FDA Oncology Drug Approval Endpoints Effectiveness and Approval



































































- Slides: 92

FDA Oncology Drug Approval Endpoints, Effectiveness, and Approval Gerald H. Sokol MD, MS, FCP* and Robert Kane, MD, FACP Division of Drug Oncology Products Office of Oncology Drug Products CDER, FDA Not an official FDA policy Asst. Prof Medicine and Clinical Pharmacology Uniformed Services University or the Health Sciences 1

The medical reviewer in his natural habitat 2

3

The “White Oak” Consolidation 4

5

FDA - Oncology Drugs • • Not – Drug imports from Canada Not – Vioxx, Celebrex, Plan B Not – dietary supplements -Foods No stock market tips 6

FDA Oncology Drug Approval • FDA – U. S. Food and Drug Administration – In the Dept of HHS – Executive Branch • Created by Congress because of prior unsafe drugs being marketed • FDA charged by Congress to evaluate all prescription drugs seeking marketing in the U. S. • Federal Laws, Regs govern these activities 7

Communication is the Key 8

Overview for oncology drug approval • • Evidence for efficacy and safety Good Evidence = Approval Endpoints for trial design Endpoints for FDA approval Trial design issues for efficacy Trial results analysis “Targeted” therapy approval and problems Interplay of disease state, existing Rx options, endpoint options, strength of evidence 9

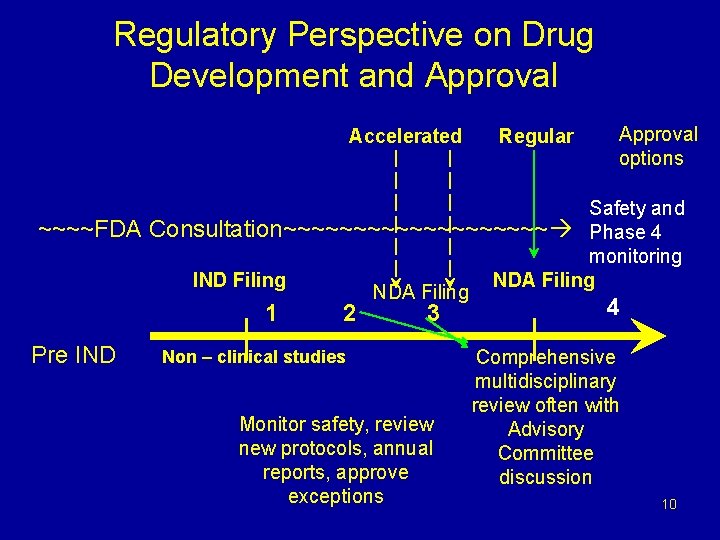
Regulatory Perspective on Drug Development and Approval Accelerated ~~~~FDA Approval options Safety and Consultation~~~~~~~~~~ Phase 4 monitoring IND Filing NDA Filing 1 Pre IND Regular 2 3 Non – clinical studies Monitor safety, review new protocols, annual reports, approve exceptions 4 Comprehensive multidisciplinary review often with Advisory Committee discussion 10

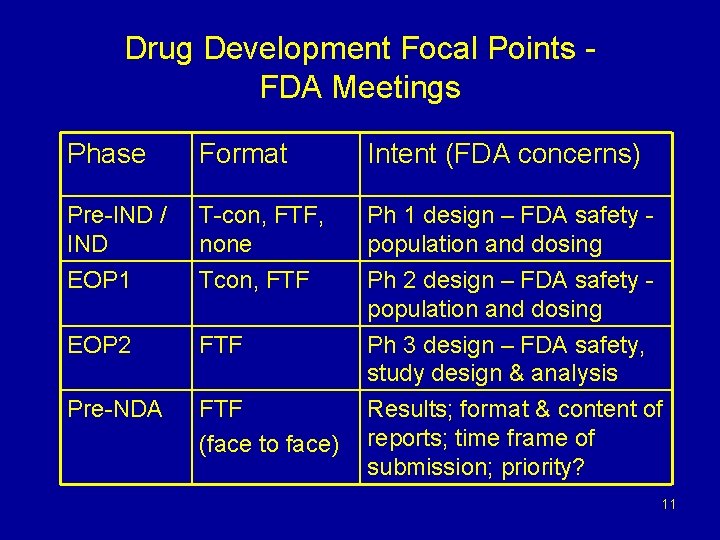
Drug Development Focal Points FDA Meetings Phase Format Intent (FDA concerns) Pre-IND / IND T-con, FTF, none Ph 1 design – FDA safety population and dosing EOP 1 Tcon, FTF EOP 2 FTF Ph 2 design – FDA safety population and dosing Ph 3 design – FDA safety, study design & analysis Pre-NDA FTF (face to face) Results; format & content of reports; time frame of submission; priority? 11

FDA - Oncology Drugs • Office of Oncology Drug Products – OODP • Three divisions DDOP- Chemotherapy drugs for Cancer DBOP- Biologic oncology therapies BLAs DMIHP- Medical Imaging and hematology 12

Requirements for Drug Approval U. S. Statutes –Congress Labeling 1906 Pure Food & Drug Act Safety 1938 Food, Drug, and Cosmetic Act Efficacy 1962 FDC Amendments Harris-Kefauver FDAMA Amdnts. 1997 Code of Federal Regulations (CFRs) Regulations from FDA provide interpretation of the laws 13

Drug Approval Tracks • FDA Modernization Act 1997 (FDAMA): CFR 312 subpart E regulations • To expedite development for life-threatening illnesses – Fast track • process for meeting FDA -unmet med need – Priority review • Determined by FDA after NDA submitted • 6 month NDA review time frame for products addressing unmet medical need 14

Drug Approval Tracks (2) • Accelerated approval “CFR 314 subpart H” – 1992 • Special Protocol Assessment – agreement between FDA and sponsor on clinical protocols for phase 3 studies forming primary basis for demonstrating efficacy for NDA; 45 day clock 15

FDA Oncology Drug Approval • How do you achieve approval ? • Provide substantial evidence of Efficacy and Safety • What is substantial evidence for Effectiveness – “A & WC investigations” CFR 314. 126 • 2 or more studies – required by 1962 amend. to FFDCA Source = Controlled clinical trials 16

Basis for New Drug Approval • Demonstration of efficacy with acceptable safety in adequate and well-controlled studies CFR 314 - NDA Regulations • Ability to generate product labeling that – Defines an appropriate patient population for treatment with the drug – Provides adequate information to enable safe and effective use – prescribing of the drug • Analogous rules for Biologics - BLA 17

FDA Oncology Drug Approval • Clinical trials: – When we do not know which therapy is better – Patients are fully informed of the uncertainty about which therapy is better and give consent – We can compare one treatment with another in a controlled way – Today’s “standard” therapy was last year’s investigational treatment 18

FDA Oncology Drug Approval • Controlled clinical trials: – Can be verified, repeated if necessary – Can allow us to be convinced a new therapy is effective – or it is not. – Allow us to compare how well tolerated (how safe) a new therapy is versus a standard • Efficacy with Safety => Approval 19

Some trial considerations • Usual situation for many FDA drug approvals – – – Multiple studies Studies are large 1, 000 – 5, 000 patients Placebo control group Double Blinded or a blinded independent assessment Highly significant p values (0. 001) • Oncology drug data submitted for approval – One study, 100 – 800 patients – No blinding, no placebo control – Heterogeneous patient group – Statistical evidence ~ 0. 03 – 0. 05 *Concern – how confident can we be that results are real 20

The FDA mission is not a new idea! "The aim. . . is not simply to accept the statements of others, but to investigate the causes that are at work in nature. " Albertus Magnus, de Mineralibus circa 1250 21

FDA Oncology Drug Approval • Suppose YOU are the FDA – • What benefits should a new drug have to allow its marketing approval in the U. S. ? • How “safe” should the drug be? 22

