue, pockmarks, tearing, “witness material” • Chemical analysis, GC/MS • Water soluble fuel?

Boston Marathon Bombing 2013 • • • 2 Pressure Cooker Bombs Explosives and Blasting Cap Electronic Device Initiator Shrapnel, Ball Bearings, Nails 3 Killed, 264 injured One suspect killed, one pled guilty

Mark Hoffmann. Salt Lake City • http: //investigation. discovery. co m/videos/solved-secrets-andbombs. html
 Chapter 6 fingerprints
Chapter 6 fingerprints Forensic science arson activity
Forensic science arson activity Ice lined refrigerator ppt
Ice lined refrigerator ppt Pathologist and anthropologist
Pathologist and anthropologist Who is this
Who is this Alligatoring burn pattern
Alligatoring burn pattern Burned fire scientist questions arson
Burned fire scientist questions arson The boundary between charred and uncharred material
The boundary between charred and uncharred material Arson pour patterns
Arson pour patterns Arson
Arson Study of fire
Study of fire David berkowitz arson
David berkowitz arson A four alarm fire damaged 14 stores today
A four alarm fire damaged 14 stores today Why is arson considered a low priority crime
Why is arson considered a low priority crime Marking bad clusters data hiding technique
Marking bad clusters data hiding technique Digital forensic lab floor plan
Digital forensic lab floor plan Tasks performed by computer forensics tools
Tasks performed by computer forensics tools Chapter 2 legal and ethical aspects of nursing
Chapter 2 legal and ethical aspects of nursing Why aren t descriptive investigations repeatable
Why aren t descriptive investigations repeatable Year 6 maths investigations
Year 6 maths investigations Craigslist wayne
Craigslist wayne Numerical datum crossword puzzle clue
Numerical datum crossword puzzle clue Statistical investigations examples
Statistical investigations examples Bmv hours heatherdowns
Bmv hours heatherdowns Conclusion for scientific method
Conclusion for scientific method Pasco child protective services
Pasco child protective services Chs investigations
Chs investigations Right iliac fossa mass investigations
Right iliac fossa mass investigations Jarrod bowditch
Jarrod bowditch Antenatal investigations
Antenatal investigations Investigations
Investigations Chs investigations
Chs investigations F&ei
F&ei What is a textile in forensic science
What is a textile in forensic science Chapter 1 introduction to forensic science and the law
Chapter 1 introduction to forensic science and the law The explosion larkin
The explosion larkin Indian burial ground candy bar
Indian burial ground candy bar Evangelism explosion outline
Evangelism explosion outline The concept of population
The concept of population A freight train is being assembled in a switching yard
A freight train is being assembled in a switching yard Mark 48 torpedo
Mark 48 torpedo Thinking routine step inside
Thinking routine step inside Dmc explosion welding
Dmc explosion welding Mass explosion hazard placard
Mass explosion hazard placard Georg grosz explosion
Georg grosz explosion Population growth factors
Population growth factors Evangelism skills
Evangelism skills Diagrama de flujo que calcule el area de un triangulo
Diagrama de flujo que calcule el area de un triangulo Tdg class 1 signs
Tdg class 1 signs Iowa turret explosion
Iowa turret explosion Bleve san juanico
Bleve san juanico Population explosion
Population explosion Mexico city explosion 1984
Mexico city explosion 1984 Texas tech chemistry department
Texas tech chemistry department











































































