Overview of New and Emerging Therapies in Cancer


































![Reference 17. Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology Inc; 2018 18. Choudhry Reference 17. Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology Inc; 2018 18. Choudhry](https://slidetodoc.com/presentation_image_h2/4fd2df055913d48e8d33d55006b88363/image-59.jpg)
![Reference 33. Iclusig [package insert]. Cambridge, MA: Takeda Pharmaceuticals; 2018 34. Bosulif [package insert]. Reference 33. Iclusig [package insert]. Cambridge, MA: Takeda Pharmaceuticals; 2018 34. Bosulif [package insert].](https://slidetodoc.com/presentation_image_h2/4fd2df055913d48e8d33d55006b88363/image-60.jpg)
![Reference 48. Alecensa [package insert]. South San Francisco, CA: Genentech USA, Inc; 2018 49. Reference 48. Alecensa [package insert]. South San Francisco, CA: Genentech USA, Inc; 2018 49.](https://slidetodoc.com/presentation_image_h2/4fd2df055913d48e8d33d55006b88363/image-61.jpg)


- Slides: 63

Overview of New and Emerging Therapies in Cancer Treatment Minhee Kang, Pharm. D, BCOP, BCPS Med. Star Washington Hospital Center

Disclosure § Dr. Kang has nothing to disclose

Objectives § Review the history of cancer therapy development § Recognize the use of targeted therapies § Describe the use of immunotherapy § Identify emerging therapies

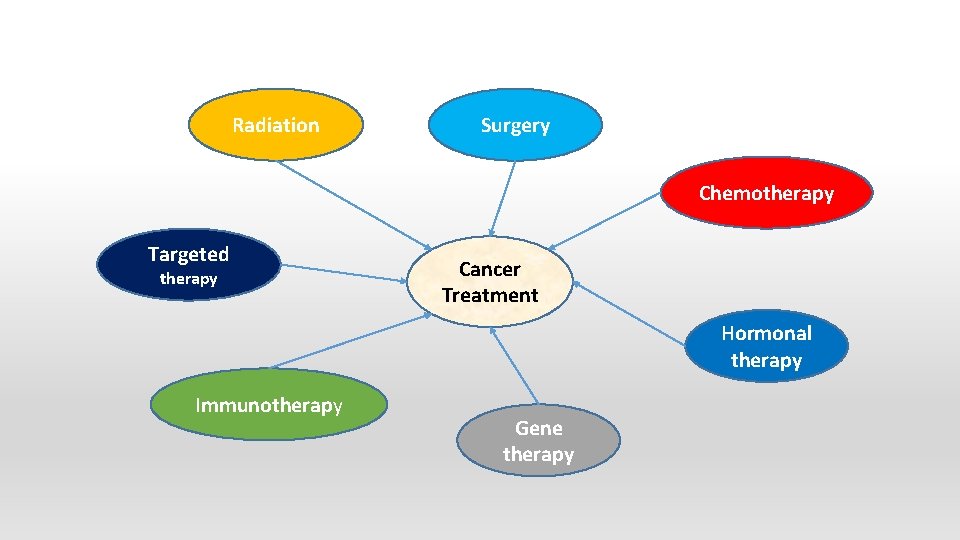
Radiation Surgery Chemotherapy Targeted therapy Cancer Treatment Hormonal therapy Immunotherapy Gene therapy

Hallmarks of cancer § Sustaining proliferative signaling § Resisting cell death § Inducing angiogenesis § Enabling replicative immortality § Evading growth suppressors § Activating invasion and metastasis § Genome instability and mutation § Tumor promoting inflammation § Regulating cellular energetics § Avoiding immune destruction

Chemotherapy

Chemotherapy § Drug therapy to slow or stop the growth of rapidly dividing cancer cells in the body. § Newly approved chemotherapy in recent years § Eribulin (Halaven) for breast cancer and liposarcoma § Trifluridine/tipiracil (Lonsurf) for colorectal cancer (CRC) and gastric and gastroesophageal junction (GEJ) adenocarcinoma § Daunorubicin/cytarabine (Vyxeos) for acute myeloid leukemia (AML) § Calaspargase pegol-mknl (Asparlas) for acute lymphoblastic leukemia (ALL)

Hormone therapy § Slow or stop the growth of hormone-sensitive tumors § Prevent body from producing the hormones or by interfering with the action of the hormones § Breast cancer § Prostate cancer § Enzalutamide (Xtandi) § Apalutamide (Erleada)

Targeted therapy

Targeted therapy § Molecules targeting specific enzymes, growth factor receptors, and signal transducers § Interfere oncogenic cellular processes § Block signal transduction § Antiangiogenesis § Promote cell death (apoptosis) or inactivity (senescence)

Two main types of targeted therapy § Small molecules: attack targets inside and outside of the cell, as well as targets within the same family or class of protein kinases § Monoclonal antibodies § Bind to their molecular target, mostly membrane bound receptors § Prevent ligand binding § Stimulate immune system to kill the targeted cell, such as complementmediated cytotoxicity, immune modulation, antibody dependent cellular cytotoxicity

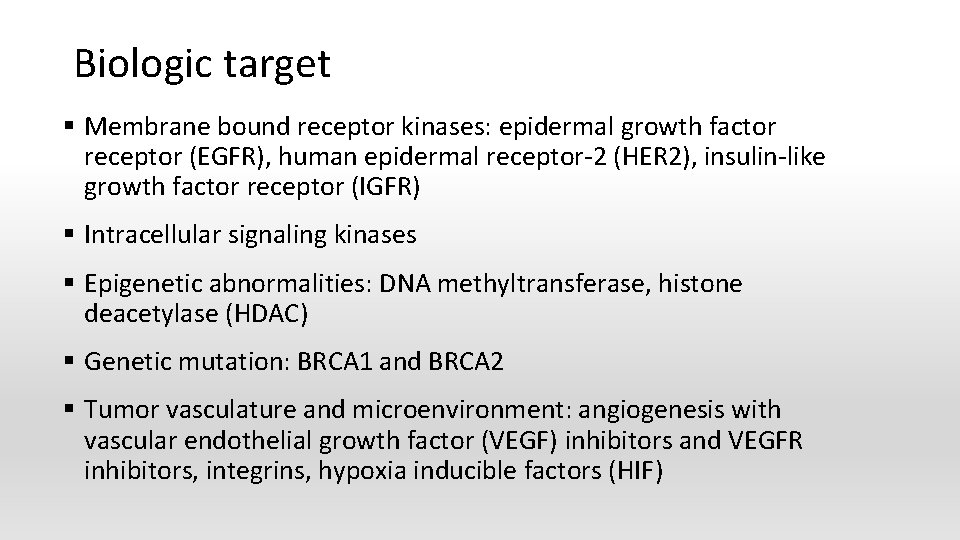
Biologic target § Membrane bound receptor kinases: epidermal growth factor receptor (EGFR), human epidermal receptor-2 (HER 2), insulin-like growth factor receptor (IGFR) § Intracellular signaling kinases § Epigenetic abnormalities: DNA methyltransferase, histone deacetylase (HDAC) § Genetic mutation: BRCA 1 and BRCA 2 § Tumor vasculature and microenvironment: angiogenesis with vascular endothelial growth factor (VEGF) inhibitors and VEGFR inhibitors, integrins, hypoxia inducible factors (HIF)