FDA Oncology Drug Approval • What benefits should a new drug have for marketing approval in the U. S. ? • LIVE LONGER -----------Effective • LIVE BETTER – QUALITY ------Effective 23

FDA Oncology Drug Approval • What benefits should a new drug have for marketing approval in the U. S. ? • LIVE LONGER -----------Effective • LIVE BETTER – QUALITY ------Effective • Improved safety - with efficacy • Benefits outweigh Risks (312. 84) • B / R assessment - in disease context • COST ? Less expensive? / Reimbursement ? – Not purview of FDA 24

FDA Oncology Drug Approval • Judgment of Benefits versus Risks • But - NEVER Have ALL the Data • Some benefits or adverse effects occur: – Rarely – After long time interval • How long to study and wait before Approval • Too slow or too fast to approve? 25

What Drugs Are Safe ? 26

What Drugs Are Safe ? None of them 27

What Drugs Might Help Someone? 28

What Drugs Might Help Someone? All of them 29

Can we tell in advance who might be helped and who might be hurt by a drug? 30

Can we tell in advance who might be helped and who might be hurt by a drug? NO But we hope to be able to soon 31

Two Types of Drug Approval: Regular or Accelerated Endpoints Supporting Regular Approval Demonstrate Clinical Benefit – Longer life – Better life (relief of tumor-related Sx) - PRO • Requires a valid measure of how a patient feels or functions – Favorable effect on established surrogate 32

Accelerated Approval (AA) Only applies in the setting of a new drug for a serious or life-threatening illness: • Improvement over available therapy • Study may use a surrogate endpoint, reasonably likely to predict clinical benefit • Requires confirmation of benefit Fed Register 1992 33

Oncology Trial Endpoints (1) CLINICAL BENEFIT ENDPOINTS • LIVE LONGER – Measure Survival- OS Efficacy, reassures for safety, unbiased endpt but – subsequent therapy, long time required • LIVE BETTER – Measure QOL – PRO Important: control group + blinding needed (bias) hard to measure, scale problems, missing data Drug Tox. symptoms versus tumor symptoms => COMPARISONS ARE NECESSARY Note – These endpoints do not measure the tumor 34

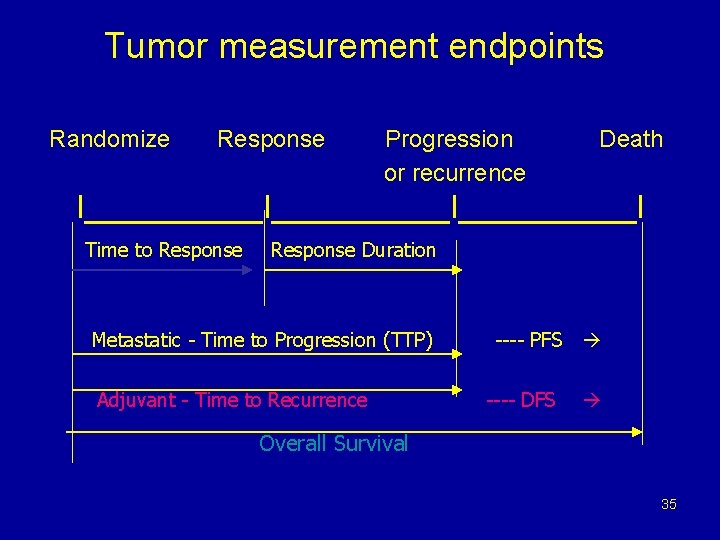
Tumor measurement endpoints Randomize Response Progression or recurrence Death I__________I_____I Time to Response Duration Metastatic - Time to Progression (TTP) Adjuvant - Time to Recurrence ---- PFS ---- DFS Overall Survival 35

Oncology Endpoints (2) • DISEASE-FREE SURVIVAL (DFS) – Composite of survival and NED – Recurrence is associated with symptoms, new therapies, cognitive effects – Adjuvant treatment setting • Breast cancer • Colorectal cancer – 3 year DFS (p ≤ 0. 03) => 5 year OS • New areas likely – lung, prostate, brain, etc 36

Endpoints - Regular Approval (3) • Survival • QOL – PRO • Safety improved- with efficacy • DFS – adjuvant and leukemia settings • Improvements convey/are clinical benefits • Improvements show effectiveness Demonstrate these endpoints = (Full) Regular Approval (RA) 37

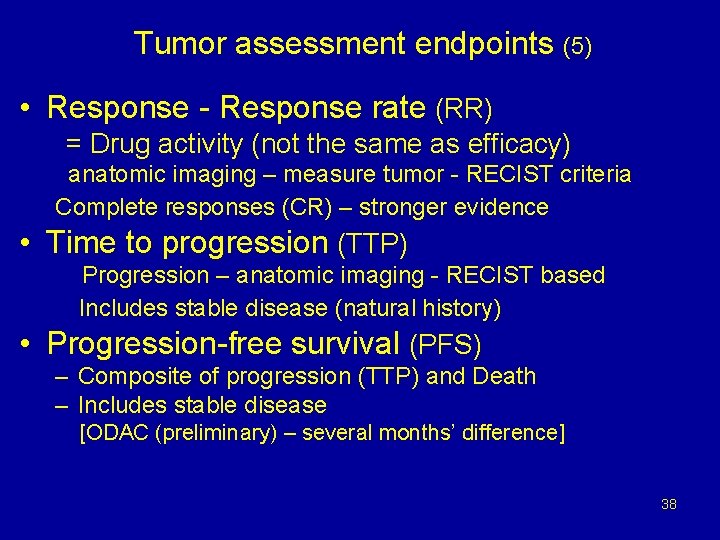
Tumor assessment endpoints (5) • Response - Response rate (RR) = Drug activity (not the same as efficacy) anatomic imaging – measure tumor - RECIST criteria Complete responses (CR) – stronger evidence • Time to progression (TTP) Progression – anatomic imaging - RECIST based Includes stable disease (natural history) • Progression-free survival (PFS) – Composite of progression (TTP) and Death – Includes stable disease [ODAC (preliminary) – several months’ difference] 38

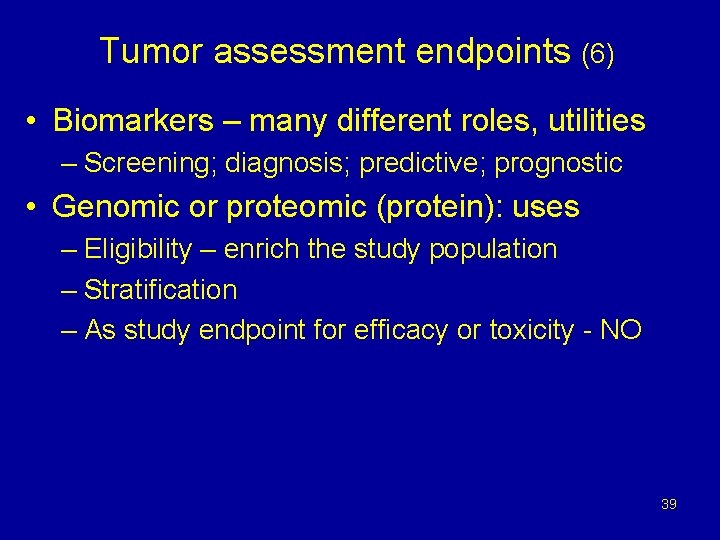
Tumor assessment endpoints (6) • Biomarkers – many different roles, utilities – Screening; diagnosis; predictive; prognostic • Genomic or proteomic (protein): uses – Eligibility – enrich the study population – Stratification – As study endpoint for efficacy or toxicity - NO 39

