Clinical Trials Overview Clinical Trials A clinical trial










































- Slides: 64

Clinical Trials Overview

Clinical Trials • A clinical trial is a prospectively planned experiment for the purpose of evaluating one or more potentially beneficial therapies or treatments • In general these studies are conducted under as many controlled conditions as possible in order to provide definitive answers to well-defined questions

Primary vs. Secondary Questions • Primary – most important, central question – ideally, only one – stated in advance – basis for design and sample size • Secondary – related to primary – stated in advance – limited in number

Examples • Physicians Health Study (PHS) started in fall 1982 – risks and benefits of aspirin and beta carotene in the prevention of cardiovascular disease and cancer – low-dose aspirin vs placebo – Primary: total mortality – Secondary: fatal + nonfatal myocardial infarction • Eastern Cooperative Oncology Group (ECOG) – tamoxifen vs placebo – Primary: tumor recurrence/relapse, disease-free survival – Secondary: total mortality

Definitions • Single Blind Study: A clinical trial where the participant does not know the identity of the treatment received • Double Blind Study: A clinical trial in which neither the patient nor the treating investigators know the identity of the treatment being administered.

Definitions • Placebo: – Used as a control treatment 1. An inert substance made up to physically resemble a treatment being investigated 2. Best standard of care if “placebo” unethical 3. “Sham control”

Definitions • Adverse event: – An incident in which harm resulted to a person receiving health care. – Examples: Death, irreversible damage to liver, nausea – Not always easy to specify in advance because many variables will be measured – May be known adverse effects from earlier trials

Adverse Events • Challenges – Long term follow-up versus early benefit – Rare AEs may be seen only with very large numbers of exposed patients and long term followup • Example – COX II inhibitors – Vioxx & Celebrex – Immediate pain reduction vs longer term increase in cardiovascular risk

Surrogate Endpoints • Response variables used to address questions often called endpoints • Surrogates used as alternative to desired or ideal clinical response to save time and/or resources • Examples – Suppression of arrhythmia (sudden death) – T 4 cell counts (AIDS or ARC) • Often used in therapeutic exploratory trials • Use with caution in therapeutic confirmatory trials

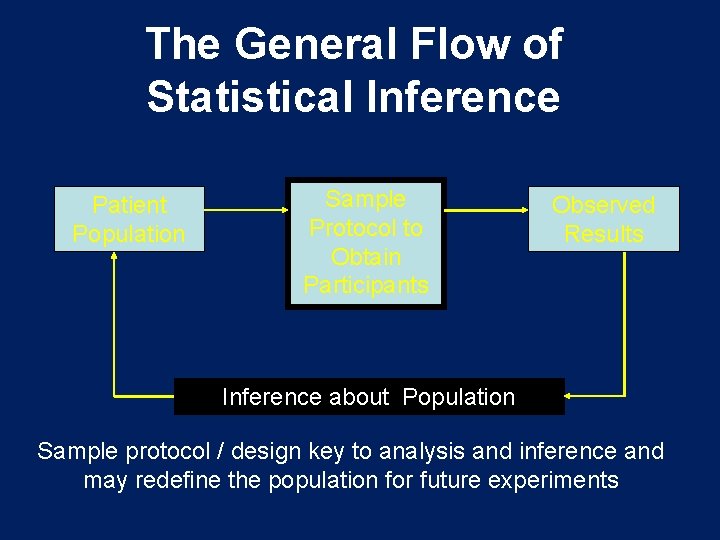
The General Flow of Statistical Inference Patient Population Sample Protocol to Obtain Participants Observed Results Inference about Population Sample protocol / design key to analysis and inference and may redefine the population for future experiments

Types of Clinical Trials • Randomized • Non-Randomized • Single-Center • Multi-Center • Phase I, III Trials

Phase I Trial • Objective : To determine an acceptable range of doses and schedules for a new drug • Usually seeking maximum tolerated dose (MTD) • Participants often those that have failed other treatments • Important, however, that they still have “normal” organ functions

Phase II Trial • Objective: To determine if new drug has any beneficial activity and thus worthy of further testing / investment of resources. • Doses and schedules may not be optimum • Begin to focus on population for whom this drug will likely show favorable effect

Phase III Trial • Objective : To compare experimental or new therapies with standard therapy or competitive therapies. • Very large, expensive studies • Required by FDA for drug approval • If drug approved, usually followed by Phase IV trials to follow-up on long-range adverse events – concern is safety


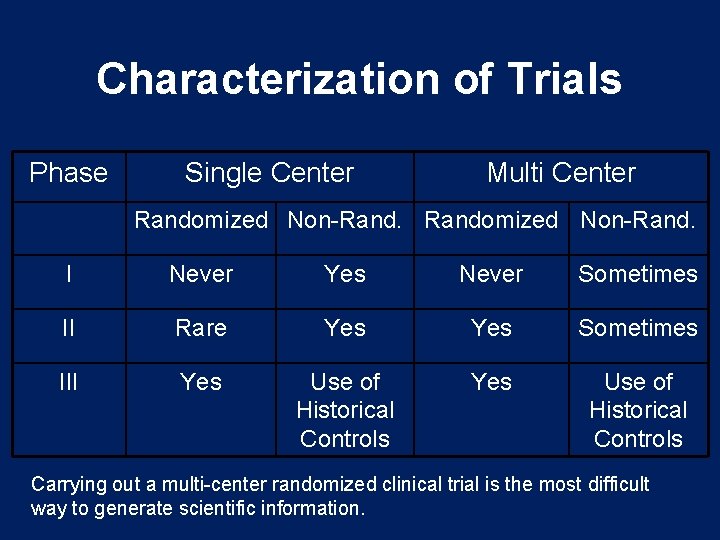
Characterization of Trials Phase Single Center Multi Center Randomized Non-Rand. I Never Yes Never Sometimes II Rare Yes Sometimes III Yes Use of Historical Controls Carrying out a multi-center randomized clinical trial is the most difficult way to generate scientific information.

