What is a MDDT Clinical Outcome Assessments MDDT








- Slides: 24

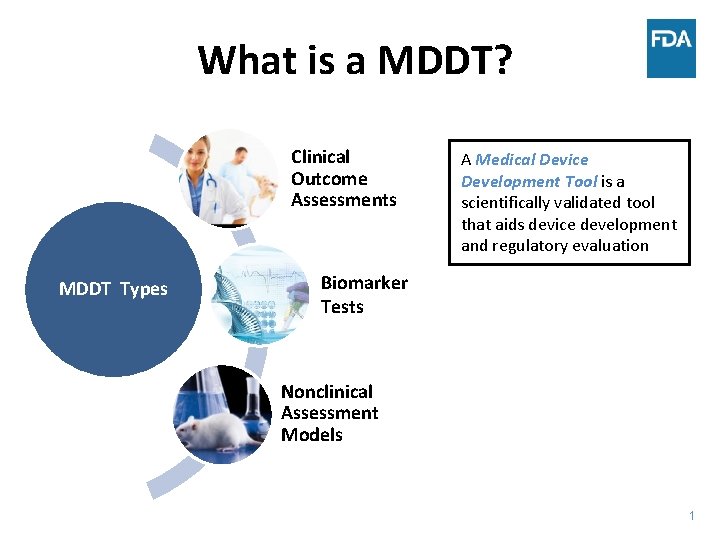
What is a MDDT? Clinical Outcome Assessments MDDT Types A Medical Device Development Tool is a scientifically validated tool that aids device development and regulatory evaluation Biomarker Tests Nonclinical Assessment Models 1

What is a MDDT? A Medical Device Development Tool is a scientifically validated tool that aids device development and regulatory evaluation Clinical Outcome Assessments MDDT Types Biomarker Tests Nonclinical Assessment Models 2

MDDT Types Clinical Outcome Assessments MDDT Types Biomarker Tests Nonclinical Assessment Models • Patient selection • Clinical study outcomes • Objective measure of biologic process or response to an intervention • Patient selection • Predict or identify outcomes • New method to measure or predict a parameter of interest • Replace/ reduce animal testing 3

What does FDA Qualification of a MDDT mean? The results of an assessment from a qualified MDDT, within a specified context of use, can be relied upon by the medical device industry in support of their device submissions • Device industry need not reconfirm the suitability of a qualified MDDT • Device industry users may need to demonstrate the tool is used according to the specified context of use 4

Why are we qualifying tools? TODAY: Tools considered and evaluated on a case-by-case basis TOMORROW: Tools qualified for regulatory purposes, within defined context of use BENEFITS: § Allow for new methods to be assessed outside of the medical device application § Minimize uncertainty in the review process § Promote innovation in medical device development and regulatory science to help bridge the gap between research and the delivery of devices to patients 5

Benefits of MDDT Qualification For device industry: More predictable and efficient product evaluation / reduced regulatory risk For FDA product evaluators: More efficient process / reduced review time spent arguing about endpoints and other methods of measuring. FDA’s efforts to qualify one MDDT could by surpassed by the time and resources saved when the MDDT is applied to several device submissions or device development programs. For FDA regulatory scientists: Standing program to bridge advances in regulatory science into regulatory practice For tool developers: Foster adoption of tools, facilitate collaboration in a pre-competitive setting to amplify evidence collection and reduce individual resource expenditure For patients: Increased adoption of outcome assessments with patient-centered treatment benefit, effective and safe medical devices coming to market more quickly 6

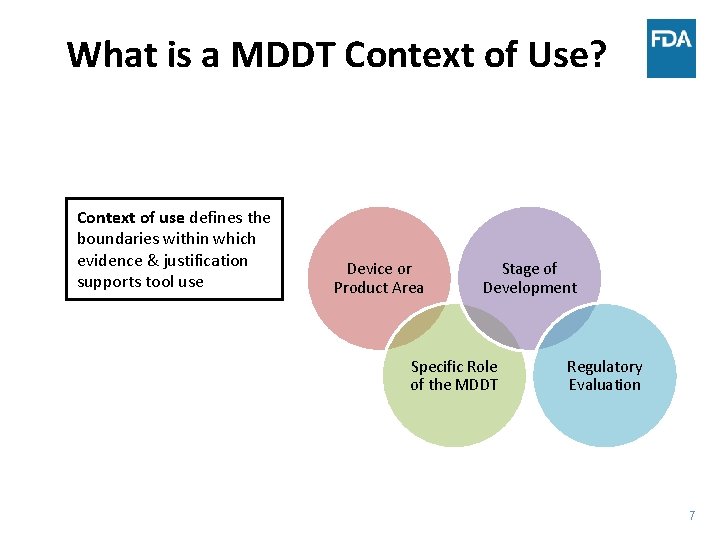
What is a MDDT Context of Use? Context of use defines the boundaries within which evidence & justification supports tool use Device or Product Area Stage of Development Specific Role of the MDDT Regulatory Evaluation 7