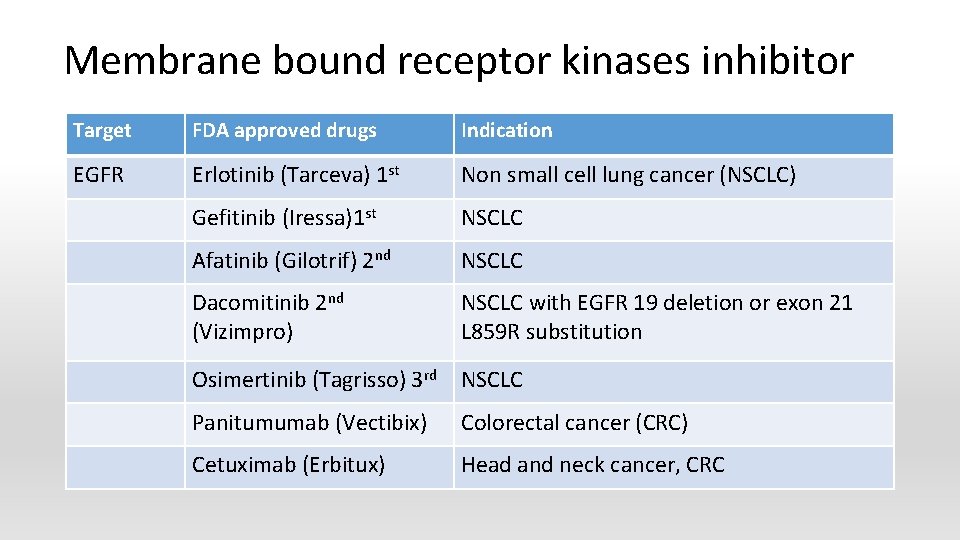
Membrane bound receptor kinases inhibitor Target FDA approved drugs Indication EGFR Erlotinib (Tarceva) 1 st Non small cell lung cancer (NSCLC) Gefitinib (Iressa)1 st NSCLC Afatinib (Gilotrif) 2 nd NSCLC Dacomitinib 2 nd (Vizimpro) NSCLC with EGFR 19 deletion or exon 21 L 859 R substitution Osimertinib (Tagrisso) 3 rd NSCLC Panitumumab (Vectibix) Colorectal cancer (CRC) Cetuximab (Erbitux) Head and neck cancer, CRC

Membrane bound receptor kinases inhibitor (cont. ) Target FDA approved drug Indication EGFR, VGFR, RET Vandetanib (Caprelsa) Thyroid cancer HER 2 Pertuzumab (Perjeta) Breast cancer Trastuzumab (Herceptin) Breast cancer Lapatinib (Tykerb) Breast cancer Neratinib (Nerlynx) Breast cancer HER 2, EGFR

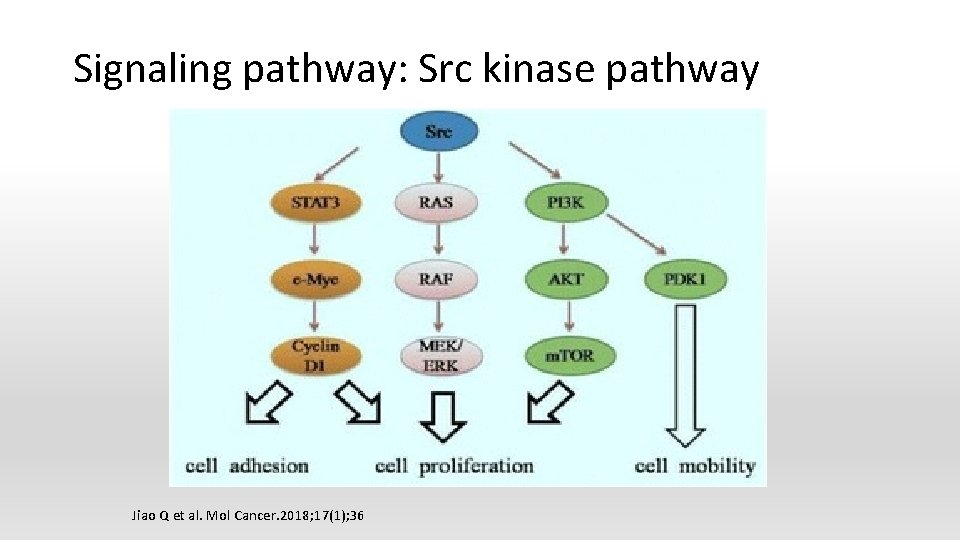
Intracellular signaling kinases inhibitor § Src /PI 3 k/AKT/m. TOR pathway inhibitors § Mitogen-activated protein kinase (MAPK) pathway inhibitors § Sonic hedgehog pathway inhibitors

Signaling pathway: Src kinase pathway Jiao Q et al. Mol Cancer. 2018; 17(1); 36

Epigenetic abnormalities § DNA methyltransferase § Histone deacetylase (HDAC) inhibitors § § § HDAC regulates the acetylation of target protein Decrease expression of oncogenes such as BCR-ABL Stop cell cycle Induce apoptosis Stop angiogenesis and cell motility

Histone deacetylase (HDAC) inhibitors • Vorinostat (Zolinza) for cutaneous T cell lymphoma (CTCL) • Romidepsin (Istodax) for CTCL • Panobinostat (Farydak) for multiple myeloma

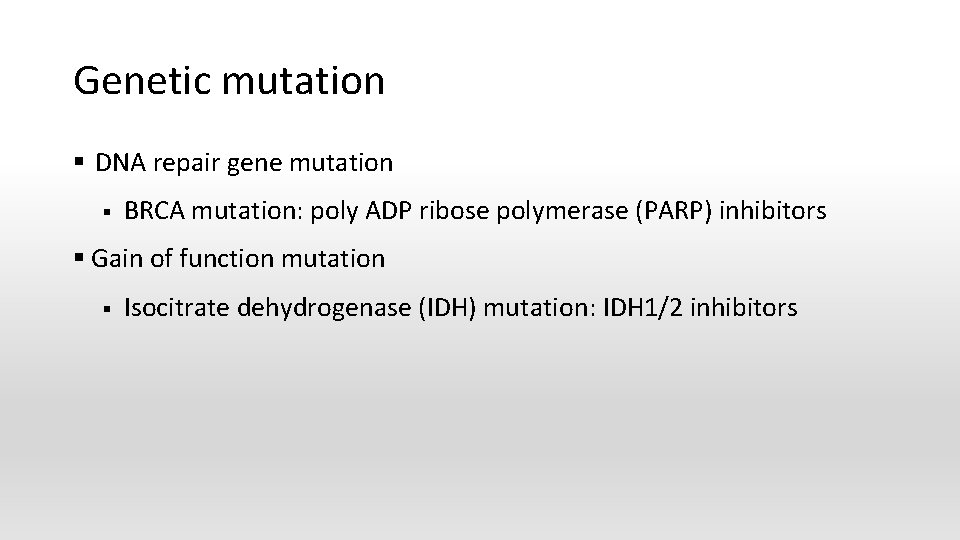
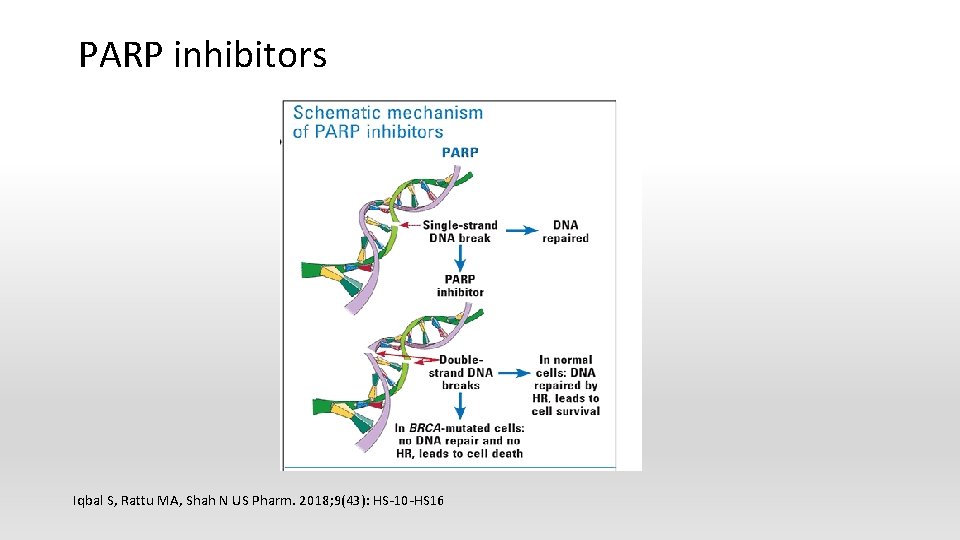
Genetic mutation § DNA repair gene mutation § BRCA mutation: poly ADP ribose polymerase (PARP) inhibitors § Gain of function mutation § Isocitrate dehydrogenase (IDH) mutation: IDH 1/2 inhibitors

