The FDA Early Feasibility Study EFS Program Update

The FDA Early Feasibility Study (EFS) Program Update on How the Program is Changing the Landscape Andrew Farb, MD and Dorothy Abel, BSBME EFS Program Co-Leaders Division of Cardiovascular Devices Center for Devices and Radiological Health (CDRH) Food and Drug Administration FDA Town Hall CRT 2015 Washington, DC February 24, 2015
Conflict of Interest No conflicts of interest to report. 2

Worldwide Device Development 1 Design device or identify a new use 2 Do preliminary non-clinical testing 3 Initiate clinical use outside of the US Conduct additional non-clinical testing 4 Initiate animal studies in the US Get CE Mark and market outside of the US 5 Optional – Initiate traditional feasibility study in the US 6 Conduct pivotal study in the US Complete non-clinical testing (e. g. , durability) 3 7 Market the device in the US

Worldwide Device Development: The Goal 1 Design device or identify a new use 2 Do bench and animal testing to justify an early feasibility study (EFS) 3 Initiate the EFS Conduct additional bench testing and animal studies to support a larger clinical study 4 Optional – Initiate traditional feasibility study 5 Initiate pivotal study Complete non-clinical testing (e. g. , durability) 6 Market worldwide 4

Worldwide Device Development: The Goal 1 Design device or identify a new use 2 Do bench and animal testing to justify an early feasibility study (EFS) 3 Initiate the EFS Conduct additional bench testing and animal studies to support a larger clinical study 4 Optional – Initiate traditional feasibility study 5 Initiate pivotal study Complete non-clinical testing (e. g. , durability) 6 Market worldwide 5

Worldwide Device Development: The Goal 1 Design device or identify a new use 2 Do bench and animal testing to justify an early feasibility study (EFS) 3 Initiate the EFS in the US Conduct additional bench testing and animal studies to support a larger clinical study 4 Optional – Initiate traditional feasibility study 5 Initiate pivotal study Complete non-clinical testing (e. g. , durability) 6 Market worldwide 6

Early Feasibility Study (EFS) • Small number of subjects • Device may be early in development, typically before the device design has been finalized – Does not necessarily involve the first clinical use of a device • May involve a new intended use for a device that has already been in clinical use • Needed when the information to advance device development cannot be practically obtained with additional nonclinical assessments, or if nonclinical tests are unavailable • May be done concurrently or in conjunction with non. US clinical studies 7

EFS Program • Goal - To facilitate US EFS, encouraging the development of useful devices, while providing protection of public health and safety under the IDE regulations Patient-centered focus – Consider the totality of the benefit/risk for the study (i. e. , not just the potential benefits and risks of the device), and keeping the clinical context at the forefront – Disease condition (e. g. , life-limiting, life-threatening) – Limitations of and risks associated with alternative treatments or diagnostic modalities – Patient tolerance for risk and perspective on benefits – Risk mitigation strategies when balancing benefits and risks, 8

EFS Guidance • Key Guidance Principle - Approval of an early feasibility study IDE may be based on less nonclinical data than would be needed to support the initiation of a larger clinical study of a more final device design 9

Guidance Provision Documenting the Appropriate Testing • Promotes consideration of the clinical context for the EFS and all available information • Emphasizes the need to consider the rationale for the testing strategy (i. e. , what the testing is intended to address) before determining whether the results are supportive – Supports Just-in-Time Testing, that is, doing the right testing at the right time – Recommends a Device Evaluation Strategy (DES) to provide a systematic, tabular approach to identify the information needed to support study initiation 10
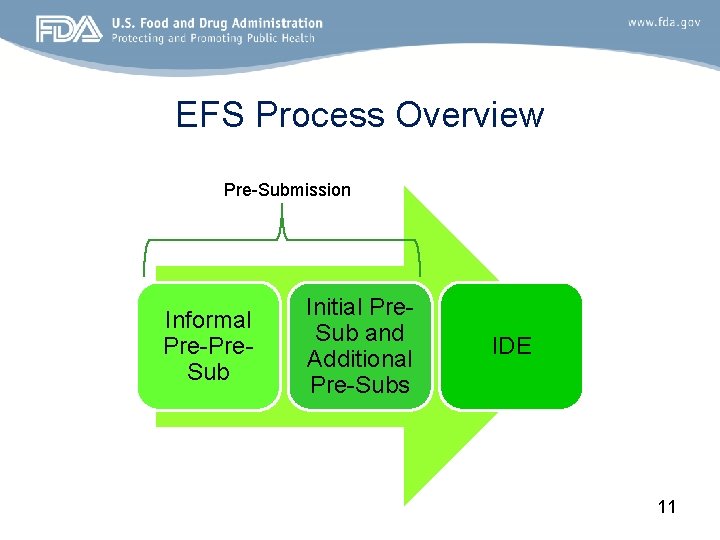
EFS Process Overview Pre-Submission Informal Pre-Pre. Sub Initial Pre. Sub and Additional Pre-Subs IDE 11
The EFS Process q Informal Interaction q EFS Pre-Submission q EFS IDE
Contact Your EFS Representative! Names Andrew Farb, M. D. Dorothy Abel Joel Anderson, Ph. D. DAGRID Joy Samuels-Reid, M. D. Tejashri Purohit-Sheth, M. D. DCD Carmen Gacchina Johnson, Ph. D. Deborah Castillo, Ph. D. Viveca Livezey, M. D. DNPMD Erin Keegan Dave Mc. Gurl Jemin Dedania DOD Casey Hanley, Ph. D. Tieuvi Nguyen, Ph. D. DOED Angelo Green, Ph. D. , DABT Veronique Li DRGUD Andrew Fu, Ph. D. Long Chen, Ph. D. DSD Betsy Ballard, M. D. Maureen Dreher, Ph. D. OSEL Cristin Welle, Ph. D. Leaders e-mail Andrew. Farb@fda. hhs. gov Dorothy. Abel@fda. hhs. gov Joel. Anderson@fda. hhs. gov Joy. Samuels-Reid@fda. hhs. gov Tejashri. Purohit-Sheth@fda. hhs. gov Carmen. Gacchina@fda. hhs. gov Deborah. Castillo@fda. hhs. gov Viveca. Livezey@fda. hhs. gov Erin. Keegan@fda. hhs. gov David. Mc. Gurl@fda. hhs. gov Jemin. Dedania@fda. hhs. gov Casey. Hanley@fda. hhs. gov Tieuvi. Nguyen@fda. hhs. gov Angelo. Green@fda. hhs. gov Veronique. Li@fda. hhs. gov Andrew. Fu@fda. hhs. gov Long. Chen@fda. hhs. gov Betsy. Ballard@fda. hhs. gov Maureen. Dreher@fda. hhs. gov Cristin. Welle@fda. hhs. gov 13

