CLINICAL REG AFFAIRS What is Clinical Clinical research









- Slides: 16

CLINICAL & REG. AFFAIRS

What is Clinical • Clinical research is the study of health and illness in people. It is the way we learn how to prevent, diagnose and treat illness. • It involves human participants and helps translate basic research into new treatments and information to benefit patients.

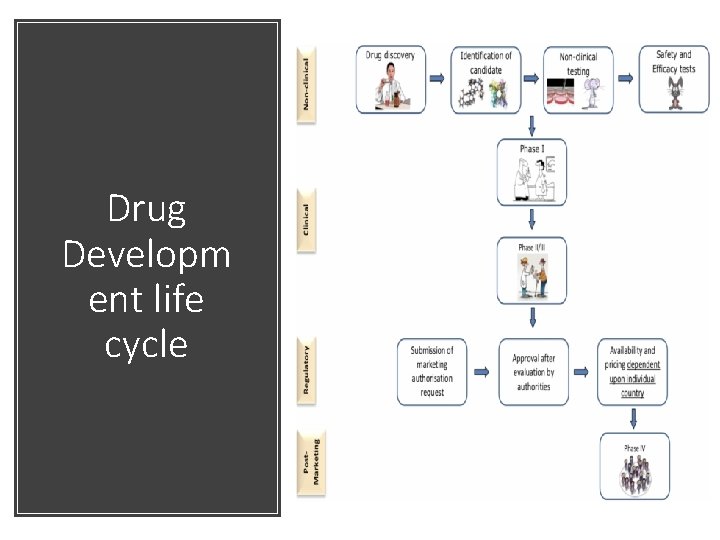
With a higher demand of quality health services and shorts of resources is necessary to have a higher frequency of evaluation of interventions that have been effective reducing risk and unnecessary cost. WHY & HOW Before approving a new drug for prescription for the general use is necessary for that drug be have been tested to check its efficiency. The drug will be also continue to be monitored after approval for long term side effects or for possible improvements. Normally we call clinical to what we know under the phases I, III & IV