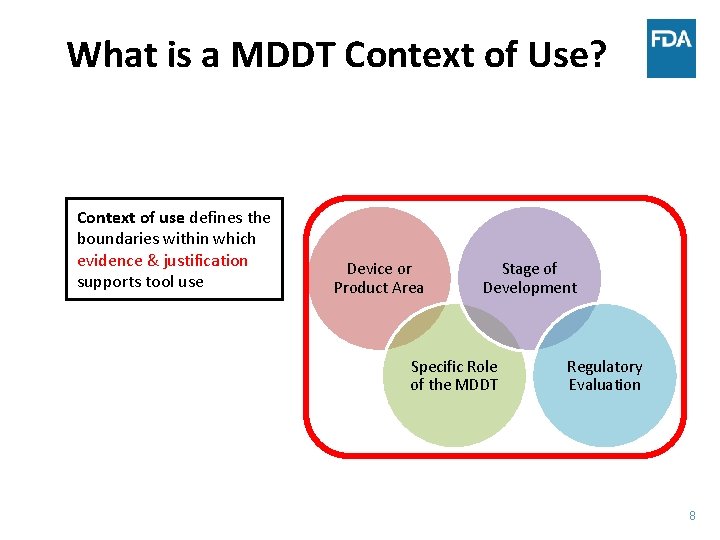
What is a MDDT Context of Use? Context of use defines the boundaries within which evidence & justification supports tool use Device or Product Area Stage of Development Specific Role of the MDDT Regulatory Evaluation 8

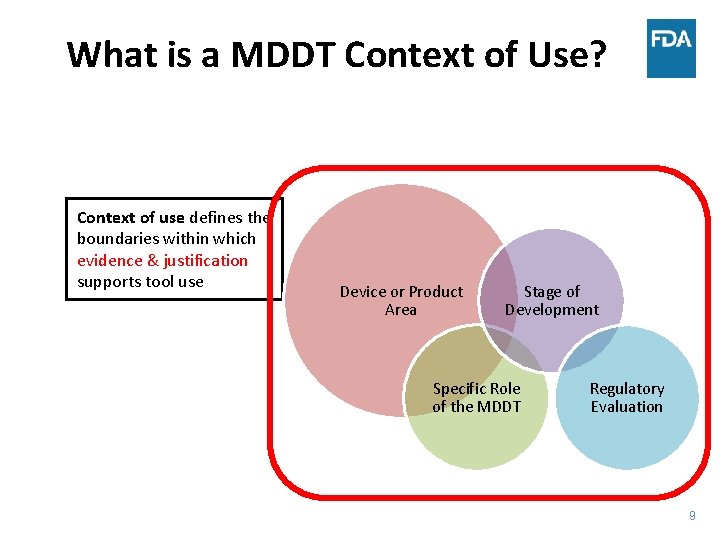
What is a MDDT Context of Use? Context of use defines the boundaries within which evidence & justification supports tool use Device or Product Area Stage of Development Specific Role of the MDDT Regulatory Evaluation 9

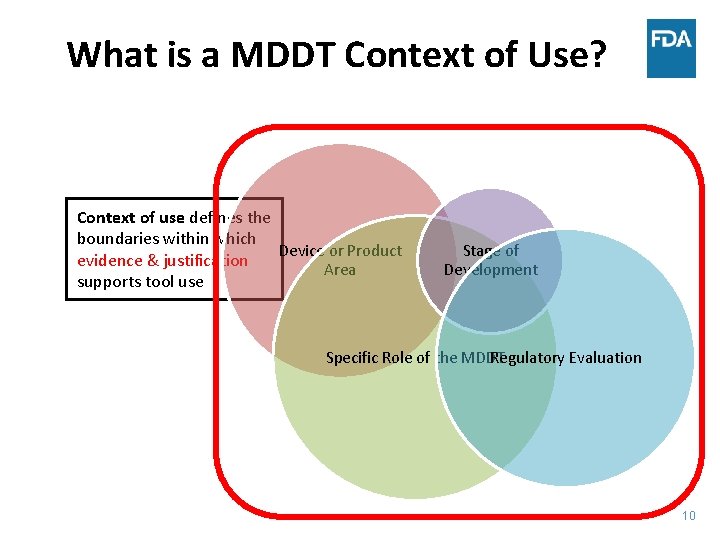
What is a MDDT Context of Use? Context of use defines the boundaries within which Device or Product evidence & justification Area supports tool use Stage of Development Regulatory Evaluation Specific Role of the MDDT 10