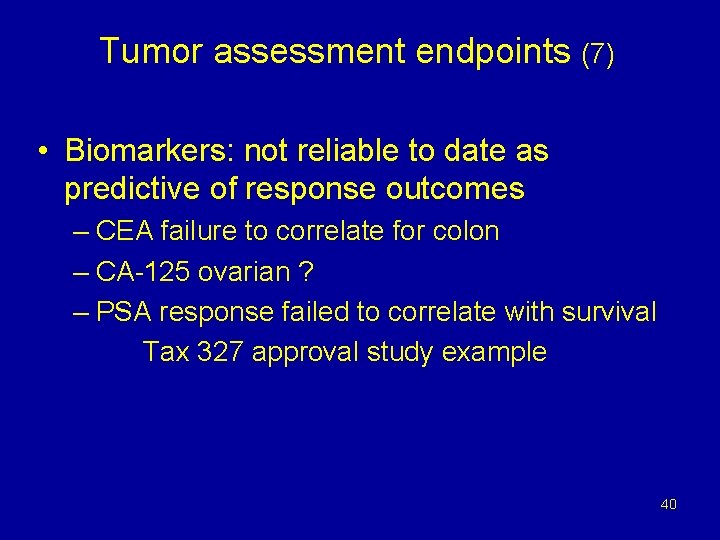
Tumor assessment endpoints (7) • Biomarkers: not reliable to date as predictive of response outcomes – CEA failure to correlate for colon – CA-125 ovarian ? – PSA response failed to correlate with survival Tax 327 approval study example 40

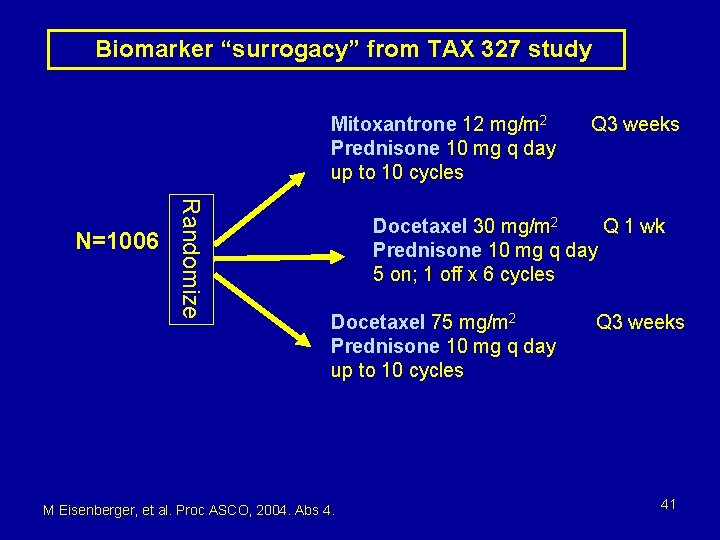
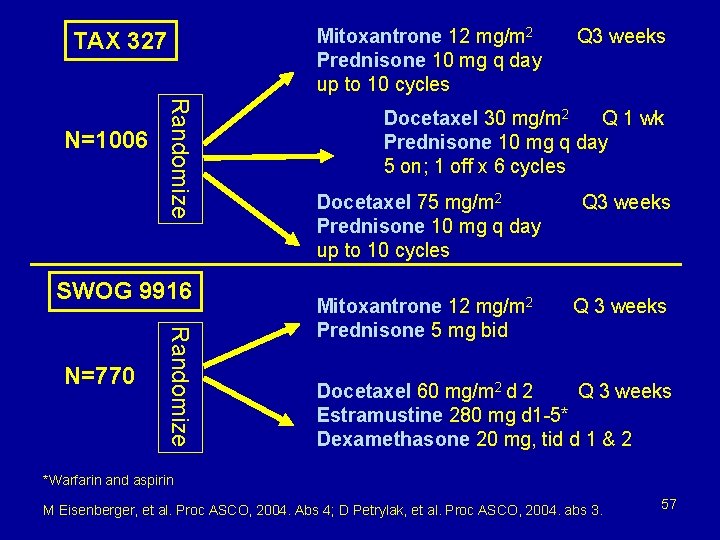
Biomarker “surrogacy” from TAX 327 study Mitoxantrone 12 mg/m 2 Prednisone 10 mg q day up to 10 cycles Randomize N=1006 Q 3 weeks Docetaxel 30 mg/m 2 Q 1 wk Prednisone 10 mg q day 5 on; 1 off x 6 cycles Docetaxel 75 mg/m 2 Prednisone 10 mg q day up to 10 cycles M Eisenberger, et al. Proc ASCO, 2004. Abs 4. Q 3 weeks 41

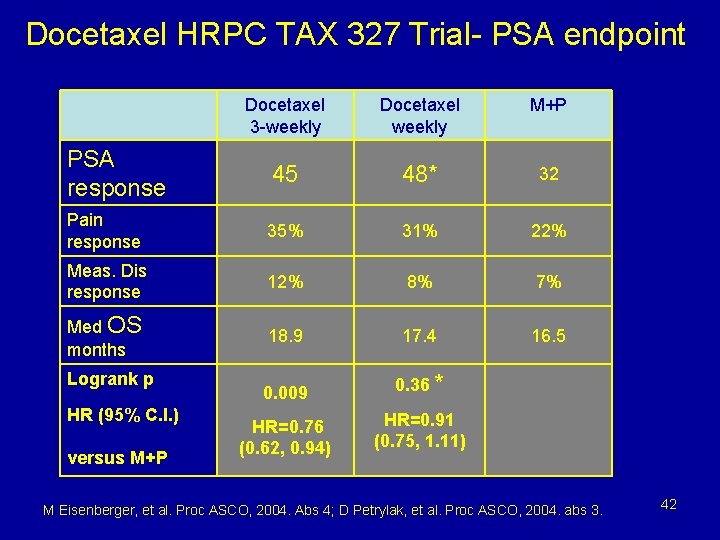
Docetaxel HRPC TAX 327 Trial- PSA endpoint Docetaxel 3 -weekly Docetaxel weekly M+P PSA response 45 48* 32 Pain response 35% 31% 22% Meas. Dis response 12% 8% 7% Med OS months 18. 9 17. 4 16. 5 0. 009 0. 36 * HR=0. 76 (0. 62, 0. 94) HR=0. 91 (0. 75, 1. 11) Logrank p HR (95% C. I. ) versus M+P M Eisenberger, et al. Proc ASCO, 2004. Abs 4; D Petrylak, et al. Proc ASCO, 2004. abs 3. 42

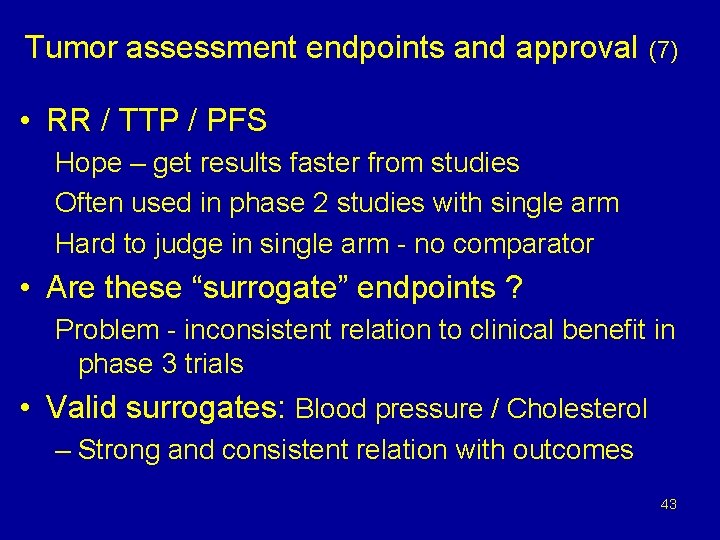
Tumor assessment endpoints and approval (7) • RR / TTP / PFS Hope – get results faster from studies Often used in phase 2 studies with single arm Hard to judge in single arm - no comparator • Are these “surrogate” endpoints ? Problem - inconsistent relation to clinical benefit in phase 3 trials • Valid surrogates: Blood pressure / Cholesterol – Strong and consistent relation with outcomes 43