Purposes of the Initial Informal Interactions Quick Strike Response Time to Jump-Start the Process • Discuss the EFS guidance principles and concepts • Prepare for initial interactions with the review team − Help develop the Report of Prior Investigations for an IDE, including a Device Evaluation Strategy (DES) table − Assist in drafting a Pre-Sub of sufficient quality for FDA’s subject matter experts 14

The EFS Process þ Informal Interaction q EFS Pre-Submission q EFS IDE

EFS Pre-Submission • Initial Pre-Submission – Reach agreement on the information needed to support study initiation • Supplements to the Pre-Submission – As needed to obtain feedback on other aspects of the EFS proposal 16

Suggested Content of the Initial Pre-Sub 1. Present the unique aspects of the device design and intended patient population – Device design concept – Clinical context 2. Explain why an EFS is needed to advance device development. 3. Provide history of the device development to date 17

Suggested Initial Pre-Sub 4. Summarize the information that will be provided in the Report of Prior Investigations in a future IDE submission and an explanation as to why this information should be adequate to support study initiation 5. Include Pre-Sub questions 18
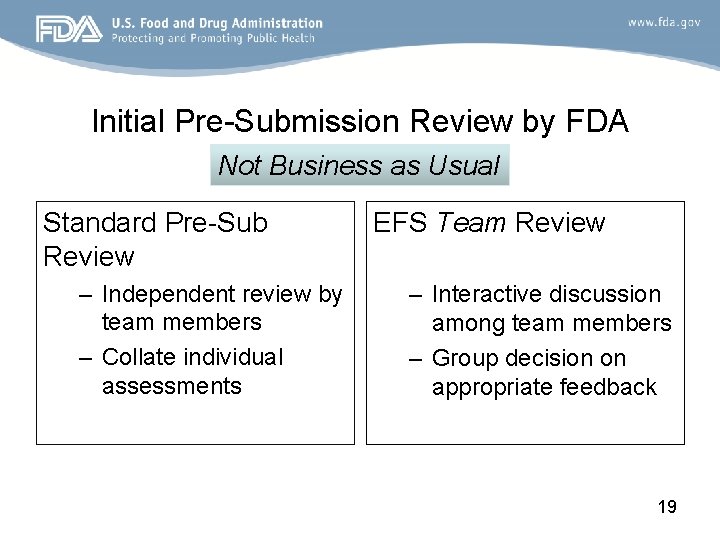
Initial Pre-Submission Review by FDA Not Business as Usual Standard Pre-Sub Review – Independent review by team members – Collate individual assessments EFS Team Review – Interactive discussion among team members – Group decision on appropriate feedback 19

Initial Pre-Sub Review Procedure • Initial review team meeting within 2 weeks to introduce the EFS Program, as needed – Discuss whether the project is appropriate to include in the EFS Program – Review the purpose of the initial Pre-Sub • Have an understanding of the device design, intended patient population, and the information needed in the Report of Prior Investigations to allow for the use of the device in that population in a small clinical study • Team review meeting within one month – Discuss questions and concerns regarding the device design concept, the clinical context, and the device evaluation strategy – Work together to identify any required clarification or areas of disagreement with the sponsor • The goal is to provide written comments to the sponsor within 45 days of receipt of the Pre-Sub 20