Drug Developm ent life cycle

Cv example Covance Clinical Development, S. A. (Formerly Chiltern International, S. L. (Jan 2018, ongoing) Sr. PM Therapeutic area: • Oncology early phase, UK and Spain Pivotal, S. L. (July 2004, Dec 2017) Project manager (May 2013, Dec 2018) Therapeutic Area: • Endocrinology (Pediatric population), Int. phase III study GH • ICU: Phase II international trial with ICU patients (Enteral Feeding Intolerance) • Neurosurgery International phase III study in a SAH • Oncology: International phase III study in metastatic Breast Ca PM responsibilities: Team coordination and supervision of EU countries, supporting milestone achievement and managing study issues and obstacles. Study implementation, reporting continuously the study progress, ensuring consistent use of study tools and training materials and compliance with standard processes, policies and procedures. European Regulatory process overview in the phase II trial, including EC and CA submissions, site contract management, site initiations process, site activation process, etc. . . Acting as resourcing manager to allocate % FTE, control of budget activities referencing the Scope of Work, collaborate with the Director Clinical Operations in the decision-making process regarding the use of internal and external resources for the project. Selection of the investigators and all activities associated with study initiation. Management of clinical and non-clinical supplies, investigational products included, as assigned/needed. Collaborate with the DOC in identifying and analysing risks and planning risk mitigation strategies as well as fostering and implementing studies critical success factors. Ensure that the study is performed according to the ICH-GCP guidelines and Sponsor SOPs. Ensure that the clinical study meets timelines and achieve deliverables. Participate actively in Investigator Meetings, Study Monitor Meetings and Study Team Meetings. Attending and preparing slides for bid defence meetings, discussing the strategy with the Sponsor, defending the proposal are tasks also included. Liaise on an ongoing basis with Sponsor’s window person assigned. Oversee study termination activities. Clinical Trial Manager (April 2012 - April 2014) Therapeutic area: • Oncology: International phase III study coordination 3 EU teams (Spain, Italy and Hungary) • Medical Devices: International Clinical Investigation Plan in Cardiology. Coordination and supervision of 4 EU teams (Germany, Netherlands, Poland Belgium) CTL Responsibilities: Coordination and supervision of EU teams. Ensure smooth running of clinical trial while maintaining the study compliance, guarantee all Lines Management are informed about study status and any relevant issues and implement corrective actions if needed. Generating monthly metrics, mentoring of SCRAs, CRA trainees is also a responsibility. Ensure all study activities are performed in accordance with GCPs, CRO and/or Sponsor SOPs and local/International regulations and applicable quality procedures. Local PM at CAIBER outsourced from Aug 2011 until March 2012 Therapeutic areas: Endocrinology, Ophthalmology and Oncology Supporting the startup phase of 5 local phase II studies at CAIBER (Clinical Trial Spanish Network). Responsible of the EC and CA submissions, site contracts and budget management, independent researching, public funding, mentoring and coordination of site teams were also duties. Lead CRA (Mar 2007 until Nov 2008)/Clinical Trial Manager (Dec 08 until Jan 2011) Therapeutic area: Oncology, International Phase 3 study in Prostate Ca. Responsibilities: Monitoring at Coordinator PI site. Coordination activities in Spain, back up for the UK when necessary. Assist with the development of project study plans and resource strategies, control of budget activities and mentoring of CRAs. Tool: IL 2 (Infolink 2. 0). Monthly metrics provided to the EU PM. Senior CRA (July 2004 until Feb 2007) • Local Phase III study in Oncology (Head &Neck Cancer) Logitest, S. L. (Jan 2001 until June 2004) CRA (Jan 2001 until June 2004) SODIBER Yoplait-Spain (Jan 2001 until June 2001) Laboratory Technician working on the quality control of products. Beltran y Camporredondo S. L. Spain (Oct 2000 until December 2000) Quality System implementation. Abelló Farmacia-Spain (Jan 2000 until Sep 2000) 8 months grant at the Quality &Assurance Department. Studies

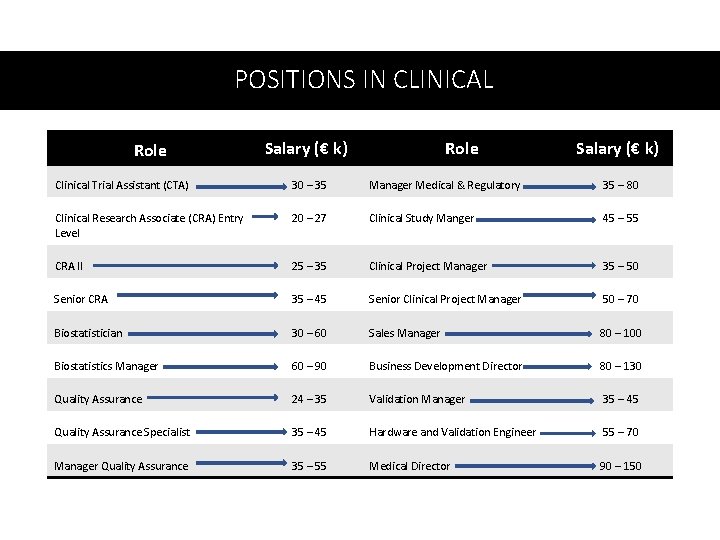
POSITIONS IN CLINICAL Role Salary (€ k) Clinical Trial Assistant (CTA) 30 – 35 Manager Medical & Regulatory 35 – 80 Clinical Research Associate (CRA) Entry Level 20 – 27 Clinical Study Manger 45 – 55 CRA II 25 – 35 Clinical Project Manager 35 – 50 Senior CRA 35 – 45 Senior Clinical Project Manager 50 – 70 Biostatistician 30 – 60 Sales Manager 80 – 100 Biostatistics Manager 60 – 90 Business Development Director 80 – 130 Quality Assurance 24 – 35 Validation Manager 35 – 45 Quality Assurance Specialist 35 – 45 Hardware and Validation Engineer 55 – 70 Manager Quality Assurance 35 – 55 Medical Director 90 – 150