PARP inhibitors Iqbal S, Rattu MA, Shah N US Pharm. 2018; 9(43): HS-10 -HS 16

Angiogenesis inhibitors § Block the growth of new blood vessels to tumors (a process called tumor angiogenesis) § Inhibit tumor growth and restrain metastasis § Vascular endothelial growth factor (VEGF) is commonly expressed in many solid tumors § VEGF inhibitors § VEGF receptor (VEGFR) inhibitors

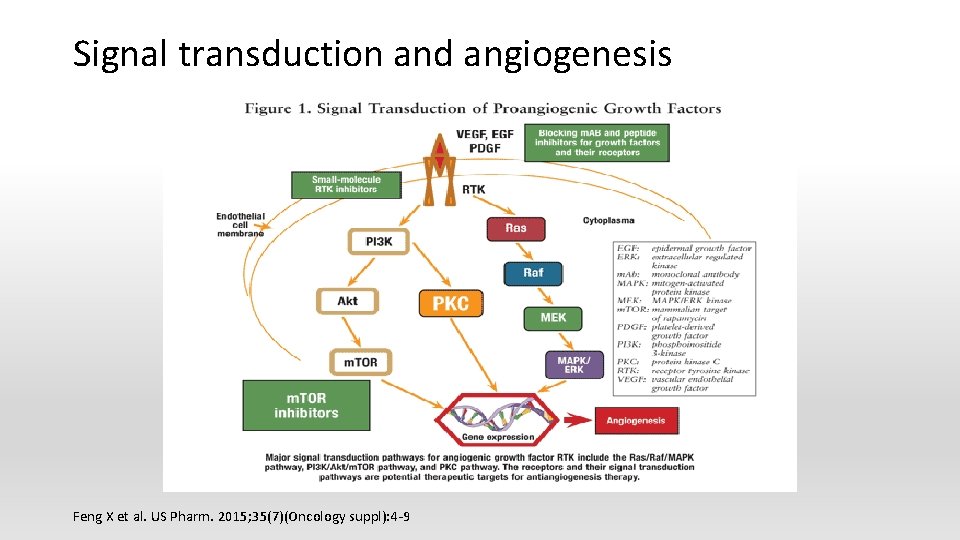
Signal transduction and angiogenesis Feng X et al. US Pharm. 2015; 35(7)(Oncology suppl): 4 -9

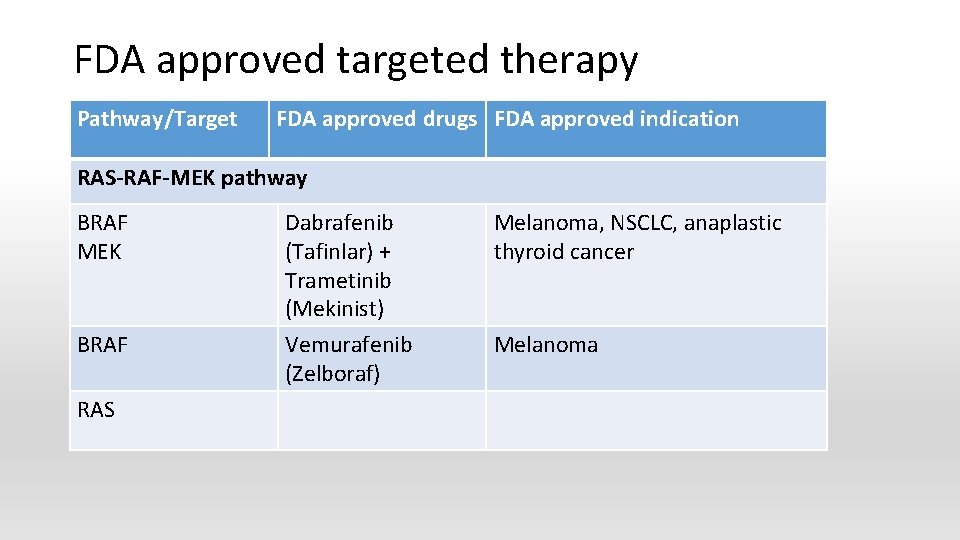
FDA approved targeted therapy Pathway/Target FDA approved drugs FDA approved indication RAS-RAF-MEK pathway BRAF MEK BRAF RAS Dabrafenib (Tafinlar) + Trametinib (Mekinist) Vemurafenib (Zelboraf) Melanoma, NSCLC, anaplastic thyroid cancer Melanoma

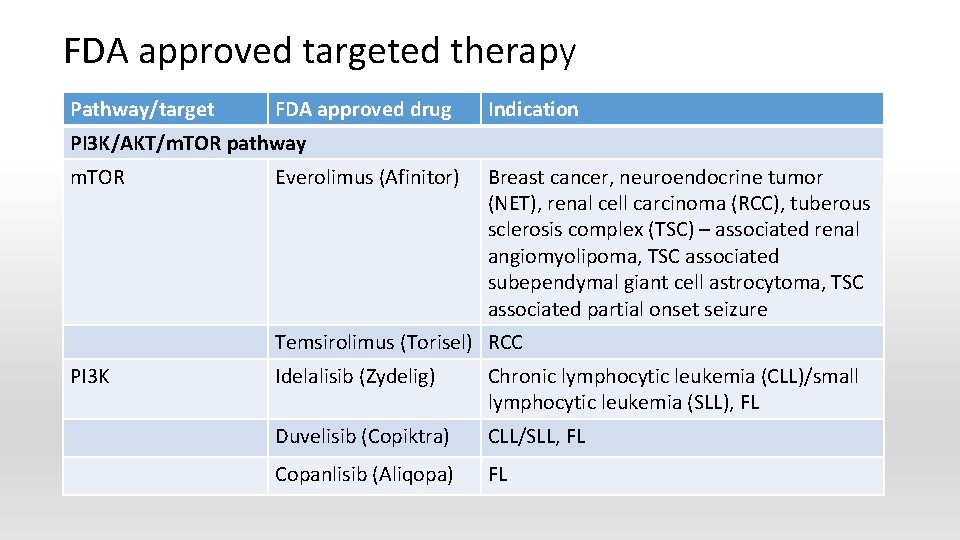
FDA approved targeted therapy Pathway/target FDA approved drug Indication PI 3 K/AKT/m. TOR pathway m. TOR Everolimus (Afinitor) Breast cancer, neuroendocrine tumor (NET), renal cell carcinoma (RCC), tuberous sclerosis complex (TSC) – associated renal angiomyolipoma, TSC associated subependymal giant cell astrocytoma, TSC associated partial onset seizure Temsirolimus (Torisel) RCC PI 3 K Idelalisib (Zydelig) Chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL), FL Duvelisib (Copiktra) CLL/SLL, FL Copanlisib (Aliqopa) FL

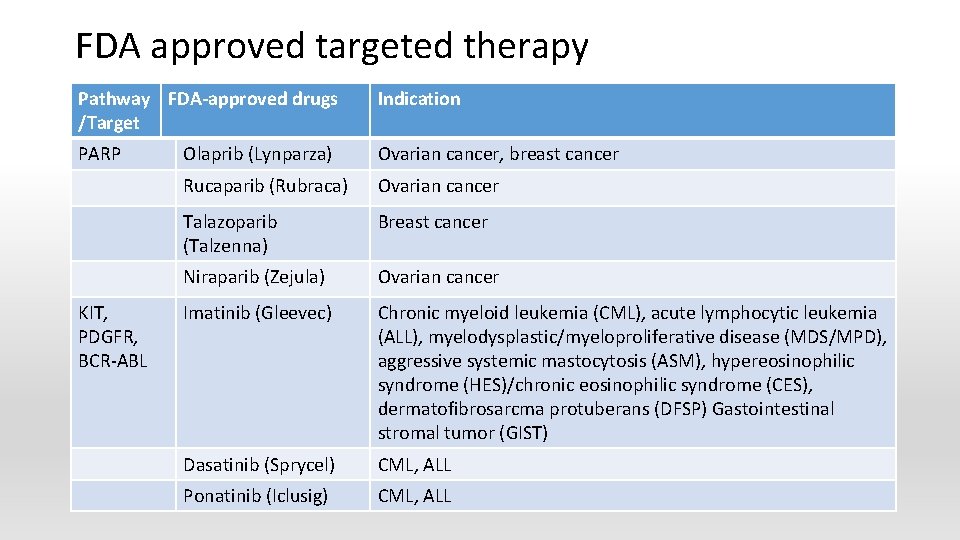
FDA approved targeted therapy Pathway FDA-approved drugs /Target Indication PARP Olaprib (Lynparza) Ovarian cancer, breast cancer Rucaparib (Rubraca) Ovarian cancer Talazoparib (Talzenna) Breast cancer Niraparib (Zejula) Ovarian cancer Imatinib (Gleevec) Chronic myeloid leukemia (CML), acute lymphocytic leukemia (ALL), myelodysplastic/myeloproliferative disease (MDS/MPD), aggressive systemic mastocytosis (ASM), hypereosinophilic syndrome (HES)/chronic eosinophilic syndrome (CES), dermatofibrosarcma protuberans (DFSP) Gastointestinal stromal tumor (GIST) Dasatinib (Sprycel) CML, ALL Ponatinib (Iclusig) CML, ALL KIT, PDGFR, BCR-ABL