Why Clinical Trials? 1. Most definitive method to determine whether a treatment is effective. – Other designs have more potential biases – One cannot determine in an uncontrolled setting whether an intervention has made a difference in the outcome.

Observational Studies • Correlation vs. Causation Examples of False Positives 1. High cholesterol diet and rectal cancer 2. Smoking and breast cancer 3. Vasectomy and prostate cancer 4. Red meat and colon cancer 5. Red meat and breast cancer 6. Drinking water frequently and bladder cancer 7. Not consuming olive oil and breast cancer – Replication of observational studies may not overcome confounding and bias

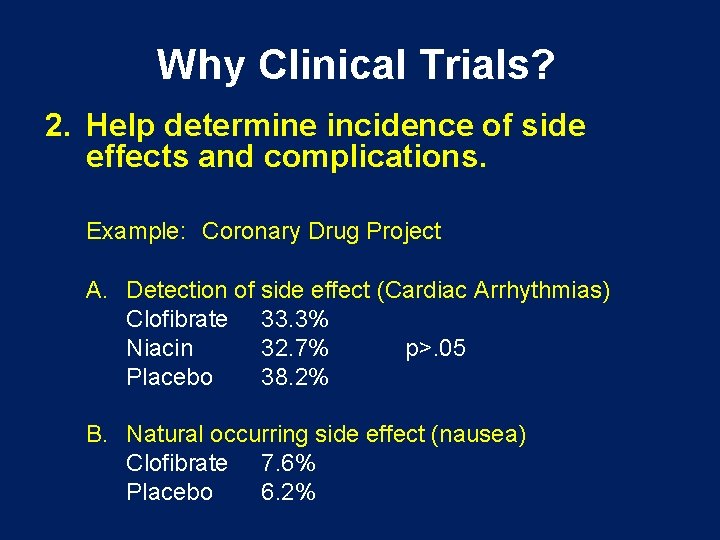
Why Clinical Trials? 2. Help determine incidence of side effects and complications. Example: Coronary Drug Project A. Detection of side effect (Cardiac Arrhythmias) Clofibrate 33. 3% Niacin 32. 7% p>. 05 Placebo 38. 2% B. Natural occurring side effect (nausea) Clofibrate 7. 6% Placebo 6. 2%

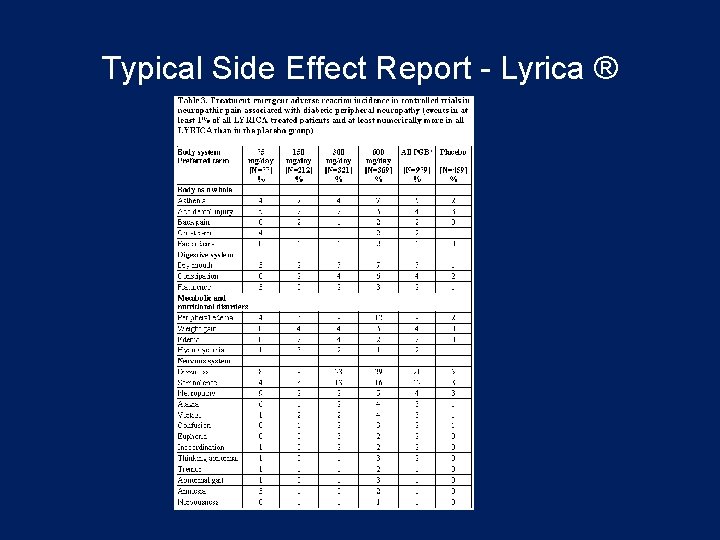
Typical Side Effect Report - Lyrica ®

Why Clinical Trials? 3. Theory not always best path • Intermittent positive pressure breathing (IPPB) reduced use, no benefit • High [O 2] in premature infants Retrolental Fibroplasia, Harmful • Tonsillectomy Reduced use • Bypass Surgery Restricted use

Phase I Design Strategy • Designs based largely on tradition • Typically do some sort of dose escalation to reach maximum tolerated dose (MTD) • Has been shown to be safe and reasonably effective • Dose escalation often based on Fibonacci series – 1 2 3 5 8 13. .