Approval and tumor assessment endpoints (8) Regular Approval – RA – If Clinical Benefit shown (live longer, better, safer) - or – Benefit on Established surrogate - (DFS, Heme CR) Accelerated Approval - AA For Serious or Life Threatening illnesses • Show meaningful therapeutic benefit over existing therapy or improved patient response over available therapy • May be based on a surrogate endpoint which is reasonably likely to predict clinical benefit - or • a clinical endpoint other than survival or irreversible morbidity 44

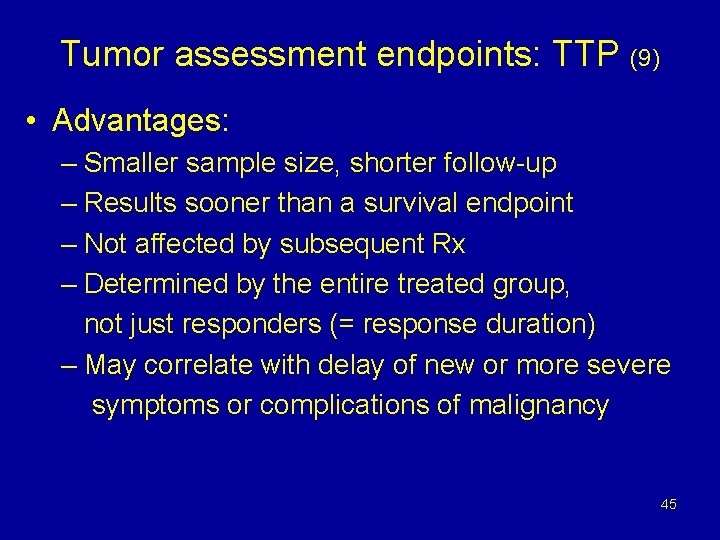
Tumor assessment endpoints: TTP (9) • Advantages: – Smaller sample size, shorter follow-up – Results sooner than a survival endpoint – Not affected by subsequent Rx – Determined by the entire treated group, not just responders (= response duration) – May correlate with delay of new or more severe symptoms or complications of malignancy 45

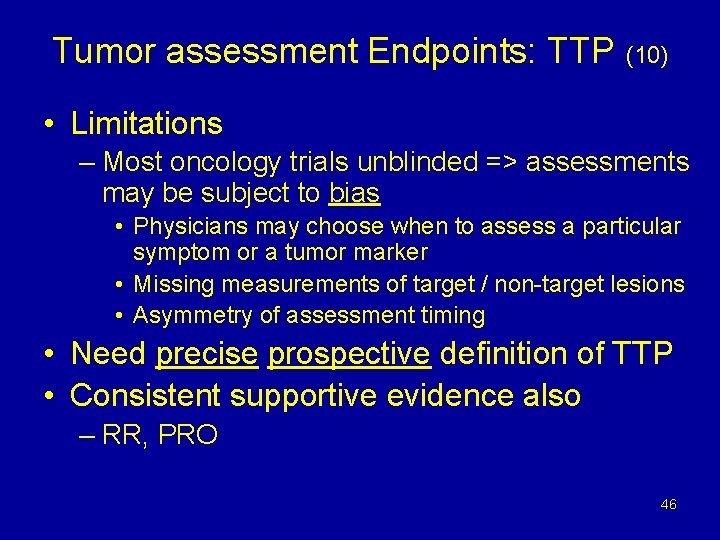
Tumor assessment Endpoints: TTP (10) • Limitations – Most oncology trials unblinded => assessments may be subject to bias • Physicians may choose when to assess a particular symptom or a tumor marker • Missing measurements of target / non-target lesions • Asymmetry of assessment timing • Need precise prospective definition of TTP • Consistent supportive evidence also – RR, PRO 46

TTP in Breast Cancer (11) • ODAC Meeting 6 -99 examined TTP in MBC • MBC using First-line cytotoxic treatment • TTP not shown to correlate with OS or QOL – May be a problem of TTP magnitude, later Rx • ODAC advised: TTP not be used alone for RA – Epirubicin: not approved for MBC – study used primary endpoint TTP • Potential use as surrogate endpoint for accelerated approval in RCTs with same study continued to survival endpoint 47

What about Response - CR or PR (12) • CR = complete response PR = partial response – assessment must be prospectively defined: when, how – published criteria available to define response • RECIST, NCI-WG, IBMT, etc – In phase 2, response => ACTIVITY, not benefit – Is it associated with symptom benefit? – Durability of response is important • MBC with hormone Rx: RR + TTP may => RA • Durable CRs in Hematologic malignancies – fewer infections, visits, transfusions – – may => RA 48

What about Response - CR or PR (13) • Examples of CR based regular approvals: – Cladribine for hairy cell leukemia (CRs > 8 mo) – Pentostatin for hairy cell leukemia CRs > 24 mo – Ifosfamide in combination for 3 rd-line therapy of germ cell testicular tumors (CRs > 2 years) – IL-2 for renal cell carcinoma / melanoma – cures ? – As. O 3 and ATRA for APL – RA: small, single arm 49

Approval and tumor assessment endpoints (14) • AA uses tumor assessment endpoints Magnitude and consistency of effect important RR + TTP may achieve AA TTP alone unlikely to be sufficient RR + TTP with hormone Rx – breast cancer may => RA CRs with Duration – Hematologic cancers may => RA Endpoints and FDA Oncology Drug Approvals Johnson, Williams, Pazdur Journal of Clinical Oncology 2003; 21: 1404 -1411 • Disease - Endpoint – Drug - Approval 50

Effectiveness: Oncology Trial Design (1) How to show effectiveness – 1. Choose appropriate endpoint (with FDA) • Disease, therapy, and regulatory context 2. Choose appropriate study design (with FDA) – Phase 2 or phase 3 – many phase 2 results not confirmed in phase 3! – Usually phase 3 with comparator arm of: • Standard of care treatment or Placebo • Prospective, randomized, blinded * comparison – Blinding or masking: allocation, investigator, patient, sponsor 51

Effectiveness: Oncology Trial Design (2) 2. Appropriate study design: How to compare? • Type of comparison – Superiority vs. Non-inferiority – Superiority: • versus placebo or add-on design • “head to head” with an active control Rx (risky) – Non-inferiority – problems IN ONCOLOGY • • Estimating effect of control treatment Constancy assumption - historical control Retention margin Large sample sizes required 52

Effectiveness: Oncology Trial Design (3) 3. Appropriate Analysis Plan – Pre-specified - why? • Control error rate (chance of false positive conclusion) • Not post-hoc, data-driven – Estimate the difference to be detected • What can your new drug do? – Size the study – what power do you want to have to demonstrate the primary endpoint – ITT population for comparison of arms – Why ? 53

Effectiveness: Oncology Trial Design (4) – ITT population for comparison of arms – Reduces Bias • Cannot define the analysis population AFTER data examined – ITT to see differences between arms 54

Effectiveness: Oncology Trial Design (5) 4. Statistical meaning – Null hypothesis = assume no difference between groups – Stat test – How likely is this difference a result of chance? – If unlikely due to chance, maybe due to treatment? – FDA concern - Error of a false positive conclusion (1/20) • ? Acceptable error rate – pre-specified = Alpha • two-sided 0. 05 - generates our P < 0. 05 – If there’s no difference found? ≡ Equivalence? • No! –Only means you cannot reject null hypothesis – Bayesian perspectives 4. Clinical meaning beyond statistical meaning 55

Oncology Approval Example - Prostate • 2004 Docetaxel (Taxotere) approval for HRPC • 2003 - Approved drugs for treatment of HRPC – Estramustine 1981 – Mitoxantrone 1996 * – Zoledronic acid 2003 * * approvals based on QOL-PRO not on survival 56