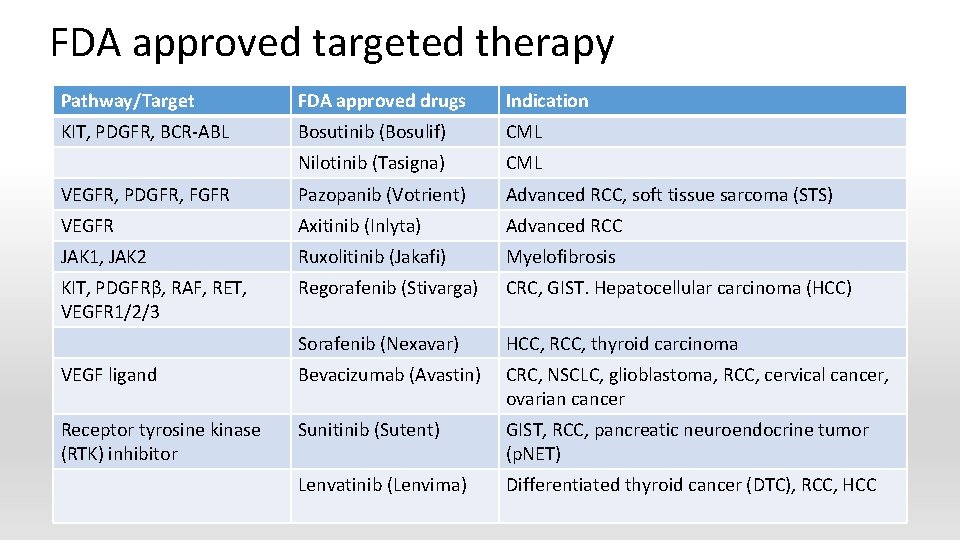
FDA approved targeted therapy Pathway/Target FDA approved drugs Indication KIT, PDGFR, BCR-ABL Bosutinib (Bosulif) CML Nilotinib (Tasigna) CML VEGFR, PDGFR, FGFR Pazopanib (Votrient) Advanced RCC, soft tissue sarcoma (STS) VEGFR Axitinib (Inlyta) Advanced RCC JAK 1, JAK 2 Ruxolitinib (Jakafi) Myelofibrosis KIT, PDGFRβ, RAF, RET, VEGFR 1/2/3 Regorafenib (Stivarga) CRC, GIST. Hepatocellular carcinoma (HCC) Sorafenib (Nexavar) HCC, RCC, thyroid carcinoma VEGF ligand Bevacizumab (Avastin) CRC, NSCLC, glioblastoma, RCC, cervical cancer, ovarian cancer Receptor tyrosine kinase (RTK) inhibitor Sunitinib (Sutent) GIST, RCC, pancreatic neuroendocrine tumor (p. NET) Lenvatinib (Lenvima) Differentiated thyroid cancer (DTC), RCC, HCC

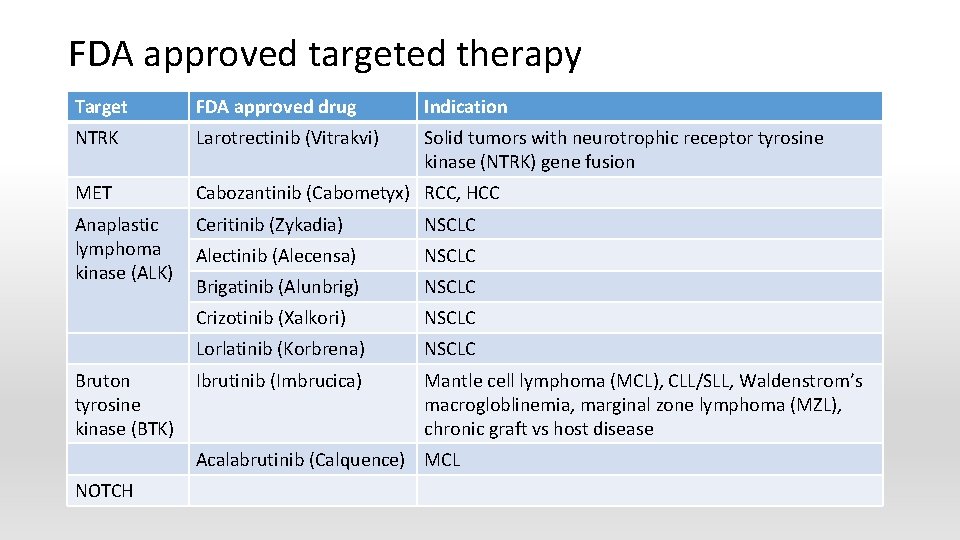
FDA approved targeted therapy Target FDA approved drug Indication NTRK Larotrectinib (Vitrakvi) Solid tumors with neurotrophic receptor tyrosine kinase (NTRK) gene fusion MET Cabozantinib (Cabometyx) RCC, HCC Anaplastic lymphoma kinase (ALK) Ceritinib (Zykadia) NSCLC Alectinib (Alecensa) NSCLC Brigatinib (Alunbrig) NSCLC Crizotinib (Xalkori) NSCLC Lorlatinib (Korbrena) NSCLC Ibrutinib (Imbrucica) Mantle cell lymphoma (MCL), CLL/SLL, Waldenstrom’s macrogloblinemia, marginal zone lymphoma (MZL), chronic graft vs host disease Bruton tyrosine kinase (BTK) Acalabrutinib (Calquence) MCL NOTCH

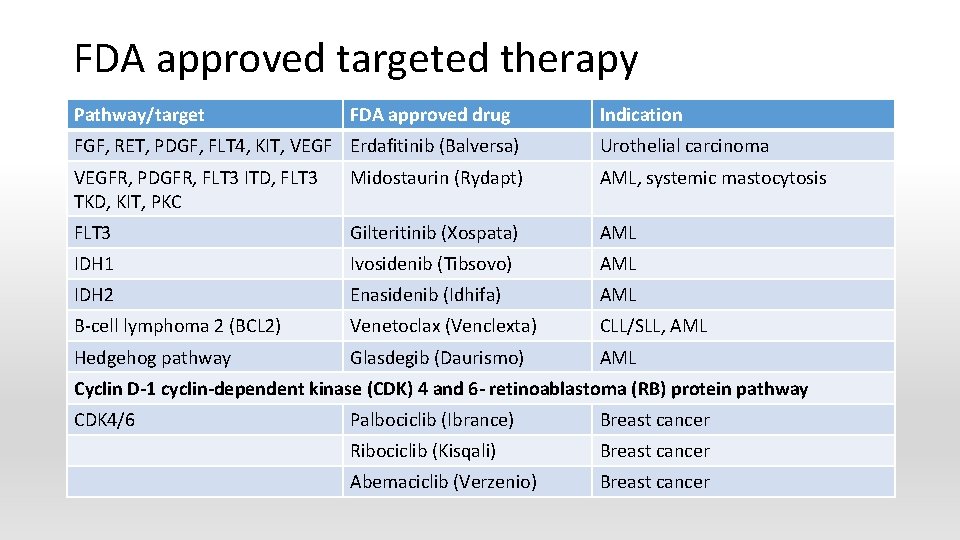
FDA approved targeted therapy Pathway/target FDA approved drug Indication FGF, RET, PDGF, FLT 4, KIT, VEGF Erdafitinib (Balversa) Urothelial carcinoma VEGFR, PDGFR, FLT 3 ITD, FLT 3 TKD, KIT, PKC Midostaurin (Rydapt) AML, systemic mastocytosis FLT 3 Gilteritinib (Xospata) AML IDH 1 Ivosidenib (Tibsovo) AML IDH 2 Enasidenib (Idhifa) AML B-cell lymphoma 2 (BCL 2) Venetoclax (Venclexta) CLL/SLL, AML Hedgehog pathway Glasdegib (Daurismo) AML Cyclin D-1 cyclin-dependent kinase (CDK) 4 and 6 - retinoablastoma (RB) protein pathway CDK 4/6 Palbociclib (Ibrance) Breast cancer Ribociclib (Kisqali) Breast cancer Abemaciclib (Verzenio) Breast cancer