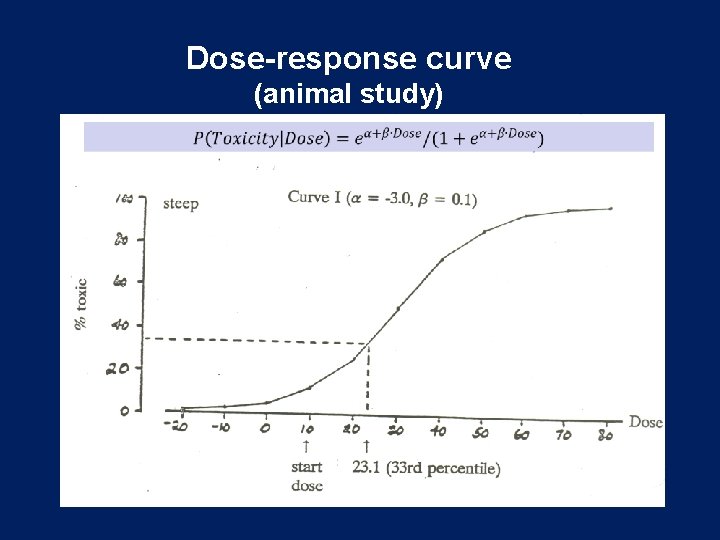
Dose-response curve (animal study)

Typical Scheme 1. Enter 3 patients at a given dose 2. If no toxicity, go to next dosage and repeat step 1 3. a. If 1 patient has serious toxicity, add 3 more patients at that dose (go to 4) b. If 2/3 have serious toxicity, consider MTD 4. a. If 2 or more of 6 patients have toxicity, MTD reached b. If 1 of 6 has toxicity, increase dose and go back to step 1

Summary of Schemes (Storer, Biometrics 45: 925 -37, 1989) A. “Standard” – Observe group of 3 patients – No toxicity increase dose – Any toxicity observe 3 or more • One toxicity out of 6 increase dose • Two or more toxicity stop B. “ 1 Up, 1 Down” – Observe single patients – No toxicity increase dose – Toxicity decrease dose

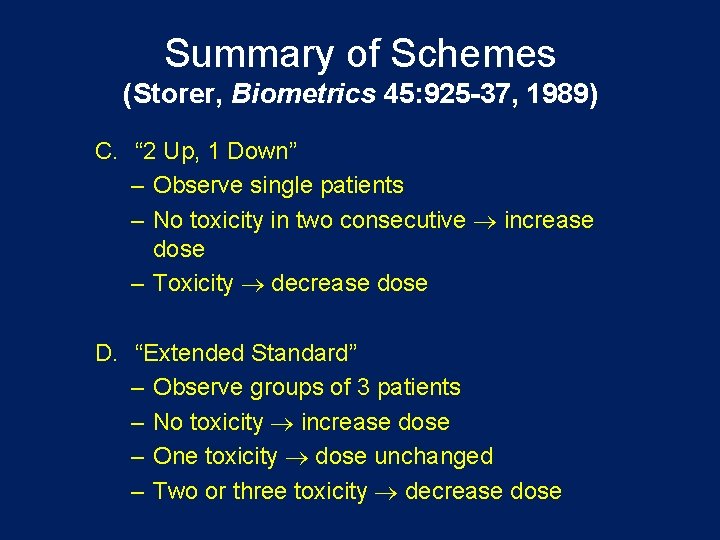
Summary of Schemes (Storer, Biometrics 45: 925 -37, 1989) C. “ 2 Up, 1 Down” – Observe single patients – No toxicity in two consecutive increase dose – Toxicity decrease dose D. “Extended Standard” – Observe groups of 3 patients – No toxicity increase dose – One toxicity dose unchanged – Two or three toxicity decrease dose

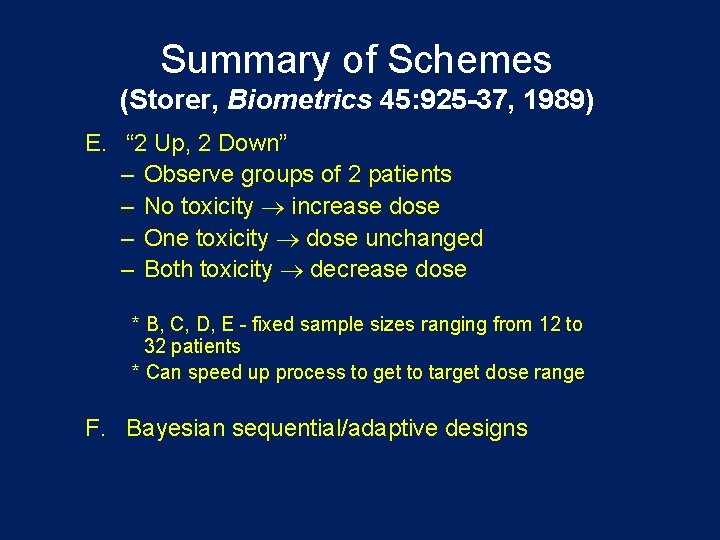
Summary of Schemes (Storer, Biometrics 45: 925 -37, 1989) E. “ 2 Up, 2 Down” – Observe groups of 2 patients – No toxicity increase dose – One toxicity dose unchanged – Both toxicity decrease dose * B, C, D, E - fixed sample sizes ranging from 12 to 32 patients * Can speed up process to get to target dose range F. Bayesian sequential/adaptive designs

Phase II Designs References: Gehan (1961) Journal of Chronic Disorders Fleming (1982) Biometrics Storer (1989) Statistics in Medicine • Goal – – Screen for therapeutic activity Further evaluate toxicity Test using MTD from Phase I If drug passes screen, test further

Phase II Design • Design of Gehan – No control (is this wise? ) – Two-stage (small initial sample, observe at least one benefit take a second larger sample) – Goal is to reject ineffective drugs ASAP Decision I: Drug is unlikely to be effective in x% of patients Decision II: Drug could be effective in x% of patients