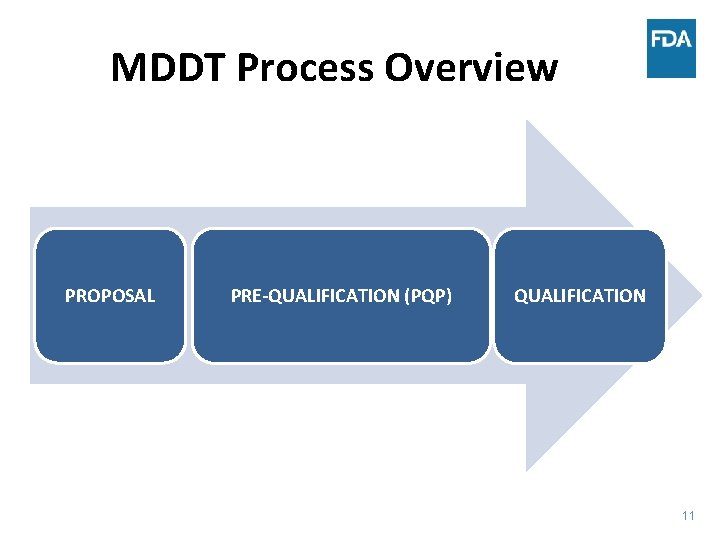
MDDT Process Overview PROPOSAL PRE-QUALIFICATION (PQP) QUALIFICATION 11

Proposal Pre-Qualification PQP Qualification Prioritization/acceptance to the MDDT Program will depend on the following factors: • Tool readiness: Does the tool exist in prototype or final form? • Proposed context of use: Does available information support acceptance of the tool principle/method of measurement for the proposed context of use, or for any use? • Timeline: What is the expected timeline to submission of a qualification package? • Potential for Public Health Impact: What type of impact will MDDT have on product development? Will the tool facilitate development of devices that will address unmet public health need? Proposal is sent as an email to MDDT@fda. hhs. gov 12

Proposal Pre-Qualification PQP Qualification • Opportunity to work interactively with FDA to develop the Qualification Plan. The Pre-Qualification Phase is Optional. • Sent as an “informational meeting” request, pre-Submission (Q-submission), based on FDA guidance document: Requests for Feedback on Medical Device Submissions: The Pre-Submission Program and Meetings with Food and Drug Administration Staff • If evidence supporting an MDDT COU is not yet available, the Pre-Qualification Phase is highly recommended. Otherwise, it’s utility will depend on the stage of MDDT development. 13

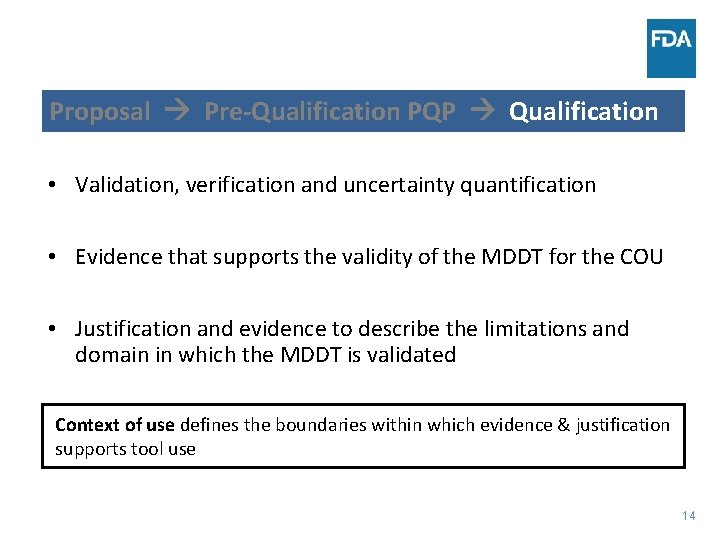
Proposal Pre-Qualification PQP Qualification • Validation, verification and uncertainty quantification • Evidence that supports the validity of the MDDT for the COU • Justification and evidence to describe the limitations and domain in which the MDDT is validated Context of use defines the boundaries within which evidence & justification supports tool use 14