Apoptosis inducers § Apoptosis is one method the body uses to get rid of unneeded or abnormal cells, but cancer cells have strategies to avoid apoptosis. § Apoptosis inducers can get around these strategies to cause the death of cancer cells. § Target: Bcl 2, PI 3 k, NFk. B, proteasome § § Bcl 2 inhibitor: venetoclax Proteasome inhibitors

Venetoclax (Venclexta) § Selective and orally bioavailable small-molecule inhibitor of BCL-2, an antiapoptotic protein. § BCL-2 proto-oncogene encodes a mitochondrial protein that blocks programmed cell death (PD) § Known to be present in lymphoid malignancies § High expression of BCL-2 associated with lower complete response (CR) rate after intensive chemotherapy § Indication: CLL/SLL, AML

Proteasome inhibitors § Proteasome pathway § § § Degradation of intracellular proteins Maintenance of protein homeostasis Clearance of misfolded/unfolded and cytotoxic proteins § Proteins degraded by the proteasome include mediators of cell-cycle progression and apoptosis § Proteasome inhibitors: inhibit proliferation and induce apoptosis in multiple myeloma cell lines § Bortezomib, carfilzomib, ixazomib

Monoclonal antibody Target Drug Indication CD 20 Rituximab (Rituxan) Non Hodgkin’s lymphoma (NHL), CLL, rheumatoid arthritis (RA), graulomatosis with polyangitis (GPA) and microscopic polyangitis (MPA), pemphigus vulgaris (PV) Obinutuzumab (Gazyva) CLL, FL Ofatumumab (Arzerra) CLL CD 33 Gemtuzumab ozogamicin (Mylotarg) AML CD 38 Daratumumab (Darzalex) Multiple myeloma CD 22 Moxetumomab paudotox-tdfk (Lumoxiti) Hairy cell leukemia (HCL) CD 30 Inotuzumab ozogamicin (Besponsa) Acute lymphocytic leukemia (ALL) Brentuximab vedotin (Adcetris) Anaplastic large cell lymphoma or other CD 30 expressing peripheral T cell lymphoma

Monoclonal antibody Target Drug Indication CD 19 and CD 3 Blinatumomab (Blincyto) B cell precursor ALL CD 52 Alemtuzumab (Campath) B cell chronic lymphocytic leukemia SLAMF 7 Elotuzumab (Empliciti) Multiple myeloma VEGF Ramicirumab (Cyramza) Gastric cancer, NSCLC, CRC PDGFRα Olaratumab (Lartruvo) Soft tissue sarcoma (STS) EGFR Panitumumab (Vectibix) CRC Cetuximab (Erbitux) Head and neck cancer, CRC Trastuzumab (Herceptin) Breast cancer, gastric cancer Pertuzumab (Perjeta) Breast cancer HER 2 SLAM 7: signaling lymphocytic activation molecule family member 7

Antibody drug conjugates (ADC) § Antibody + toxic substance that kill cancer cells § Gemtuzumab ozogamicin (Mylotarg) § § Anti- CD 33 antibody fragment linked to the antitumor agent calicheamicin Inidcation: CD-33 positive AML § Inotuzumab ozogamicin (Besponsa) § § Anti- CD 22 antibody fragment linked to the antitumor agent calicheamicin Indication: adults with relapsed or refractory B-cell precursor ALL

Antibody drug conjugates (ADC) § Moxetumomab paudotox-tdfk (Lumoxiti) § § Anti-CD 22 antibody + protein toxin PE 38 Indication: HCL § Ado-trastuzumab emtansine (T-DM 1) § § Monoclonal antibody trastuzumab + the cytotoxic agent emtansine, which inhibits cell proliferation by blocking the formation of microtubules. Indication: HER 2 -positive breast cancer § Brentuximab vedotin (Adcetris) § § Anti-CD 30 antibody + microtubule disrupting agent, MMAE Indication: HL, anaplastic large cell lymphoma

Application

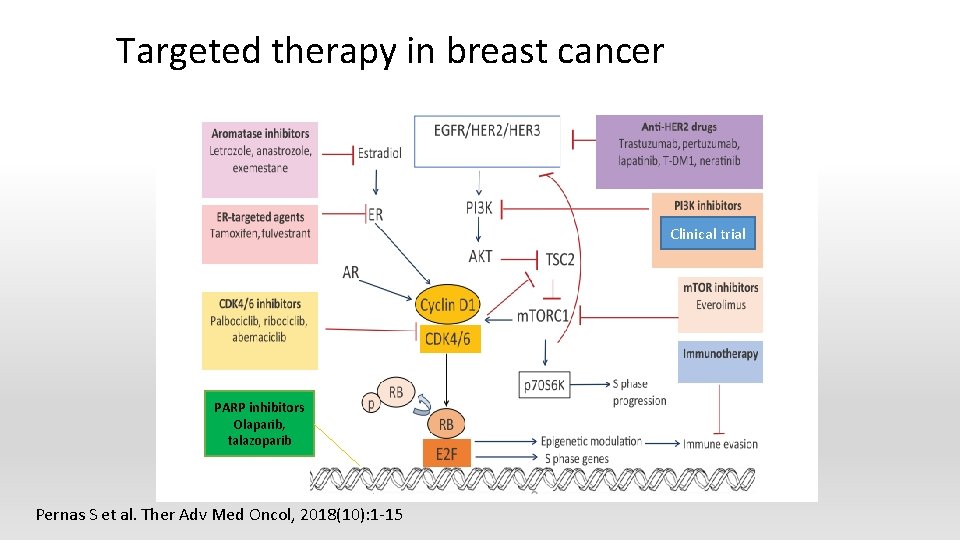
Targeted therapy in breast cancer § § § Hormonal therapy HER 2/neu inhibitor CDK 4/6 inhibitor Poly ADP-ribose polymerase (PARP) inhibitor Immunotherapy

Targeted therapy in breast cancer Clinical trial PARP inhibitors Olaparib, talazoparib Pernas S et al. Ther Adv Med Oncol, 2018(10): 1 -15

Targeted therapy in AML § Targets § Cell surface epitopes: CD 33, CD 123, NGK 2 D § Activated kinases: FLT 3, KIT § Other gain-of-function mutations: mutant RAS, IDH 1/2 § Spliceosome inhibition: U 2 AF 1, SF 3 B 1 § CD 33 targeting monoclonal antibody: gemtuzumab ozogamicin § FLT 3 inhibitor: midostaurin, gilteritinib § IDH 1 IDH 2 inhibitors: enasidenib, ivosidenib

Side effects of targeted therapies § Skin problems: acneiform rash, dry skin, nail changes, hair depigmentation § GI perforation § Diarrhea § Hepatitis, elevated liver enzymes § Interstitial lung disease § Problems with blood clotting or wound healing § High blood pressure

Limitation of targeted therapies § Resistance § § Mutation in target Develop a new pathway to achieve tumor growth that dose not depend on the target § Overcome resistance § § Two different targeted therapies combination Combination with chemotherapy: i. e. trastuzumab + docetaxel