Initial Pre. Sub FDA/Sponsor Pre-Sub Meeting • The goal is to have a meeting with the sponsor within 60 days of receipt of Pre-Sub • Discussion should include: – Comments on the DES – Areas needing clarification or disagreement based on the feedback provided by FDA – Additional questions included in the Pre-Sub – Identifying the need for additional Pre-Subs or interactions • Further work on the DES • Submission and review of nonclinical testing protocols • Clinical study plan and informed consent 21
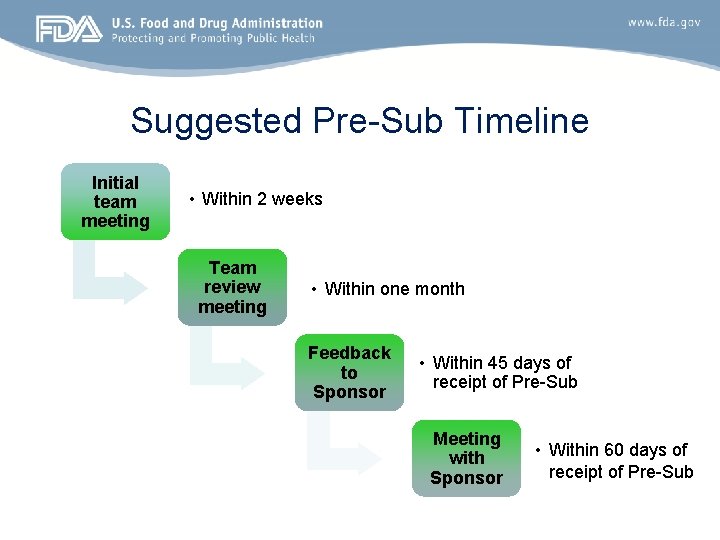
Suggested Pre-Sub Timeline Initial team meeting • Within 2 weeks Team review meeting • Within one month Feedback to Sponsor • Within 45 days of receipt of Pre-Sub Meeting with Sponsor • Within 60 days of receipt of Pre-Sub

The EFS Process þ Informal Interaction þ EFS Pre-Submission q EFS IDE

EFS IDE’s • Goals: – Increased number of EFS IDEs submitted – Approval to initiate the clinical study within the first 30 day review cycle • Lay groundwork for approval via Pre-Sub collaboration • Interactive review to identify and communicate major concerns • Interactive study conduct – Frequent communication between FDA and sponsors regarding study progress, outcomes, and proposed changes 24

IDE Next Steps • Subsequent clinical evaluation depends on: – the stability of the device design; – the availability of data to justify the next study; and – the purpose of the clinical study • Options: – submission of an IDE supplement requesting expansion of EFS; – initiation of a traditional feasibility study; or – initiation of a pivotal study, as appropriate 25
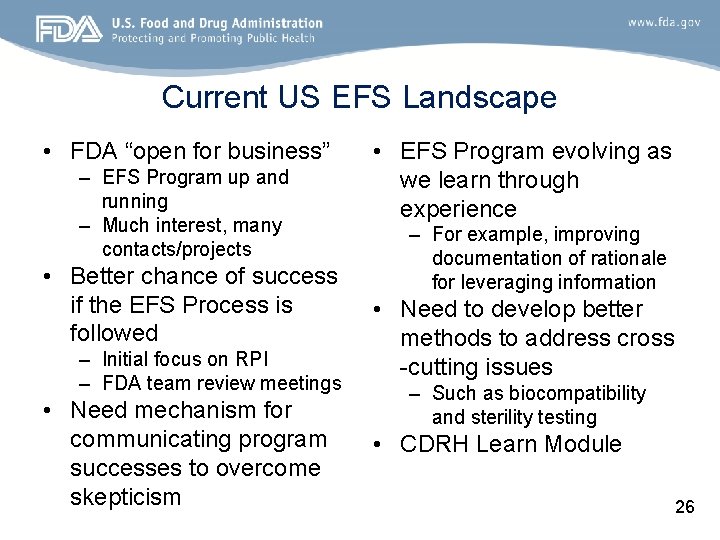
Current US EFS Landscape • FDA “open for business” – EFS Program up and running – Much interest, many contacts/projects • Better chance of success if the EFS Process is followed – Initial focus on RPI – FDA team review meetings • Need mechanism for communicating program successes to overcome skepticism • EFS Program evolving as we learn through experience – For example, improving documentation of rationale for leveraging information • Need to develop better methods to address cross -cutting issues – Such as biocompatibility and sterility testing • CDRH Learn Module 26

Resources • Early Feasibility Study Guidance http: //www. fda. gov/downloads/Medical. Devices/Device. Regulationand. Gui dance/Guidance. Documents/UCM 279103. pdf 2 • Pre-Submission Guidance http: //www. fda. gov/downloads/Medical. Devices/Device. Regulationand. Gui dance/Guidance. Documents/UCM 311176. pdf Contacts: andrew. farb@fda. hhs. gov and dorothy. abel@fda. hhs. gov 27
- Slides: 27