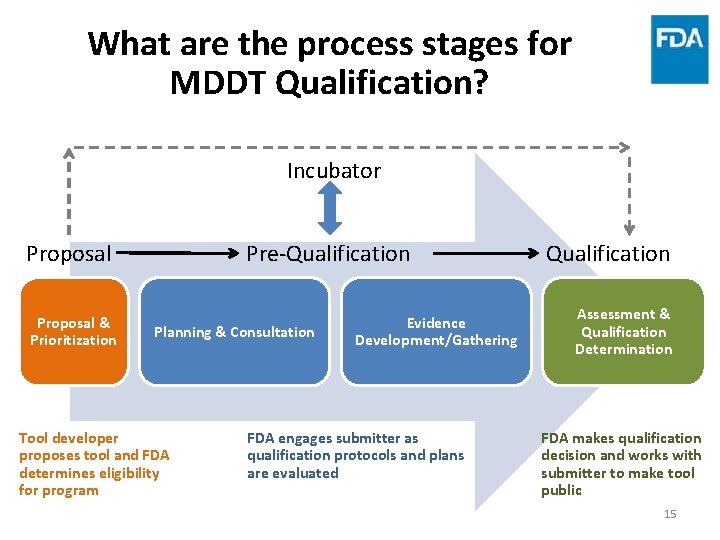
What are the process stages for MDDT Qualification? Incubator Proposal & Prioritization Pre-Qualification Planning & Consultation Tool developer proposes tool and FDA determines eligibility for program Evidence Development/Gathering FDA engages submitter as qualification protocols and plans are evaluated Qualification Assessment & Qualification Determination FDA makes qualification decision and works with submitter to make tool public 15

MDDT Pilot Goals The proposed MDDT qualification voluntary process is intended to support the development of MDDTs • Pressure test the process ― Develop needed organizational procedures and resources ― Identify challenges, barriers to adoption ― Best practices and training • Qualify a variety of tools 16

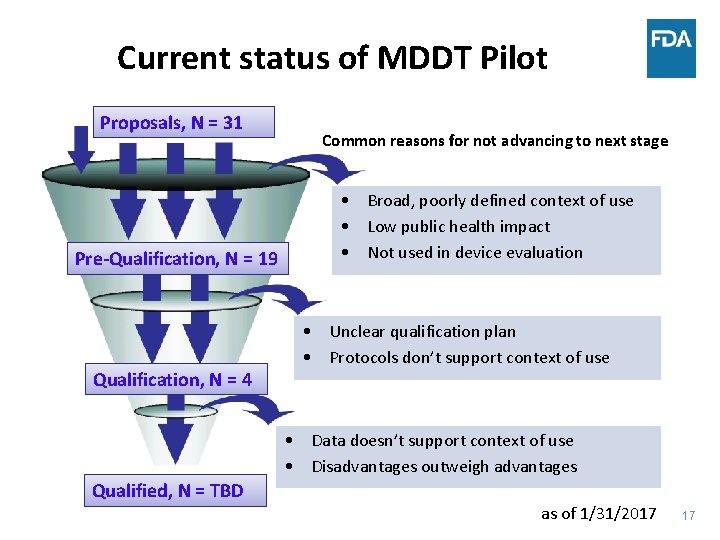
Current status of MDDT Pilot Proposals, N = 31 Pre-Qualification, N = 19 Qualification, N = 4 Common reasons for not advancing to next stage • Broad, poorly defined context of use • Low public health impact • Not used in device evaluation • Unclear qualification plan • Protocols don’t support context of use • Data doesn’t support context of use • Disadvantages outweigh advantages Qualified, N = TBD as of 1/31/2017 17