Immunotherapy

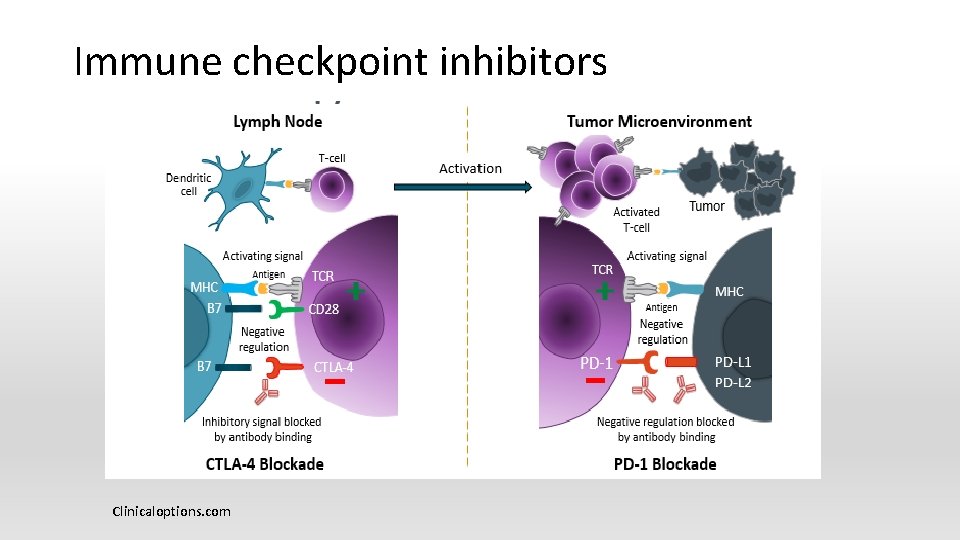
Immunotherapies § The 5 th modalities of cancer treatment after surgery, radiation, chemotherapy and targeted therapy § Stimulate one’s own immune system to fight cancer, activating immune cells or getting them to recognize cancer cells as different from normal cells

Cancer Immunotherapy approaches http: //www. media. jkstudios. tv/3 d-animation-cgi/2 d-3 d-medical-animation-cancerimmunotherapy_009. jpg 44

Immune checkpoints § Immune checkpoints reduce inappropriate responses to self-antigens § Protect self from inflammation and autoimmunity § Prevents allergy and/or hypersensitivity § Pregnancy § Gastrointestinal microbiome (commensal organisms)

Immune checkpoint inhibitors Clinicaloptions. com

CTLA-4 Blockade § CTLA-4 ligation on activated T-cells down regulates T-cell response § Blocking CTLA -4 ligation § Enhancement of T- cell activity § Inhibition or elimination of Treg activity § Iplimumab (Yervoy) 47

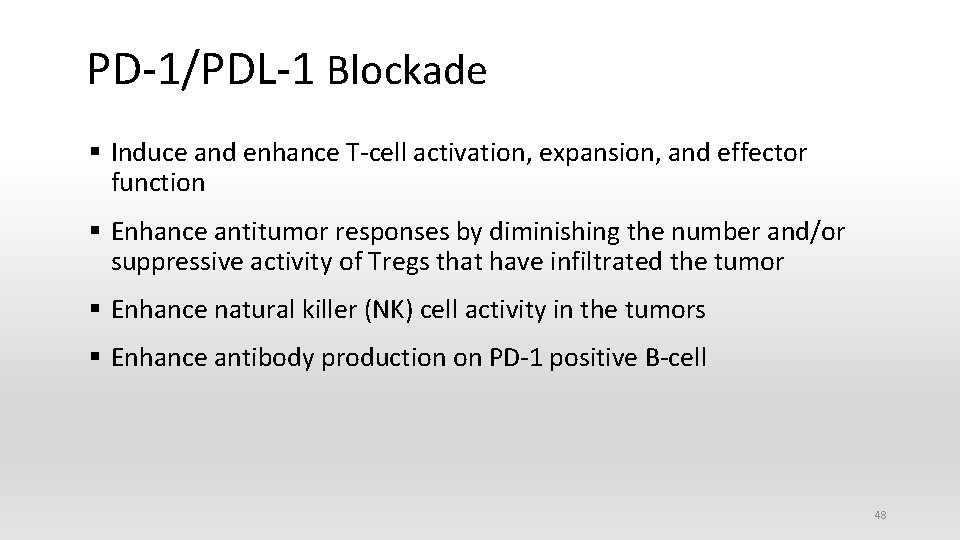
PD-1/PDL-1 Blockade § Induce and enhance T-cell activation, expansion, and effector function § Enhance antitumor responses by diminishing the number and/or suppressive activity of Tregs that have infiltrated the tumor § Enhance natural killer (NK) cell activity in the tumors § Enhance antibody production on PD-1 positive B-cell 48

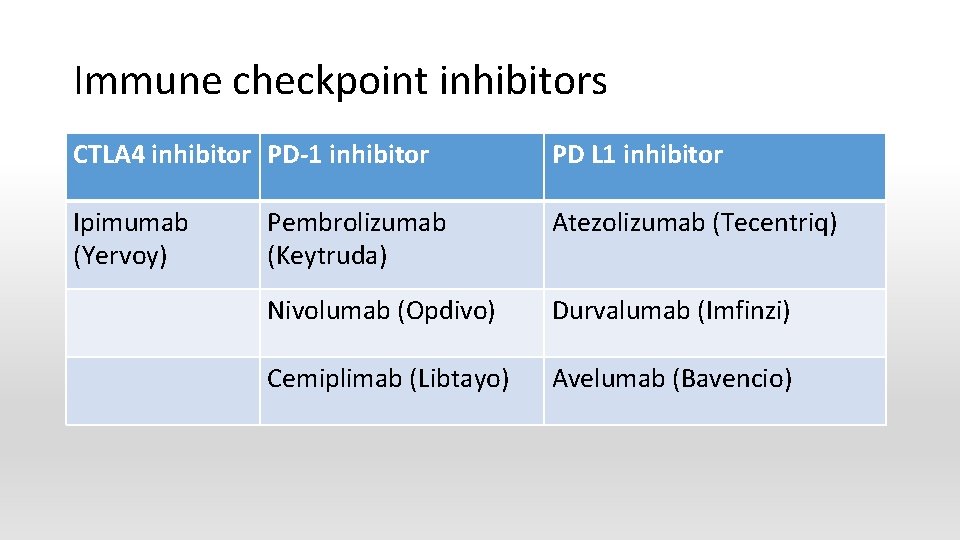
Immune checkpoint inhibitors CTLA 4 inhibitor PD-1 inhibitor PD L 1 inhibitor Ipimumab (Yervoy) Pembrolizumab (Keytruda) Atezolizumab (Tecentriq) Nivolumab (Opdivo) Durvalumab (Imfinzi) Cemiplimab (Libtayo) Avelumab (Bavencio)

Immune check point inhibitors FDA approval in multiple cancer treatment (March 2019) § Head and neck squamous cell carcinoma § Hodgkin lymphoma § Hepatocellular carcinoma § Gastric/gastroesophageal junctional cancer § Melanoma § Merkel cell carcinoma § Cutaneous squamous cell carcinoma clinicaloptions. com § MMR-deficient solid tumors § Primary mediastinal B cell lymphoma § Non small cell lung cancer § Small cell lung cancer § Renal cell carcinoma § Urothelial carcinoma § MSI-high colorectal cancer § Cervical cancer

Immune checkpoint inhibitor related side effects § Skin: vitiligo, psoriasis, Steven Johnson syndrome, drug rash with eosinophilia and systemic symptoms (DRESS) § GI: colitis, autoimmune hepatitis § Lung: pneumonitis § Heart: myocarditis § Endocrine: type I diabetes, hyperthyroidism, hypothyroidism, adrenal insufficiency § Kidney: nephritis, renal dysfunction § Nervous system: Guillain- Barre syndrome, myasthenia gravis, encephalitis

Gene therapy § Gene therapy is a technique that modifies a person’s genes to treat or cure disease § Gene therapies can work by several mechanisms § Replacing a disease-causing gene with a healthy copy of the gene § Inactivating a disease-causing gene that is not functioning properly § Introducing a new or modified gene into the body to help treat a disease