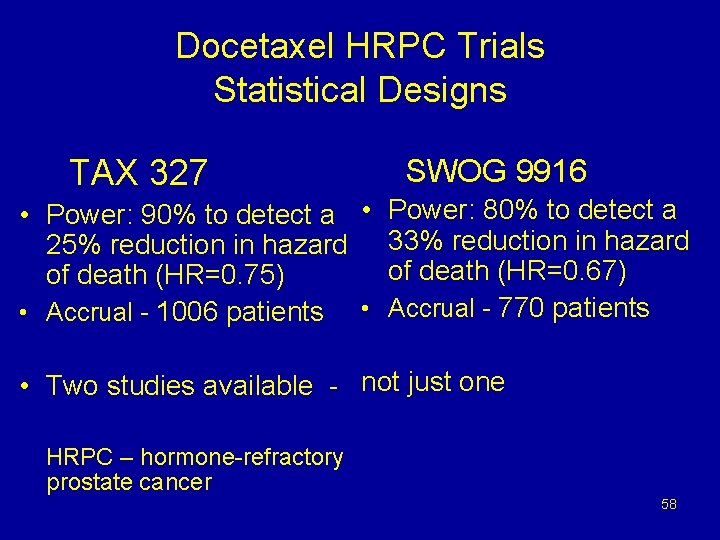
Mitoxantrone 12 mg/m 2 Prednisone 10 mg q day up to 10 cycles TAX 327 Randomize N=1006 SWOG 9916 Randomize N=770 Q 3 weeks Docetaxel 30 mg/m 2 Q 1 wk Prednisone 10 mg q day 5 on; 1 off x 6 cycles Docetaxel 75 mg/m 2 Prednisone 10 mg q day up to 10 cycles Mitoxantrone 12 mg/m 2 Prednisone 5 mg bid Q 3 weeks Docetaxel 60 mg/m 2 d 2 Q 3 weeks Estramustine 280 mg d 1 -5* Dexamethasone 20 mg, tid d 1 & 2 *Warfarin and aspirin M Eisenberger, et al. Proc ASCO, 2004. Abs 4; D Petrylak, et al. Proc ASCO, 2004. abs 3. 57

Docetaxel HRPC Trials Statistical Designs TAX 327 SWOG 9916 • Power: 90% to detect a • Power: 80% to detect a 25% reduction in hazard 33% reduction in hazard of death (HR=0. 67) of death (HR=0. 75) • Accrual - 1006 patients • Accrual - 770 patients • Two studies available - not just one HRPC – hormone-refractory prostate cancer 58

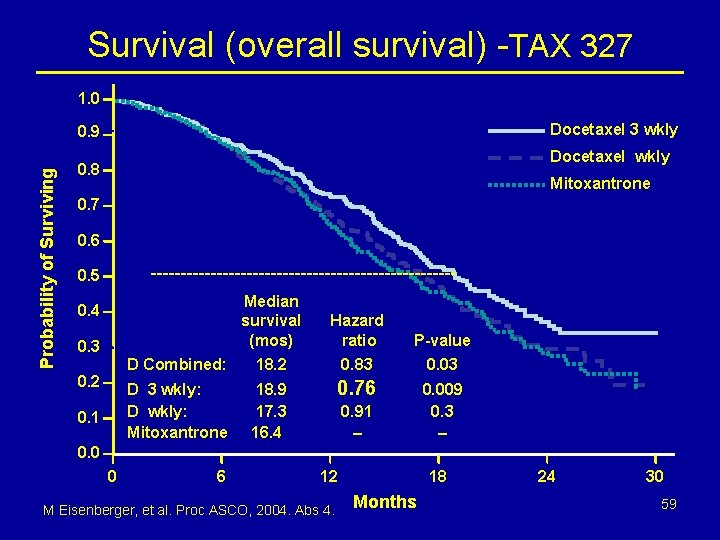
Survival (overall survival) -TAX 327 1. 0 Docetaxel 3 wkly Probability of Surviving 0. 9 Docetaxel wkly 0. 8 Mitoxantrone 0. 7 0. 6 -------------------------- 0. 5 Median survival (mos) Hazard ratio P-value D Combined: 18. 2 0. 83 0. 03 D 3 wkly: D wkly: Mitoxantrone 18. 9 17. 3 16. 4 0. 76 0. 009 0. 3 – 0. 4 0. 3 0. 2 0. 1 0. 91 – 0. 0 0 6 12 M Eisenberger, et al. Proc ASCO, 2004. Abs 4. 18 Months 24 30 59

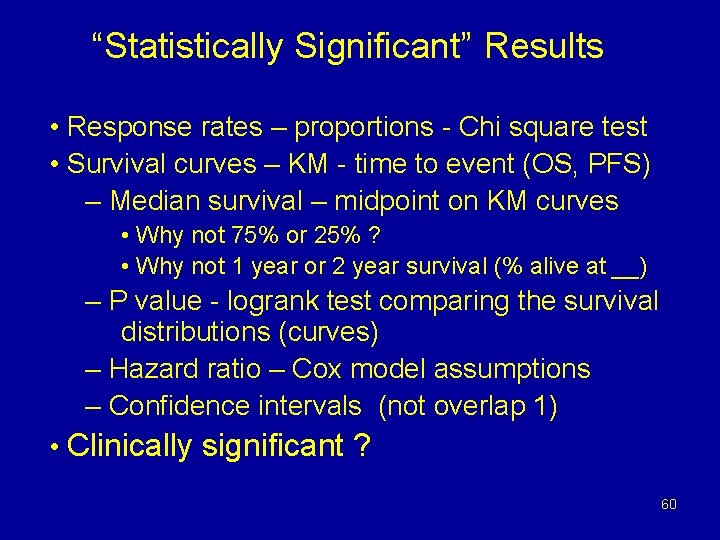
“Statistically Significant” Results • Response rates – proportions - Chi square test • Survival curves – KM - time to event (OS, PFS) – Median survival – midpoint on KM curves • Why not 75% or 25% ? • Why not 1 year or 2 year survival (% alive at __) – P value - logrank test comparing the survival distributions (curves) – Hazard ratio – Cox model assumptions – Confidence intervals (not overlap 1) • Clinically significant ? 60

Statistical / Clinical Significance ? • Best case – have both • Can you have stat sig. but not clinical - yes – Approval likely ? No • Can you have clinical sig. but not stat - yes – Approval likely ? It depends • • Phase 2 results Statistical design may become inappropriate Safety advantage, Other Clinical benefit is the goal! 61

FDA Oncology Drug Approval • Regulatory consequences of Demonstrating Efficacy • Regular Approval if: clinical benefit endpoint – OS, QOL, DFS, and occasional other • Accelerated Approval if: • Benefit over existing therapy – if any; and • If a surrogate, then “reasonably likely” to predict, and • Must verify clinical benefit later • If efficacy uncertain – ODAC likely 62

Evidence for Accelerated Approval • Substantial evidence from well controlled clinical trials regarding a surrogate endpoint • NOT: Borderline evidence regarding a clinical benefit endpoint 63

FDA Review Times • Assuming a complete application and no substantive amendments submitted during the review • Priority review completion: – 6 months – May fulfill an unmet medical need – Substantial improvement • Standard review completion: – 10 months 64

Ages of Oncology Drug Approval • Historical Era – Response rates - approval • Current Era – Statistical refinements – Clinical Benefit and surrogate endpoints • Molecular Era 65