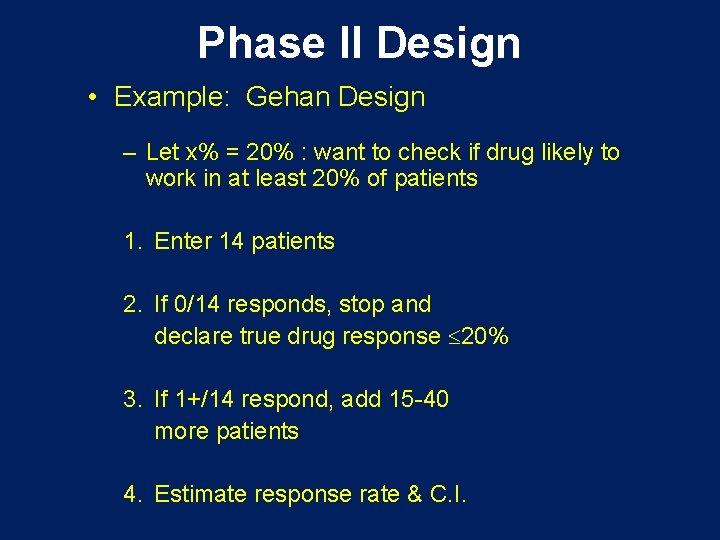
Phase II Design • Example: Gehan Design – Let x% = 20% : want to check if drug likely to work in at least 20% of patients 1. Enter 14 patients 2. If 0/14 responds, stop and declare true drug response 20% 3. If 1+/14 respond, add 15 -40 more patients 4. Estimate response rate & C. I.

Gehan Design • Why 14 patients initially? Patient 1 2 3 --8 --14 Prob 0. 8 0. 64 (0. 8 x 0. 8) 0. 512 (0. 8 x 0. 8) --0. 16 --0. 044 • If drug 20% effective, there would be ~95. 6% chance of at least one success • If 0/14 success observed, reject drug

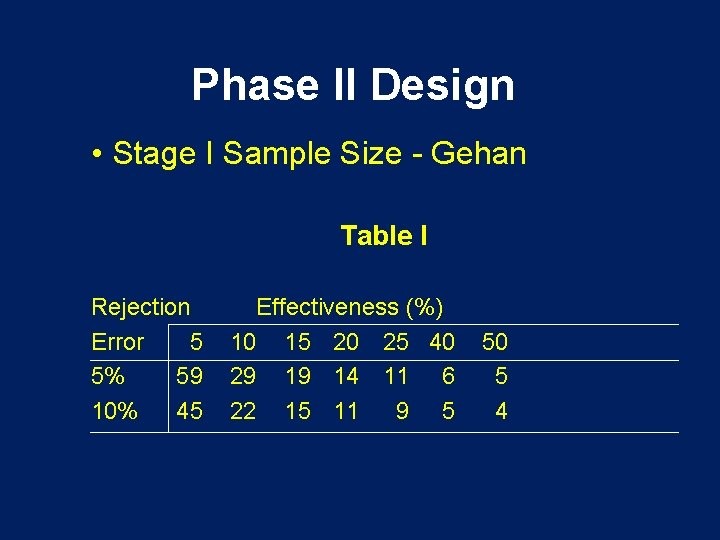
Phase II Design • Stage I Sample Size - Gehan Table I Rejection Error 5 5% 59 10% 45 Effectiveness (%) 10 15 20 25 40 29 19 14 11 6 22 15 11 9 5 50 5 4

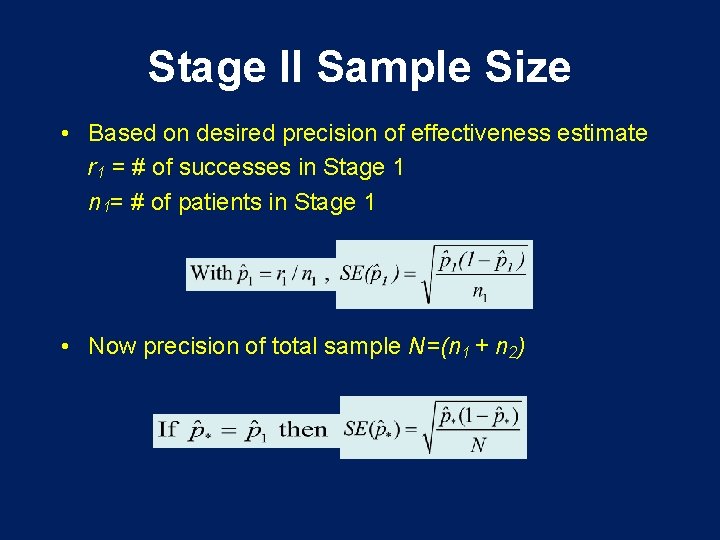
Stage II Sample Size • Based on desired precision of effectiveness estimate r 1 = # of successes in Stage 1 n 1= # of patients in Stage 1 • Now precision of total sample N=(n 1 + n 2)