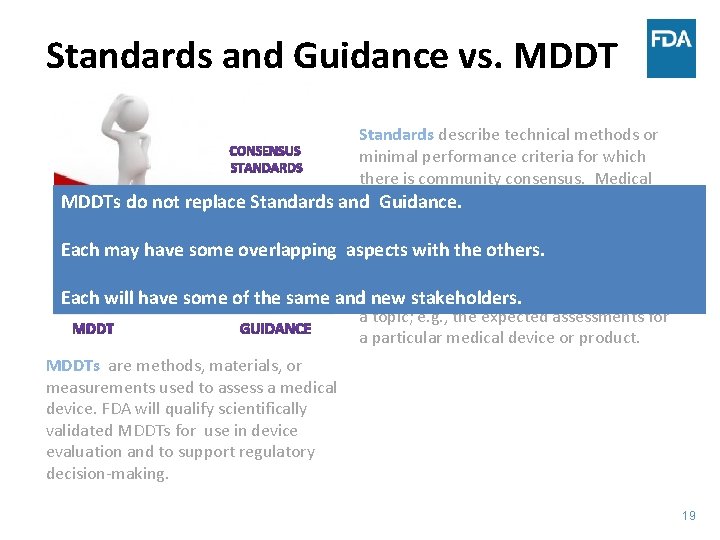
Standards and Guidance vs. MDDT Standards describe technical methods or minimal performance criteria for which there is community consensus. Medical devices and MDDTs often use standards in their development and to support regulatory decision-making. MDDTs are methods, materials, or measurements used to assess a medical device. FDA will qualify scientifically validated MDDTs for use in device evaluation and to support regulatory decision-making. Guidance provides the current thinking for a topic; e. g. , the expected assessments for a particular medical device or product. 18

Standards and Guidance vs. MDDT Standards describe technical methods or minimal performance criteria for which there is community consensus. Medical devices and MDDTs often use standards in MDDTs do not replace Standards and Guidance. their development and to support regulatory decision-making. Each may have some overlapping aspects with the others. Guidance provides the current thinking for Each will have some of the same and new stakeholders. a topic; e. g. , the expected assessments for a particular medical device or product. MDDTs are methods, materials, or measurements used to assess a medical device. FDA will qualify scientifically validated MDDTs for use in device evaluation and to support regulatory decision-making. 19

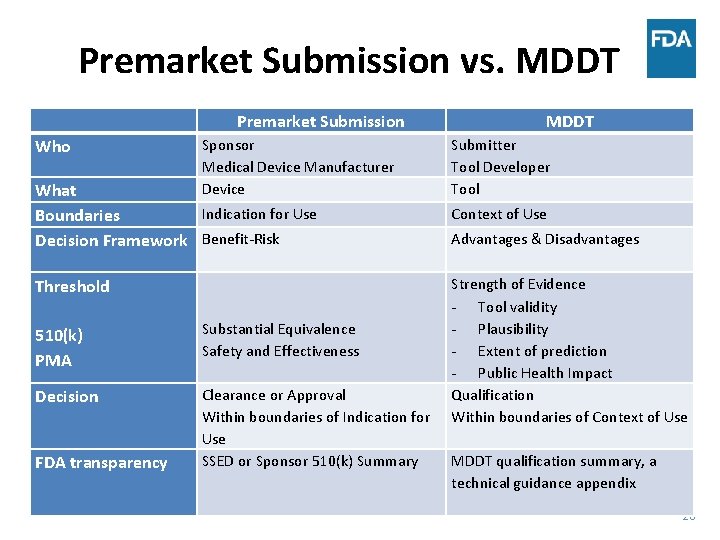
Premarket Submission vs. MDDT Who Premarket Submission Sponsor Medical Device Manufacturer Device What Indication for Use Boundaries Decision Framework Benefit-Risk Threshold 510(k) PMA Substantial Equivalence Safety and Effectiveness Decision Clearance or Approval Within boundaries of Indication for Use SSED or Sponsor 510(k) Summary FDA transparency MDDT Submitter Tool Developer Tool Context of Use Advantages & Disadvantages Strength of Evidence - Tool validity - Plausibility - Extent of prediction - Public Health Impact Qualification Within boundaries of Context of Use MDDT qualification summary, a technical guidance appendix 20

Resources • Federal Register (FR) notice announcing the MDDT Pilot Program: https: //www. federalregister. gov/articles/2014/08/15/2014 -19360/pilot-program-forqualification-of-medical-device-development-tools • FDA MDDT Guidance Document (Draft): http: //www. fda. gov/downloads/Medical. Devices/Device. Regulationand. Guidance/Guidance. Docu ments/UCM 374432. pdf • FDA MDDT Webpage: http: //www. fda. gov/Medical. Devices/Scienceand. Research/Medical. Device. Development. Tools. M DDT/default. htm 21