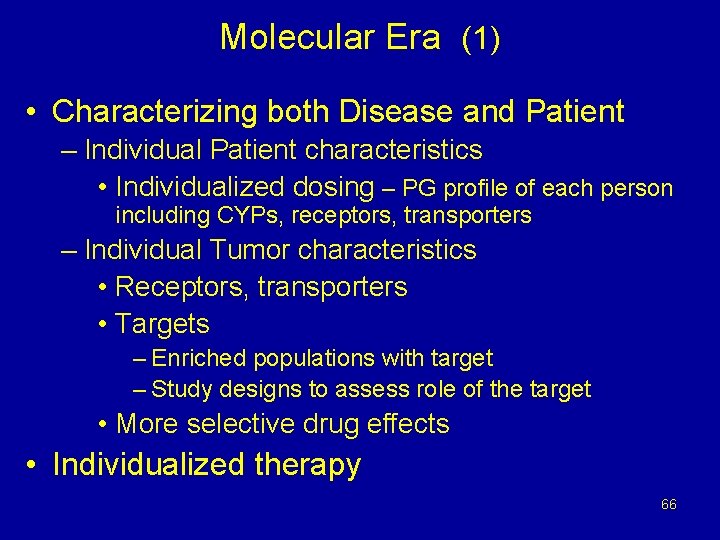
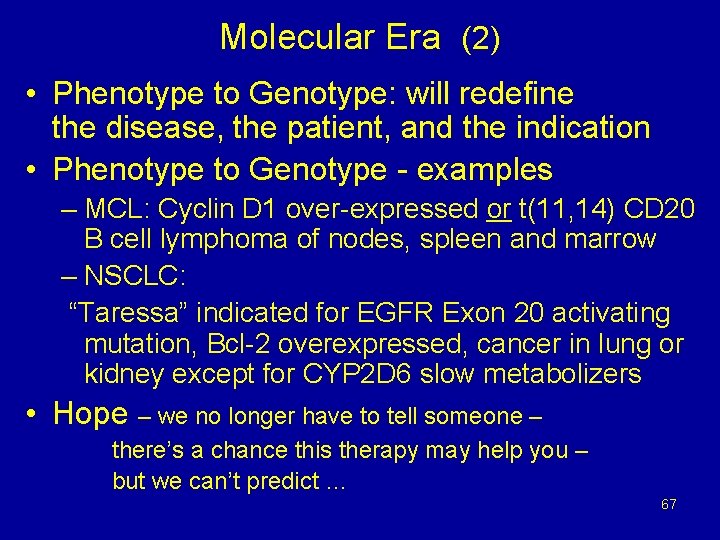
Molecular Era (1) • Characterizing both Disease and Patient – Individual Patient characteristics • Individualized dosing – PG profile of each person including CYPs, receptors, transporters – Individual Tumor characteristics • Receptors, transporters • Targets – Enriched populations with target – Study designs to assess role of the target • More selective drug effects • Individualized therapy 66

Molecular Era (2) • Phenotype to Genotype: will redefine the disease, the patient, and the indication • Phenotype to Genotype - examples – MCL: Cyclin D 1 over-expressed or t(11, 14) CD 20 B cell lymphoma of nodes, spleen and marrow – NSCLC: “Taressa” indicated for EGFR Exon 20 activating mutation, Bcl-2 overexpressed, cancer in lung or kidney except for CYP 2 D 6 slow metabolizers • Hope – we no longer have to tell someone – there’s a chance this therapy may help you – but we can’t predict … 67

Oncology targeted therapies 2006 (3) Monoclonal Antibodies: approval target label ? Rituxan (Rituximab) – lymphoma 1997 CD 20 yes Herceptin (Trastuzumab) - breast 1998 p 185 neu yes *Mylotarg (Gemtuzumab) - AML 2000 CD 33 yes *Campath (Alemtuzumab) – CLL 2001 CD 52 no *Erbitux (Cetuximab) – colon 2004 EGFR yes Avastin (Bevacizumab) – colon 2004 VEGF no Label ? - Is a test for the target included in the label indication? (May not be exactly the same test as the target assay) (Radio-immunoconjugates- Zevalin (2002) and Bexxar (2003): anti-CD 20) *AA products - Comparative, randomized trials demonstrating increased survival or clinical benefits such as improvement in disease-related symptoms have not yet been conducted. 68

Oncology targeted therapies • Small molecule inhibitors approval – Nolvadex (Tamoxifen)-Breast 1977 – Vesanoid (ATRA) ----- APL 1995 – Gleevec (Imatinib) ---- CML 2001 – *Gleevec (Imatinib) -- GIST 2002 – *Iressa (Gefitinib) ----- Lung 2003 – Tarceva (Erlotinib) ---- Lung 2004 2006 (4) target label ? ER yes RARα yes bcr/abl yes c-kit yes EGFR? no Label ? – Is a test for the target included in the label indication? *AA -comparative, randomized trials demonstrating increased survival or clinical benefits such as improvement in disease-related symptoms have not yet been conducted – AA 69

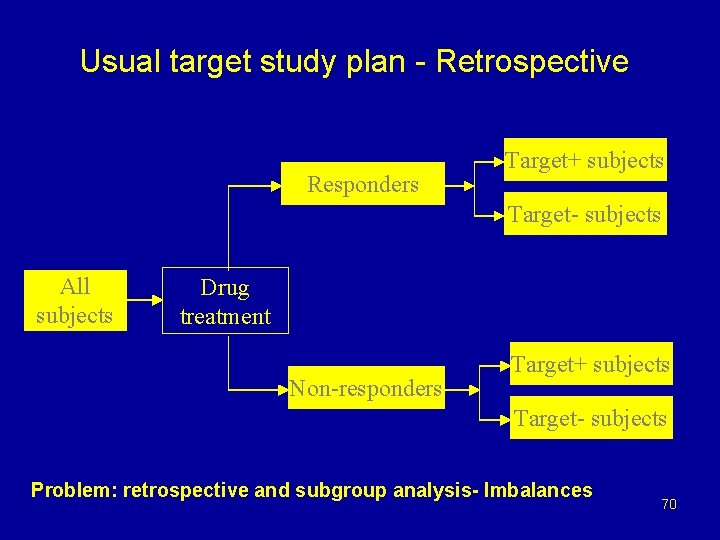
Usual target study plan - Retrospective Responders Target+ subjects Target- subjects All subjects Drug treatment Non-responders Target+ subjects Target- subjects Problem: retrospective and subgroup analysis- Imbalances 70

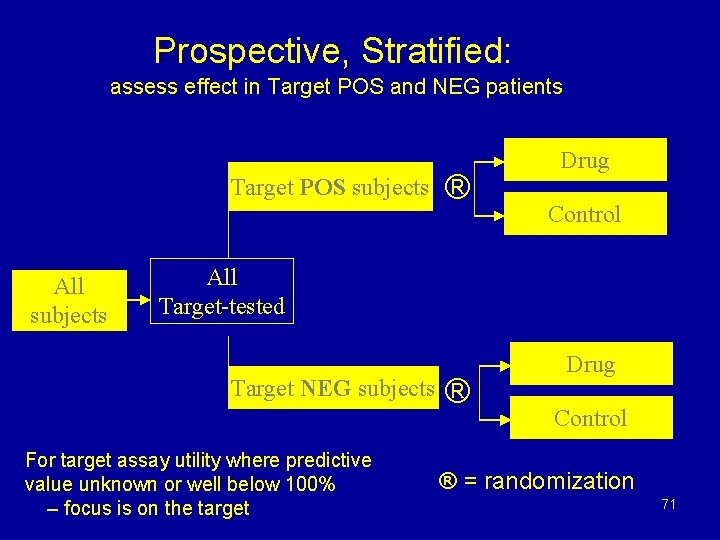
Prospective, Stratified: assess effect in Target POS and NEG patients Target POS subjects All subjects ® Drug Control All Target-tested Target NEG subjects For target assay utility where predictive value unknown or well below 100% – focus is on the target ® Drug Control ® = randomization 71