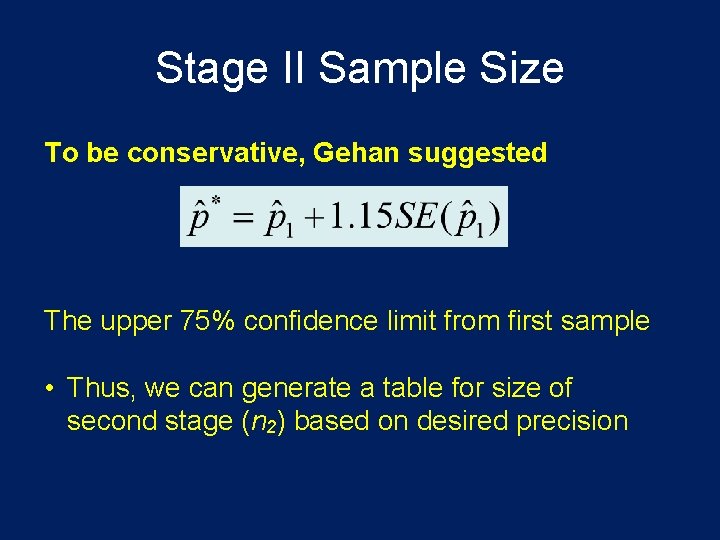
Stage II Sample Size To be conservative, Gehan suggested The upper 75% confidence limit from first sample • Thus, we can generate a table for size of second stage (n 2) based on desired precision

Additional Patients for Stage II (n 2, a 1=. 05)

Phase II Trial Designs • Many cancer Phase II trials follow Gehan design • Many other diseases could – there seems to be no standard non-cancer Phase II design • Might also randomize patients into multiple arms each with a different dose – can then get a dose response curve • Other two-stage designs based on determining p 1 -p 0 > x% where p 0 is the standard care combination

Phase III Trial Designs o The foundation for the design of controlled experiments established for agricultural experiments o The need for control groups in clinical studies recognized, but not widely accepted until 1950 s o No comparison groups needed when results dramatic: o Penicillin for pneumococcal pneumonia o Rabies vaccine o Use of proper control group necessary due to: o Natural history of most diseases o Variability of a patient's response to intervention

Phase III Design • Comparative Studies • Experimental Group vs. Control Group • Establishing a Control 1. 2. 3. Historical Concurrent Randomized • Randomized Control Trial (RCT) is the gold standard – Eliminates several sources of bias

Purpose of Control Group • To allow discrimination of patient outcomes caused by test treatment from those caused by other factors – Natural progression of disease – Observer/patient expectations – Other treatment • Fair comparisons – Necessary to be informative

Goals of Phase III Clinical Trial • Superiority Trials – A controlled trial may demonstrate efficacy of the test treatment by showing that it is superior to the control • No treatment (placebo) • Best standard of current care

Goals of Phase III Clinical Trials • Non-Inferiority Trials – Controlled trial may demonstrate efficacy by showing the test treatment is similar in efficacy to a known effective treatment • The active control has to be effective under the conditions of the trials • New treatment cannot be worse by a pre-specified amount • New treatment may not be better than the standard but may have other advantages – Cost – Toxicity and/or side effects – Invasiveness

Significance of Control Group • • Inference drawn from the trial Ethical acceptability of the trial Degree to which bias is minimized Type of subjects Kind of endpoints that can be studied Credibility of the results Acceptability of the results by regulatory authorities Other features of the trial, its conduct, and interpretation

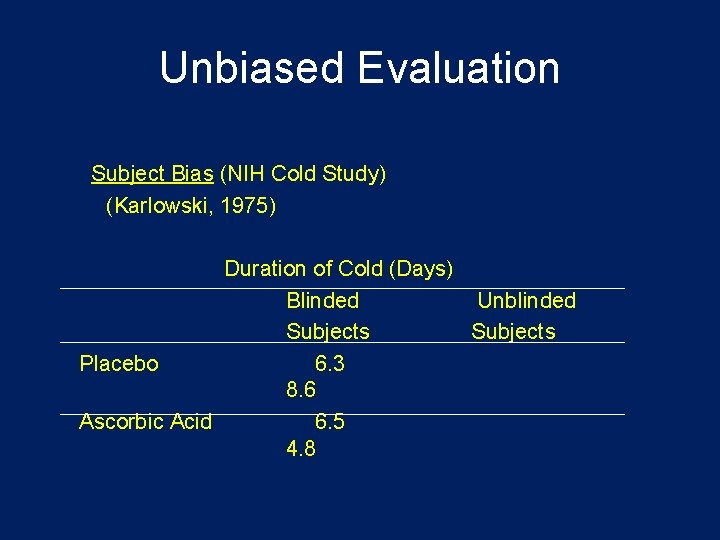
Use of Placebo Control • The “placebo effect” is well documented (as high as 33% according to some studies) • Could be – No treatment + placebo – Standard care + placebo • Matched placebos are necessary so patients and investigators cannot decode the treatment assignment • E. g. Vitamin C trial for common cold • Placebo was used, but was distinguishable – Many on placebo dropped out of study – not blinded – Those who knew they were on vitamin C reported fewer cold symptoms and duration than those on vitamin who didn't know

Unbiased Evaluation Subject Bias (NIH Cold Study) (Karlowski, 1975) Duration of Cold (Days) Blinded Unblinded Subjects Placebo 6. 3 8. 6 Ascorbic Acid 6. 5 4. 8

Historical Control Study • A new treatment used in a series of subjects • Outcome compared with previous series of comparable subjects • Non-randomized • Rapid, inexpensive, good for initial testing of new • treatments • Vulnerable to biases Different underlying populations Criteria for selecting patients Patient care Diagnostic or evaluating criteria

Historical Control Study When might we consider a historical control study? • When preliminary data strongly suggest efficacy. • When course of disease predictable, generally a consistently poor outcome. • When endpoints objective, like death or metastisization. • When impact of baseline and other variables on endpoint is well characterized.