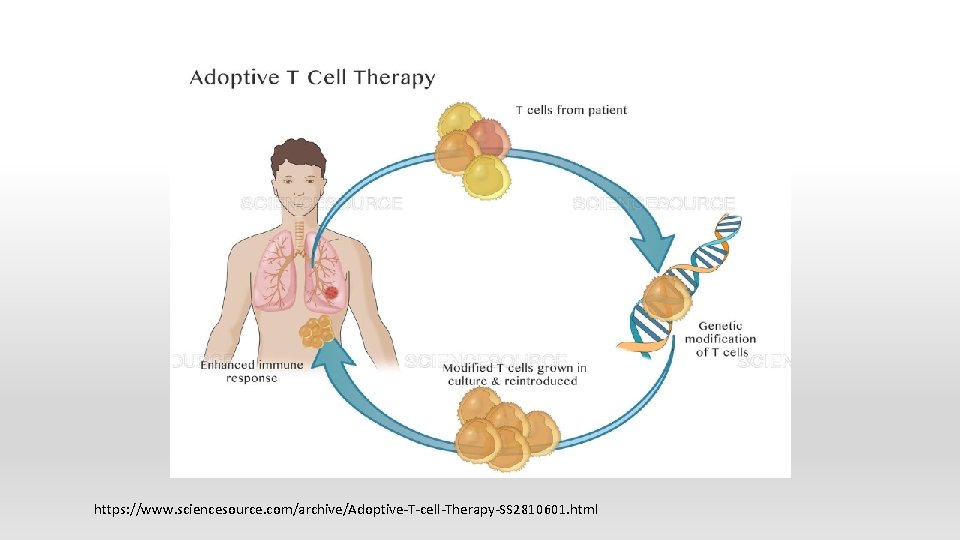
Chimeric antigen receptor T (CAR-T) cell therapy § Target CD 19 § Tisagenlecleucel (Kymriah) for ALL, B-cell non Hodgkin lymphoma § Axicabtagene ciloleucel (Yescarta) for B-cell lymphoma § Side effects: cytokine release syndrome, neurotoxicity

https: //www. sciencesource. com/archive/Adoptive-T-cell-Therapy-SS 2810601. html

Cancer treatment vaccines § Introducing cancer antigens into the body will cause an immune response that ultimately kill the cancer cells § Goal § § Delay or stop cancer cell growth Prevent cancer recurrence ▪ Tumor shrinkage ▪ Eliminate cancer cell § Sipuleucel-T (Provenge) for prostate cancer § Talimogene laherparepvec (T-VEC or Imlygic), oncolytic virus therapy, for metastatic melanoma § Side effects § Flu-like symptoms, severe allergic reaction, stroke (Sipuleucel-T), herpes virus infection (T-VEC)

Emerging therapy § Combination therapy § Targeted therapy + chemotherapy § Targeted therapy + immunotherapy § Chemotherapy + immunotherapy § Biomarker driven cancer therapy § New biomarker development in immunotherapy § “Basket” design: combining tumor tissue genomics with potential offthe-shelf therapies in drug development

Summary § Cancer treatment evolved to target specific cancer cells § Targeted therapy binds to specific receptors or ligands involved in cell proliferation, differentiation, motility and apoptosis § Targeted therapy and immunotherapy treat cancer with less toxicities compared to chemotherapy § Combining different modalities of cancer therapies may optimize treatment outcome § Gene therapy is one of new treatment options

Reference 1. Baskar R, Lee KA, Yeoh KW. Cancer and radiation therapy: Current Advances and Future Directions Int J Med Sci 2012; 9(3): 193 -9 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011 Mar 4; 144(5): 646 -74 3. Halaven [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2016 4. Lonsurf [package insert]. Princeton, NJ: Taiho Oncology, Inc; 2019 5. Vyxeos [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2017 6. Asparlas [package insert]. Boston, MA: Servier Pharmaceuticals LLC; 2018 7. Lancet JE, et al. Phase II trial of CPSX-351, a fixed 5: 1 molar ratio of cytarabine/daunorubicin vs cytarabine/daunorubcin in older adults with untereated AML. Blood. 2014; 123: 3239 -46. 8. https: //www. cancer. gov/about-cancer/treatment/types/targeted-therapies-fact-sheet Accessed on 4/10/19 9. Xtandi [package insert]. Northbrook, IL: Astellas Pharma US Inc; 201 10. Erleada [package insert]. Gurabo, PR: Janssen; 2018 11. Erbitux [package insert]. Indianapolis, IN: Eli Lilly; 2018 12. Vizimpro [package insert]. New York, NY: Pfizer Inc; 2018 13. Tagrisso [package insert]. Wilmington, DE: Astra. Zeneca; 2018 14. Vectibix [package insert]. Thousand Oaks, CA: Amgen Inc; 2017 15. Caprelsa [package insert]. Cambridge, MA: Sanofi Genzyme; 2018 16. Tykerb [package insert]. East Hanover, NJ: Novartis; 2018
![Reference 17 Nerlynx package insert Los Angeles CA Puma Biotechnology Inc 2018 18 Choudhry Reference 17. Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology Inc; 2018 18. Choudhry](https://slidetodoc.com/presentation_image_h2/4fd2df055913d48e8d33d55006b88363/image-59.jpg)
Reference 17. Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology Inc; 2018 18. Choudhry Z, Rikani AA, Choudhry AM, et al. Sonic hedgehog signalling pathway: a complex network. Ann Neurosci. 2014; 21(1): 28– 31. 19. Tafinlar [package insert]. East Hanover, NJ: Novartis; 2018 20. Meknist [package insert]. East Hanover, NJ: Novartis; 2018 21. Zelboraf [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017 22. Afinitor [package insert]. East Hanover, NJ: Novartis; 2018 23. Torisel [package insert]. New York, NY: Pfizer Inc; 2018 24. Aliqopa [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; 2017 25. Copiktra [package insert]. Needham, MA: Verastem, Inc; 2018 26 Lynparza [package insert]. Wilmington, DE: Astra. Zeneca; 2018 27. Ru braca [package insert]. Boulder, CO: Clovis Oncology, Inc; 2018 28. Talzenna [package insert]. New York, NY: Pfizer Inc; 2018 29. Zejula [package insert]. Waltham, MA: Tesaro, Inc; 2019 30. Gleevec [package insert]. East Hanover, NJ: Novartis; 2018 31. Sprycel [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2018 32. Zydelig [package insert]. Foster City, CA: Gilead Sciences, Inc; 2018
![Reference 33 Iclusig package insert Cambridge MA Takeda Pharmaceuticals 2018 34 Bosulif package insert Reference 33. Iclusig [package insert]. Cambridge, MA: Takeda Pharmaceuticals; 2018 34. Bosulif [package insert].](https://slidetodoc.com/presentation_image_h2/4fd2df055913d48e8d33d55006b88363/image-60.jpg)
Reference 33. Iclusig [package insert]. Cambridge, MA: Takeda Pharmaceuticals; 2018 34. Bosulif [package insert]. New York, NY: Pfizer Inc; 2018 35. Tasigna [package insert]. East Hanover, NJ: Novartis; 2018 36. Votrient [package insert]. East Hanover, NJ: Novartis; 2017 37. Inlyta [package insert]. New York, NY: Pfizer Inc; 2018 38. Jakafi [package insert]. Willington, DE: Incyte Corp; 2018 39. Styvarga [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; 2018 40. Nexavar [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; 2018 41. Avastin [package insert]. South San Francisco, CA: Genentech USA, Inc; 2019 42. Sutent [package insert]. New York, NY: Pfizer Inc; 2018 43. Lenvima [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2018 44. Balversa [package insert]. Horsham, PA: Janssen; 2019 45. Vitrakvi [package insert]. Stamford, CT: Loxo Oncology; 2018 46. Cabometyx [package insert]. Alameda, CA: Exelixis, inc; 2019 47. Zykadia [package insert]. East Hanover, NJ: Novartis; 2019
![Reference 48 Alecensa package insert South San Francisco CA Genentech USA Inc 2018 49 Reference 48. Alecensa [package insert]. South San Francisco, CA: Genentech USA, Inc; 2018 49.](https://slidetodoc.com/presentation_image_h2/4fd2df055913d48e8d33d55006b88363/image-61.jpg)
Reference 48. Alecensa [package insert]. South San Francisco, CA: Genentech USA, Inc; 2018 49. Alunbrig [package insert]. Cambridge, MA: Takeda Pharmaceuticals; 2018 50. Xalkori [package insert]. New York, NY: Pfizer Inc; 2019 51. Lorbrena [package insert]. New York, NY: Pfizer Inc; 2018 52. Imbruvica [package insert]. Horsham, PA: Janssen; 2019 53. Calquence [package insert]. Willmington, DE: Astra. Zeneca; 2017 54. Rydapt [package insert]. East Hanover, NJ: Novartis; 2018 55. Xospata [package insert]. Northbrook, IL: Astellas Pharma; 2018 56. Tipsovo [package insert]. Cambridge, MA: Agios Phamraceuticals, Inc; 2018 57. Idhifa [package insert]. Cambridge, MA: Agios Phamraceuticals, Inc 2017 58. Venclexta [package insert]. South San Francisco, CA: Genentech USA, Inc; 2018 59. Daurismo [package insert]. New York, NY: Pfizer Inc; 2018 60. Ibrance [package insert]. New York, NY: Pfizer Inc; 2019 61. Kisqali [package insert]. East Hanover, NJ: Novartis; 2018 62. Verzenio [package insert]. Indianapolis, IN: Eli Lilly; 2018 63. Yescarta [package insert]. Santa Monica, CA: Kite Pharma, Inc; 2017 64. Kymriah [package insert]. East Hanover, NJ: Novartis; 2018