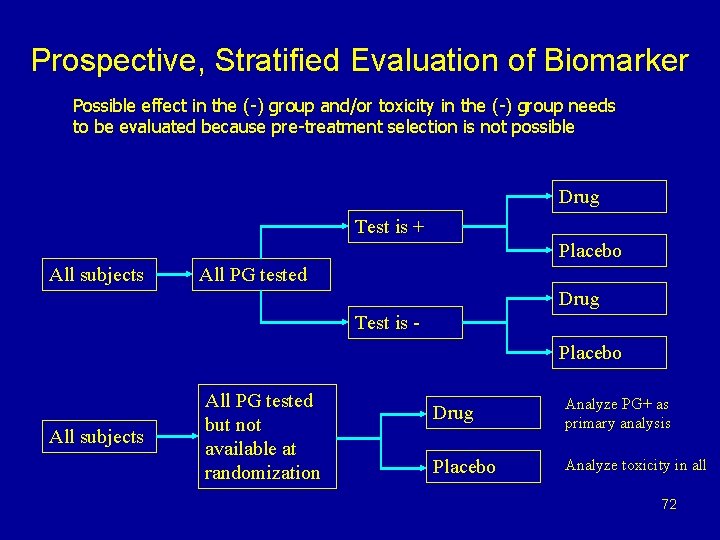
Prospective, Stratified Evaluation of Biomarker Possible effect in the (-) group and/or toxicity in the (-) group needs to be evaluated because pre-treatment selection is not possible Drug Test is + Placebo All subjects All PG tested Drug Test is Placebo All subjects All PG tested but not available at randomization Drug Analyze PG+ as primary analysis Placebo Analyze toxicity in all 72

Targeted Therapy - Problems • Which target(s) is the target? Herceptin target-gene amplification not protein “expression” • Can’t measure the target – EGFR, her-2 • Can’t correlate target inhib. with outcome – Wrong target for the disease state – Target may not be in the disease pathway – Variable “expression” “over-expression” – Target present but non-functional or variable f(x) • Can’t validate target – “Inconclusive” study design 73

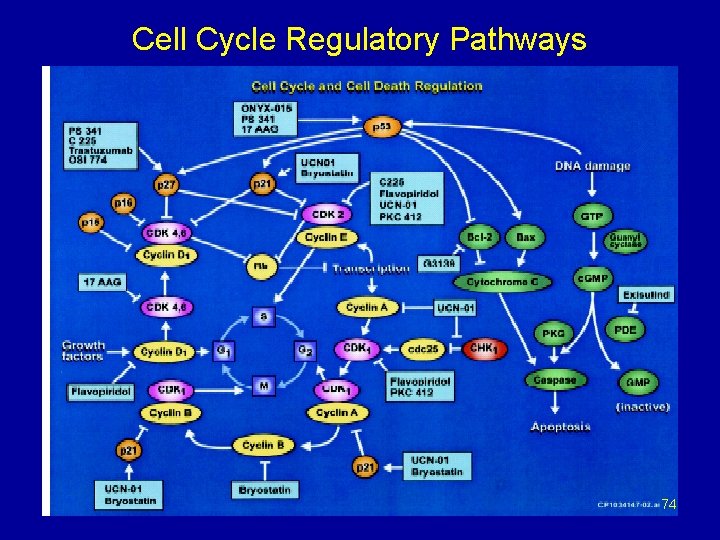
Cell Cycle Regulatory Pathways 74

FDA Oncology Drug Approval • • • Appropriate Endpoint Appropriate Design Appropriate Conduct (FDA will verify data) Appropriate Analysis Demonstrate Efficacy and Safety (Benefit - Risk assessment) DDOP - NDA => Marketing Approval DBOP - BLA => Biologics license 75

General NDA Review Procedure for a New Drug • Separate reviews by disciplines – stat, med, pharm-tox, Biopharm, CMC • All primary data reviewed • Analysis of Benefit versus Risk in the context of the disease process. • Applications may be discussed before an advisory committee (ODAC) at an open public meeting 76

Why Bad Things (non-approval) can happen to Good Drugs 77

“Tortured Data Will Eventually Confess” Some Examples of Clinical Trial Conduct “problems” – Inadequate or no controls – Missing or unclear selection/eligibility criteria – Small sample size - underpowered – Randomization process concerns – Lack of objective outcome assessment – Improper handling of dropouts – Inadequate adjustment for prognostic factors – Improper or misleading tables and graphs 78

“Complementary and Alternative Statistics” • Some improper statistical methods – Disregard of multiple comparisons – Improper (selective) censoring, exclusions – Post-hoc hypothesis selection (data-dredging) usually on a subgroup analysis not pre-specified – Claiming subgroup results when the overall study fails 79

notes • ODAC 1977: – Approval to be based on survival or, possibly, improved quality of life • Supreme Court 1979: US vs. Rutherford (Laetrile) – A drug is effective “if it … prolonged life, improved physical condition, or reduced pain” • ODAC March 24, 1983: – Reaffirmed above, and added “objective tumor response could also be used if a positive correlation between tumor response and: survival, QOL, or relief of pain could be shown. ” 80

FDA Website for Oncology Endpoints www. fda. gov/cder/drug/cancer_endpoints/default. htm FDA Guidances: www. fda. gov/cder/guidance/index. htm • Guidance on Effectiveness: http: //www. fda. gov/cder/guidance/5506 fnl. pdf • Guidance on Oncology trials endpoints- draft http: //www. fda. gov/cder/guidance/6592 dft. htm • Guidance on PRO - draft http: //www. fda. gov/cder/guidance/5460 dft. htm 81

23. This isn't an office. It's Hell with fluorescent lighting. 24. I started out with nothing & still have most of it left. 25. Sarcasm is just one more service we offer. 26. If I throw a stick, will you leave? 27. Errors have been made. Others will be blamed. 28. Whatever kind of look you were going for, you missed. 29. I'm trying to imagine you with a personality. 30. A cubicle is just a padded cell without a door. 82 31. Can I trade this job for what's behind door

stupid. 12. You are validating my inherent mistrust of strangers. 13. I have plenty of talent and vision; I just don't give a damn. 14. I'm already visualizing the duct tape over your mouth. 15. I will always cherish the initial misconceptions I had about you. 16. Thank you. We're all refreshed and challenged by your unique point of view. 17. The fact that no one understands you doesn't mean you're an artist. 18. Any connection between your reality and mine is purely coincidental. 19. What am I? Flypaper for freaks!? 20. I'm not being rude. You're just insignificant. 21. And your crybaby whiny-assed opinion would be. . . ? 83 22. Do I look like a people person?

Notes: Basis for New Drug Approval - NDA • Demonstration of efficacy with acceptable safety in adequate and well-controlled studies CFR 314 - NDA Regs • Ability to generate product labeling that – Defines an appropriate patient population for treatment with the drug – Provides adequate information to enable safe and effective use – prescribing of the drug • Analogous rules for Biologics - BLA 84

United States versus Rutherford US Supreme court 442 US 544 (1979) • A drug is effective “if it fulfills by objective indices, its sponsor’s claims of prolonged life, improved physical condition, or reduced pain, ” and safe if the drug’s potential for inflicting death or physical injury is offset by the possibility of therapeutic benefit. 85

notes • ODAC 1977: – Approval to be based on survival or, possibly, improved quality of life • Supreme Court 1979: US vs. Rutherford (Laetrile) – to be effective, a cancer drug must by objective indices, improve survival, improve the quality of life, or relieve pain • ODAC March 24, 1983: – Reaffirmed above, and added “objective tumor response could also be used if a positive correlation between tumor response and: survival, QOL, or relief of pain could be shown. ” • ODAC June 28, 1985: – Prolonged disease-free survival is an important goal of adjuvant studies and is sufficient for approval of a drug for adjuvant therapy of breast cancer 86