Randomized Control Clinical Trial • Reference: Byar et al. (1976) New England Journal of Medicine • Patients assigned at random to either treatment(s) or control • Considered to be “Gold Standard”

Disadvantages of Randomized Control Clinical Trial 1. Generalizable Results? – Subjects may not represent general patient population – volunteer effect 2. Recruitment – Twice as many new patients 3. Acceptability of Randomization Process – Some physicians will refuse – Some patients will refuse 4. Administrative Complexity

Ethics of Randomization • Statistician/clinical trialist must sell benefits of randomization • Ethics Þ MD should do what he thinks is best for his patient – Two MD's might ethically treat same patient quite differently • Chalmers & Shaw (1970) Annals New York Academy of Science 1. 2. If MD "knows" best treatment, should not participate in trial If in doubt, randomization gives each patient equal chance to receive one of therapies (i. e. best) 3. More ethical way of practicing medicine • Bayesian Adaptive designs More likely assign “better” treatment

Comparing Treatments • Fundamental principle • Groups must be alike in all important aspects and only differ in the treatment each group receives • In practical terms, “comparable treatment groups” means “alike on the average” • Randomization • Each patient has the same chance of receiving any of the treatments under study • Allocation of treatments to participants is carried out using a chance mechanism so that neither the patient nor the physician know in advance which therapy will be assigned • Blinding • Avoidance of psychological influence • Fair evaluation of outcomes

Randomized Phase III Experimental Designs Assume: • Patients enrolled in trial have satisfied eligibility criteria and have given consent • Balanced randomization: each treatment group will be assigned an equal number of patients Issue • Different experimental designs can be used to answer different therapeutic questions

Commonly Used Phase III Designs • • • Parallel Withdrawal Group/Cluster Randomized Consent Cross Over Factorial Large Simple Equivalence/Non-inferiority Sequential

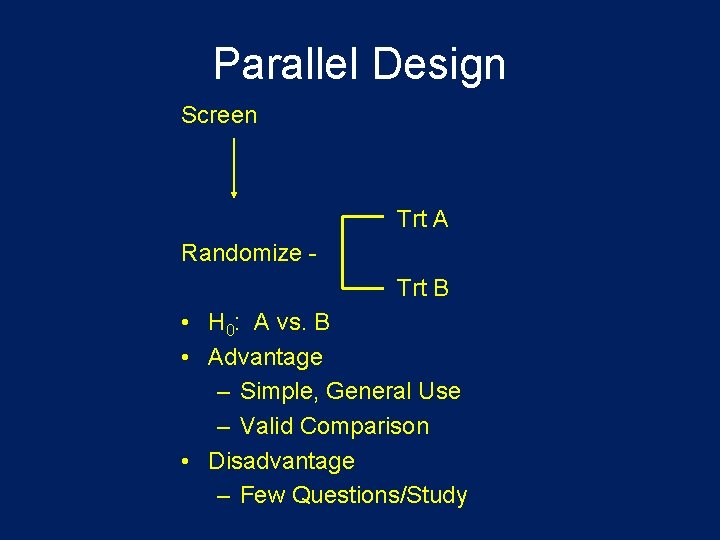
Parallel Design Screen Trt A Randomize Trt B • H 0: A vs. B • Advantage – Simple, General Use – Valid Comparison • Disadvantage – Few Questions/Study

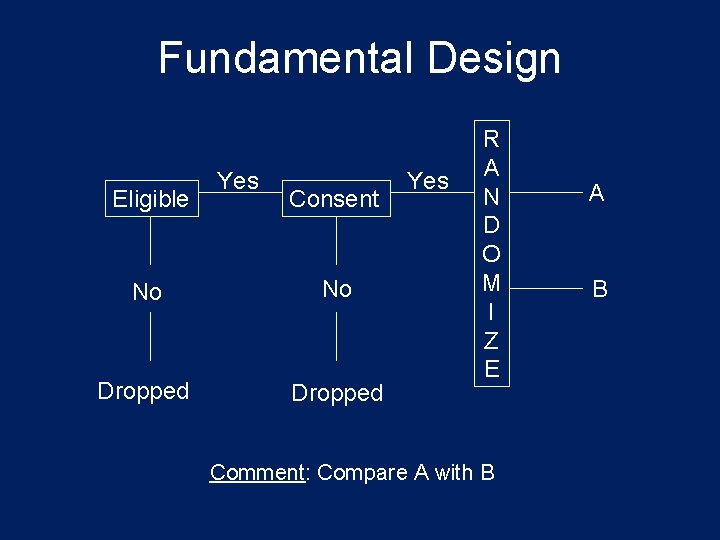
Fundamental Design Eligible Yes Consent No No Dropped Yes R A N D O M I Z E Comment: Compare A with B A B