Reference 65. https: //www. fda. gov/biologicsbloodvaccines/cellulargenetherapyproducts/ucm 573960. htm accessed on 4/16/19 66. Weiner G J. . Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007; 39: 271 -278 67. Ma W W and Adjei A A. : Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009; 59(2): 111 -36. 68. Tsimberidou A Tsimberidou, Apostolia-Maria. Targeted Therapy in Cancer. In: Kantarjian HM, Wolff RA. Kantarjian H. M. , Wolff R. A. Eds. Hagop M. Kantarjian, and Robert A. Wolff. eds. The MD Anderson Manual of Medical Oncology, 3 e New York, NY: Mc. Graw-Hill; . http: //accessmedicine. mhmedical. com/content. aspx? bookid=1772§ionid=121902560. Accessed April 21, 2019 69. Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 2018; 17(1): 36. Published 2018 Feb 19. doi: 10. 1186/s 12943 -018 -0801 -5 70. Gross S, Rahal R, Stransky N et al. Targeting cancer with kinase inhibitors. J Clin Invest 2015; 125(5): 1780 -89 71. Mondesir J et al. IDH 1 and IDH 2 mutations as novel therapeutic targets: current perspectives. J Blood Med 2016; 7: 171 -80 72. Medeiros BC et al. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017; 31: 272 -81 73. Yang H, Ye D, Guan KL, Xiong Y. IDH 1 and IDH 2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012; 18(20): 5562– 5571 74. Stein EM et al. Enasidenib in mutant IDH 2 relapsed or refractory acute myeloid leukemia. Blood. 2017; 130” 722 -31 75. https: //www. accessdata. fda. gov/drugsatfda_docs/label/2012/203415 lbl. pdf. Accessed on April 26, 2019 76. Pernas S, Tolaney SM, Winer EP et al. CDK 4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 2018 (10): 1 -15

Reference 77. Luoh SW, Flaherty KT. When tissue is no longer the issue: Tissue-agnostic cancer therapy comes of age, Ann Inter Med. 2018, 169(4): 233 -39 78. Wilkes, G. (2011). Targeted Cancer Therapy: A Handbook For Nurses. Retrieved April 25, 2019 from https: //www. r 2 library. com/Resource/Title/0763772119 79. Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006; 4(3): 218– 227 80. Rituxan [package insert], South San Francisco, CA: Genentech USA, Inc; 2019 81. Gazyva [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017 82. Darzalex [package insert]. Horsham, PA: Janssen; 2019 83. Lumoxiti [package insert]. Willington, DE: Astra. Zeneca; 2019 84. Adcetris [package insert], Bothell, WA: Seattle Genetics, Inc; 2018 85. Blincyto [package insert]. Thousand Oaks, CA: Amgen Inc; 2019 86. Empliciti [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2018 87. Weber JS. Immuno-oncology Comes of Age-Introduction. Semin Oncol 2014 Oct; 41(5); supple 5: S 1 -S-2 88, Goldberg SB, Narayan A, Kole AJ, et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin Cancer Res. 2018; 24(8): 1872– 1880. 89. June CH et al. Is autimmunity the Achilles’hel of cancer immunotherapy? Nat Med 2017; 23: 540 -47
 Systematic desensitization therapy
Systematic desensitization therapy Chapter 32 complementary and alternative therapies
Chapter 32 complementary and alternative therapies Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Pvu
Pvu Bodywork and movement therapies
Bodywork and movement therapies Pharmacological and parenteral therapies
Pharmacological and parenteral therapies Complementary and alternative medicine ppt
Complementary and alternative medicine ppt Laprp
Laprp Module 73 the biomedical therapies
Module 73 the biomedical therapies Biomedical therapy:
Biomedical therapy: Stiriti ayur therapies private limited
Stiriti ayur therapies private limited Westminster talking therapies
Westminster talking therapies Insight therapy definition
Insight therapy definition Advanced therapies apprenticeship community
Advanced therapies apprenticeship community Trafford psychological therapies
Trafford psychological therapies Flooding therapy example
Flooding therapy example Trafford psychological services
Trafford psychological services Humanistic therapies aim to boost
Humanistic therapies aim to boost What are biomedical therapies
What are biomedical therapies Astellas gene therapies
Astellas gene therapies Insight therapies involve verbal interactions
Insight therapies involve verbal interactions Trafford psychological therapies
Trafford psychological therapies Advantages of group therapy
Advantages of group therapy Trafford psychological therapies
Trafford psychological therapies Deca prepares emerging leaders and entrepreneurs
Deca prepares emerging leaders and entrepreneurs It infrastructure and emerging technologies
It infrastructure and emerging technologies Leo iii outlawed crucifixes as idolatry.
Leo iii outlawed crucifixes as idolatry. It infrastructure and emerging technologies
It infrastructure and emerging technologies Emerging bridging expanding
Emerging bridging expanding Byzantine empire 1300
Byzantine empire 1300 Chapter 5 it infrastructure and emerging technologies
Chapter 5 it infrastructure and emerging technologies It infrastructure and emerging technologies
It infrastructure and emerging technologies Computer hardware platforms in it infrastructure
Computer hardware platforms in it infrastructure Adolescence and emerging adulthood a cultural approach
Adolescence and emerging adulthood a cultural approach Emerging media definition
Emerging media definition Emerging database technologies and applications
Emerging database technologies and applications Emerging database technologies
Emerging database technologies Byzantine definition
Byzantine definition Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer The lymphatic capillaries are
The lymphatic capillaries are New york pennsylvania new jersey delaware
New york pennsylvania new jersey delaware Fresh oil new wine
Fresh oil new wine Articles of confederation strengths
Articles of confederation strengths What forces are defining the new marketing realities
What forces are defining the new marketing realities New classical macroeconomics
New classical macroeconomics Chapter 16 toward a new heaven and a new earth
Chapter 16 toward a new heaven and a new earth Leanne keene french ambassador arrives from paris
Leanne keene french ambassador arrives from paris New classical and new keynesian macroeconomics
New classical and new keynesian macroeconomics Esci web of science
Esci web of science Emerging technology synthesis
Emerging technology synthesis Emerging issues in community development
Emerging issues in community development Explain emerging patterns of state politics in india
Explain emerging patterns of state politics in india Emerging adulthood psychosocial development
Emerging adulthood psychosocial development Emerging proficient extending
Emerging proficient extending Is nigeria a newly emerging economy
Is nigeria a newly emerging economy Levinson's stages
Levinson's stages Emerging role of finance manager in india
Emerging role of finance manager in india Business intelligence market trends
Business intelligence market trends Introduction to industrial relations
Introduction to industrial relations Postformal thought
Postformal thought Mainframe and minicomputer era
Mainframe and minicomputer era Art of emerging europe summary
Art of emerging europe summary Jeffrey arnett emerging adulthood theory
Jeffrey arnett emerging adulthood theory Emerging technologies introduction
Emerging technologies introduction