FAQs 1. Who may submit an MDDT? Any individual or group may submit an MDDT. The intent of this voluntary CDRH MDDT program is to promote the development and use of tools to streamline device development and regulatory evaluation. Once an MDDT is qualified for a specific context of use, CDRH encourages developers to make their qualified tools available to the public, so that the tools can be used by any medical device sponsor for that Context of Use. 2. How can an MDDT be submitted for qualification? An MDDT Proposal can be submitted by emailing the CDRH MDDT Working Group at MDDT@fda. hhs. gov. Tool developers should follow the recommendations in the MDDT Draft Guidance Document and the FR notice announcing the MDDT Pilot Program. If you have questions or would like to preliminarily discuss the value of your tool to this program, please email the MDDT Working Group. 3. Is there a fee associated with submission? No, at this time there are no fees associated with the MDDT program. 22

FAQs 4. Are there qualified MDDT already available? No. The MDDT qualification process described in the draft guidance is a draft policy that the pilot program is designed to test and evaluate. 5. What information about the tools will the FDA make accessible to the public? When the FDA has qualified an MDDT for a context of use, the FDA’s decision and a summary basis of the decision will be provided to the public. Information that is proprietary to the tool developer will not be disclosed. 6. Once qualified, is the MDDT required to be made available for public use for free and is the tool required to be open source? The tool developer determines the conditions of use of a tool such as licensing, cost, or degree of access to Intellectual Property associated with the tool. For example, if the tool includes software, the software may be open or closed source, free or licensed. The Program intends to qualify tools that the developer will make available to the public, rather than tools that are offered only to certain parties. 23

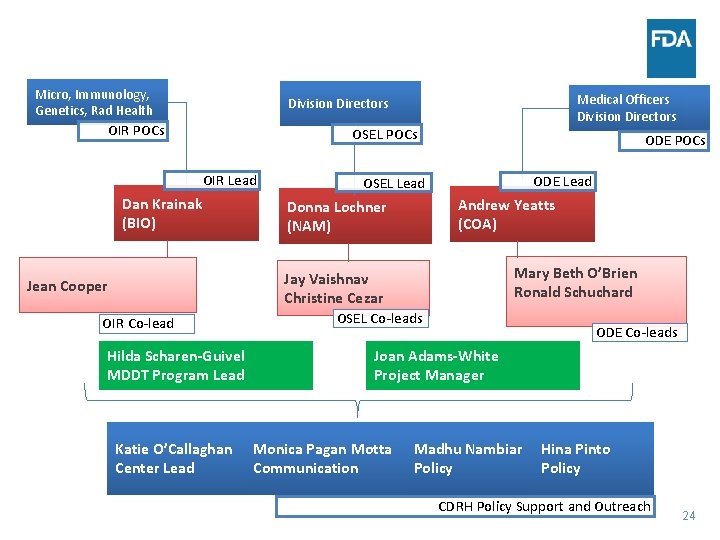
Micro, Immunology, Genetics, Rad Health Medical Officers Division Directors OIR POCs OSEL POCs OIR Lead Dan Krainak (BIO) ODE POCs ODE Lead OSEL Lead Andrew Yeatts (COA) Donna Lochner (NAM) Mary Beth O’Brien Ronald Schuchard Jay Vaishnav Christine Cezar Jean Cooper OIR Co-lead Hilda Scharen-Guivel MDDT Program Lead Katie O’Callaghan Center Lead OSEL Co-leads ODE Co-leads Joan Adams-White Project Manager Monica Pagan Motta Communication Madhu Nambiar Policy Hina Pinto Policy CDRH Policy Support and Outreach 24
 Mddt
Mddt Physical education assessments examples
Physical education assessments examples Nebraska reads approved assessments
Nebraska reads approved assessments General revision of assessments and property classification
General revision of assessments and property classification Assessments that favor one group over another are
Assessments that favor one group over another are Next generation assessments examples
Next generation assessments examples Assesment strategies
Assesment strategies Interim assessment tea
Interim assessment tea Cte technical skills assessments.azed.gov/student
Cte technical skills assessments.azed.gov/student Informal and formal assessment
Informal and formal assessment Process oriented learning competencies
Process oriented learning competencies Anet interim assessments
Anet interim assessments Common core ela assessments
Common core ela assessments Ricas math
Ricas math Informative assesment
Informative assesment Florida assessments for instruction in reading
Florida assessments for instruction in reading Apm assessment
Apm assessment Informal reading inventory examples
Informal reading inventory examples Performance assessment examples
Performance assessment examples Kentucky assessments science
Kentucky assessments science Iowa assessments
Iowa assessments Is diagnostic assessment formative or summative
Is diagnostic assessment formative or summative Creative arts grade 7 term 2: drama
Creative arts grade 7 term 2: drama Characteristics of psychological assessment
Characteristics of psychological assessment Mlpp assessments
Mlpp assessments