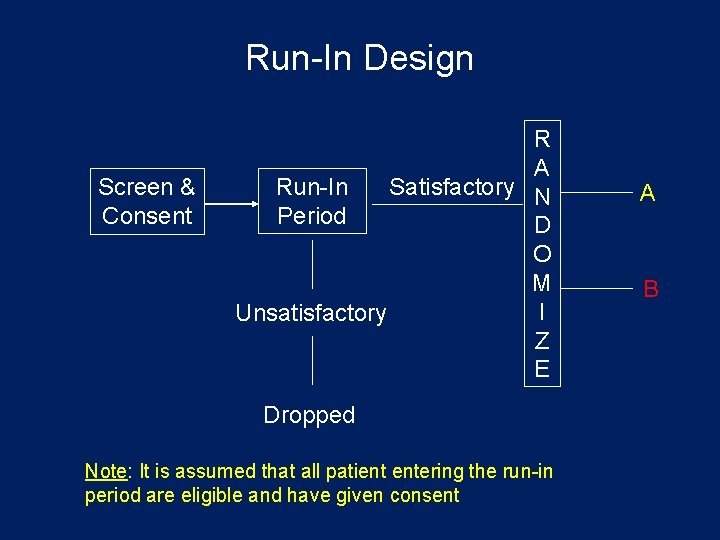
Run-In Design Problem: • Non-compliance by patient may seriously impair efficiency and possibly distort conclusions. Possible Solution: Drug Trials • Assign all eligible patients a placebo to be taken for a “brief” period of time. Patients who are “judged” compliant are enrolled into the study. This is often referred to as the “Placebo Run-In” period. • Can also use active drug to test for compliance.

Run-In Design Screen & Consent R A Run-In Satisfactory N Period D O M I Unsatisfactory Z E Dropped Note: It is assumed that all patient entering the run-in period are eligible and have given consent A B

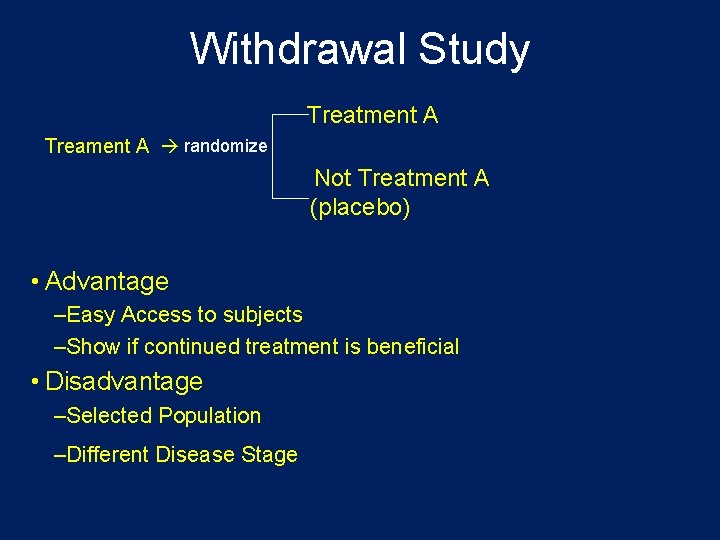
Withdrawal Study Treatment A Treament A randomize Not Treatment A (placebo) • Advantage –Easy Access to subjects –Show if continued treatment is beneficial • Disadvantage –Selected Population –Different Disease Stage

Cluster Randomization Designs • Groups (clinics, communities) are randomized to treatment or control • Examples: • Community trials on fluoridization of water • Breast self-examination programs in different clinic settings in USSR • Smoking cessation intervention trial in different school districts in the state of Washington • Advantages • Sometimes logistically more feasible • Avoid contamination • Allow mass intervention, thus “public health trial” • Disadvantages • Effective sample size less than number of subjects • Many units must participate to overcome unit-to-unit variation, thus requires larger sample size • Need cluster sampling methods

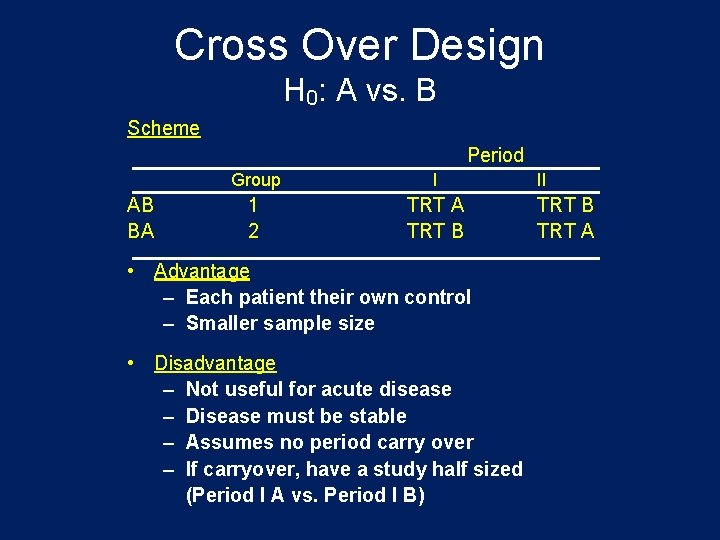
Cross Over Design H 0: A vs. B Scheme Period AB BA Group I 1 2 TRT A TRT B • Advantage – Each patient their own control – Smaller sample size • Disadvantage – Not useful for acute disease – Disease must be stable – Assumes no period carry over – If carryover, have a study half sized (Period I A vs. Period I B) II TRT B TRT A

Superiority vs. Non-Inferiority Trials Superiority Design: Show that new treatment is better than the control or standard (maybe a placebo) Non-inferiority: Show that the new treatment a) Is not worse that the standard by more than some margin b) Would have beaten placebo if a placebo arm had been included (regulatory)

Equivalence/Non-inferiority Trial • Trial with active (positive) controls. • The question is whether new (easier or cheaper) treatment is as good as the current treatment. • Must specify margin of “equivalence” or non-inferiority • Can't statistically prove equivalency -- only show that difference is less than something with specified probability. • Historical evidence of sensitivity to treatment • Sample size issues are crucial. • Small sample size, leading to low power and subsequently lack of significant difference, does not imply “equivalence”.