Notes • FDA Modernization Act 1997: FDAMA – Created CFR 312 subpart E: Procedures to expedite development for serious and life-threatening disease – Fast track – process for meeting with FDA – Priority review – 6 month NDA review time frame for products addressing unmet medical need – Endorsed as possible one high quality study • SPA – clinical protocols for phase 3 studies forming primary basis for efficacy for NDA; 45 d. 87

FDAMA 1997 – one study for efficacy • 1998 FDA Guidance: Characteristics of a single study to support effectiveness (with independent substantiation from related study data): a. Large, multicenter study no single site or investigator disproportionately responsible for result b. Consistency across study subsets consistency across key subsets, i. e. severity of disease, stage, age c. Multiple studies within the study – pairwise comparisons within the study d. Multiple endpoints involving different events somewhat unrelated endpts i. e. MI and death e. Statistically very persuasive – very low p values (not 0. 045!) 88

U. S. Legal Process • Regulations: CFRs – Interpretations of laws – by the Executive Branch Departments – FDA Regs: Full power of laws when adopted • Guidance: Issued by individual agencies (FDA or CDER) to reflect current thinking, not binding. 89

Chemo-Prevention Endpoints Current Approvals 90

Reduction in cancer incidence • Tamoxifen is approved: – to reduce the incidence of BC in women at high risk for BC – In women with DCIS, following breast surgery and RT, to reduce the risk of invasive BC – To reduce contralateral BC in patients receiving adjuvant tamoxifen therapy for BC • Basis: Reduction in cancer incidence in RCT 91

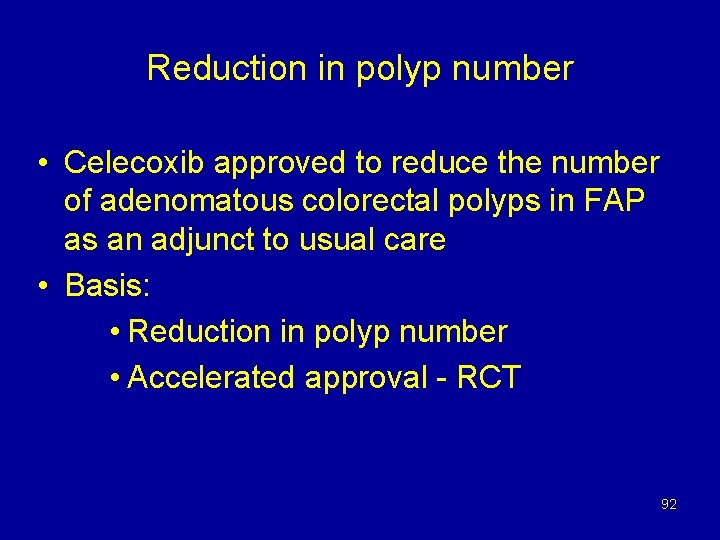
Reduction in polyp number • Celecoxib approved to reduce the number of adenomatous colorectal polyps in FAP as an adjunct to usual care • Basis: • Reduction in polyp number • Accelerated approval - RCT 92
 Fda approval means nothing
Fda approval means nothing Fda approval means nothing
Fda approval means nothing Liquid vision presbyopia
Liquid vision presbyopia New drug development and approval process
New drug development and approval process Example of substitution with exhausted drug is
Example of substitution with exhausted drug is Physical devices of a computer
Physical devices of a computer It is a segment whose endpoints lie on the circle
It is a segment whose endpoints lie on the circle Synerzip
Synerzip Fda v brown and williamson
Fda v brown and williamson The effectiveness of online and blended learning
The effectiveness of online and blended learning It is arrangement of people in an organization
It is arrangement of people in an organization Efficiency and effectiveness examples
Efficiency and effectiveness examples Efficiency and effectiveness examples
Efficiency and effectiveness examples Efficiency and effectiveness in higher education
Efficiency and effectiveness in higher education Managing productivity and marketing effectiveness
Managing productivity and marketing effectiveness Managerial behavior and effectiveness
Managerial behavior and effectiveness Sachs immuno oncology bd&l and investment forum
Sachs immuno oncology bd&l and investment forum Unc hematology oncology fellowship
Unc hematology oncology fellowship 20092006 color
20092006 color Lurbinectedin posologie
Lurbinectedin posologie Point halfway between the endpoints of a line segment
Point halfway between the endpoints of a line segment Hyperbola
Hyperbola Facts about hyperbola
Facts about hyperbola A part of a line consisting of two endpoints
A part of a line consisting of two endpoints Find the missing endpoint
Find the missing endpoint Magbigay ng tatlong fraction na katumbas ng 5/6
Magbigay ng tatlong fraction na katumbas ng 5/6 Ivt theorem
Ivt theorem Testing endpoints for convergence
Testing endpoints for convergence The shortest arc connecting two endpoints on a circle
The shortest arc connecting two endpoints on a circle A line with two arrows
A line with two arrows Study endpoints
Study endpoints Early feasibility study
Early feasibility study Nicole gillette fda
Nicole gillette fda Leonard sacks fda
Leonard sacks fda Real world evidence
Real world evidence Equipment qualification fda
Equipment qualification fda Sulmetin
Sulmetin Ryzyko stosowania w ciąży klasyfikacja fda
Ryzyko stosowania w ciąży klasyfikacja fda Fda klasifikacija lekova u trudnoci
Fda klasifikacija lekova u trudnoci Investigator's brochure
Investigator's brochure Cartisona
Cartisona Fda cgmp training
Fda cgmp training Leonard sacks fda
Leonard sacks fda Nicole ibrahim fda
Nicole ibrahim fda Fda
Fda Fda chemist
Fda chemist Efs program
Efs program Dr todd simpson
Dr todd simpson Fda ora org chart
Fda ora org chart Fda cfsan organizational chart
Fda cfsan organizational chart Fda reviewer jobs
Fda reviewer jobs Fda registration number example
Fda registration number example Qsit audit
Qsit audit Good documentation practices fda
Good documentation practices fda Nsde file
Nsde file Fda iso 14971
Fda iso 14971 Estimand meaning
Estimand meaning Fda authorizes first to
Fda authorizes first to 513g fda
513g fda Fda pre submission cover letter
Fda pre submission cover letter Clinical investigator training program
Clinical investigator training program Garnett wood fda
Garnett wood fda Fda open data
Fda open data Fda dmc guidance
Fda dmc guidance Fda ssed database
Fda ssed database Ashley boam
Ashley boam Oopd database
Oopd database Blincyto fda
Blincyto fda Melissa torres fda
Melissa torres fda Neumedix
Neumedix Thalia mills fda
Thalia mills fda Fda caers
Fda caers Hec-fda
Hec-fda Bimo audit checklist
Bimo audit checklist Irbmakeup
Irbmakeup Fda debarment list clinical investigators
Fda debarment list clinical investigators Fda bimo inspection checklist
Fda bimo inspection checklist Fda environmental assessment
Fda environmental assessment Fda form 3911
Fda form 3911 Fda cosmetic labeling guide
Fda cosmetic labeling guide Fda 1572 electronic signature
Fda 1572 electronic signature Bob temple fda
Bob temple fda Usda fda
Usda fda Fda product code builder
Fda product code builder Fda meaning
Fda meaning Yoshio nakatani
Yoshio nakatani What part of gcp mandates data integrity
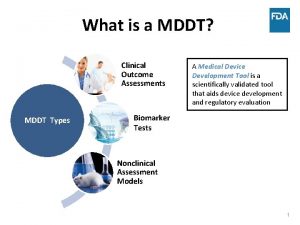
What part of gcp mandates data integrity Mddt
Mddt 78 fr 58786
78 fr 58786 Fda early feasibility study
Fda early feasibility study Epedigree fda
Epedigree fda Requisitos fda para exportar alimentos
Requisitos fda para exportar alimentos 21cfr312.23
21cfr312.23