Non-Inferiority Challenges • Requires high quality trial • Poor execution favors non-inferiority • Treatment margin somewhat arbitrary

Sequential Design • Continue to randomize subjects until H 0 is either rejected or “accepted” • A large statistical literature for classical sequential designs • Developed for industrial setting • Modified for clinical trials (e. g. Armitage 1975, Sequential Medical Trials)

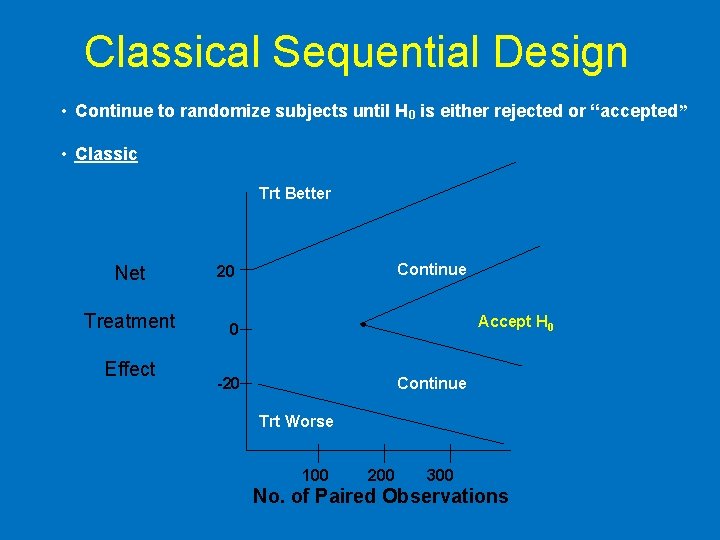
Classical Sequential Design • Continue to randomize subjects until H 0 is either rejected or “accepted” • Classic Trt Better Net Treatment Effect Continue 20 Accept H 0 0 -20 Continue Trt Worse 100 200 300 No. of Paired Observations
 Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Mpn clinical trials
Mpn clinical trials Nida clinical trial network
Nida clinical trial network Ohsu clinical trials office
Ohsu clinical trials office Clinical trials quality by design
Clinical trials quality by design York clinical trials unit
York clinical trials unit Andrew nunn
Andrew nunn Clinical trials prs
Clinical trials prs Site initiation visit powerpoint presentation
Site initiation visit powerpoint presentation Prs clinical trial
Prs clinical trial Professor claire harrison
Professor claire harrison Stratified randomization
Stratified randomization Clinical trials
Clinical trials Clinicaltrails.gov api
Clinicaltrails.gov api Clinical trials.gov login
Clinical trials.gov login Dhl clinical trials
Dhl clinical trials Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Audits and inspections of clinical trials
Audits and inspections of clinical trials Role of statistician in clinical trials
Role of statistician in clinical trials Iwr clinical trials
Iwr clinical trials Clinical trial worksheet
Clinical trial worksheet Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Morpheus clinical trial
Morpheus clinical trial Clinical trial centers alliance
Clinical trial centers alliance Clinical trial timeline
Clinical trial timeline Nnz-2566
Nnz-2566 Clinical trial budget example
Clinical trial budget example Companion diagnostic clinical trial
Companion diagnostic clinical trial Master clinical trial agreements
Master clinical trial agreements Fsfd clinical trial
Fsfd clinical trial Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Novel clinical drug trial design
Novel clinical drug trial design Clinical trial exports
Clinical trial exports Clinical trial matching service
Clinical trial matching service Rsna clinical trial processor
Rsna clinical trial processor Mosaico janssen
Mosaico janssen Clinical trial financial management
Clinical trial financial management Korean bridging phase 1 trials
Korean bridging phase 1 trials Ndsu corn variety trials
Ndsu corn variety trials Malta football trials
Malta football trials Sumaltriptan
Sumaltriptan Salem witch trials thesis ideas
Salem witch trials thesis ideas Nordic field trial
Nordic field trial Nuremberg trials
Nuremberg trials Virtual field trip salem witch trials
Virtual field trip salem witch trials Design and analysis of cross over trials
Design and analysis of cross over trials Bernoulli trials formula
Bernoulli trials formula Many kids called unfit for adult trials
Many kids called unfit for adult trials Repeated bernoulli trials
Repeated bernoulli trials Ccea
Ccea Discovery education salem witch trials
Discovery education salem witch trials How is the crucible unlike the salem witch trials?
How is the crucible unlike the salem witch trials? 6 trials of jesus
6 trials of jesus Hercules monomyth
Hercules monomyth Dr spock apush
Dr spock apush Random control trials
Random control trials Do these situations involve bernoulli trials
Do these situations involve bernoulli trials Poe trials
Poe trials National geographic salem witch trials
National geographic salem witch trials Rebecca nurse trial
Rebecca nurse trial Do we our life done
Do we our life done Future search trials
Future search trials Pediatric trials network
Pediatric trials network Salem witch trials facts
Salem witch trials facts Palo alto trial
Palo alto trial