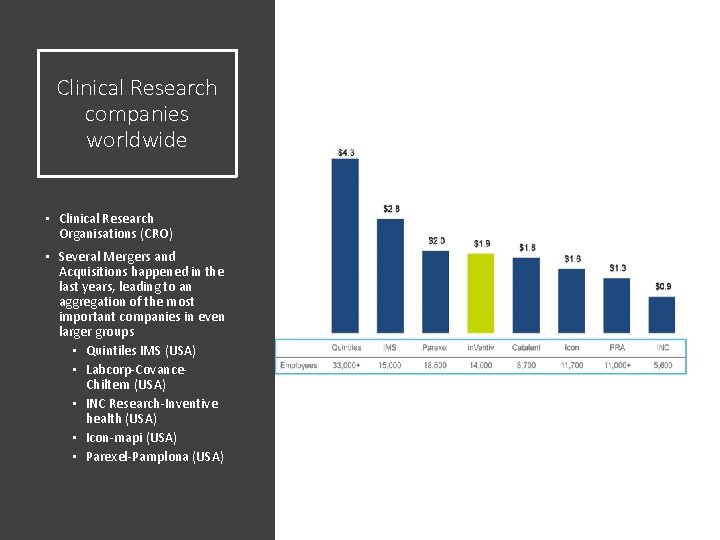
Clinical Research companies worldwide • Clinical Research Organisations (CRO) • Several Mergers and Acquisitions happened in the last years, leading to an aggregation of the most important companies in even larger groups • Quintiles IMS (USA) • Labcorp-Covance. Chiltern (USA) • INC Research-Inventive health (USA) • Icon-mapi (USA) • Parexel-Pamplona (USA)


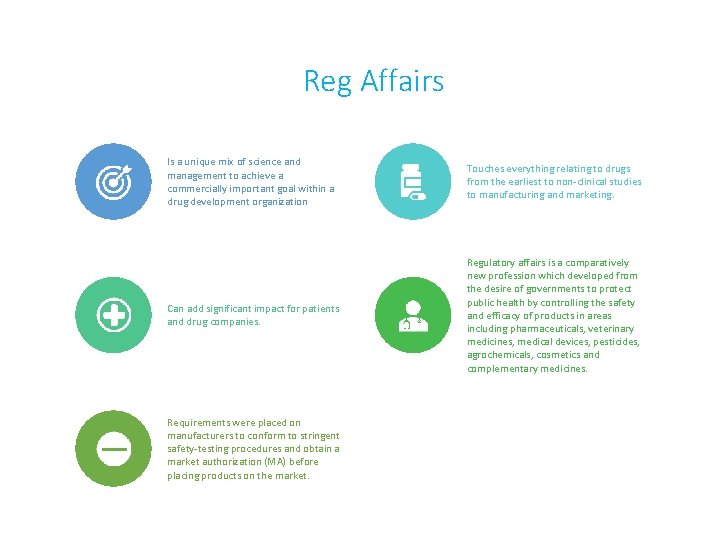
Reg Affairs Is a unique mix of science and management to achieve a commercially important goal within a drug development organization Touches everything relating to drugs from the earliest to non-clinical studies to manufacturing and marketing. Can add significant impact for patients and drug companies. Regulatory affairs is a comparatively new profession which developed from the desire of governments to protect public health by controlling the safety and efficacy of products in areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines. Requirements were placed on manufacturers to conform to stringent safety-testing procedures and obtain a market authorization (MA) before placing products on the market.

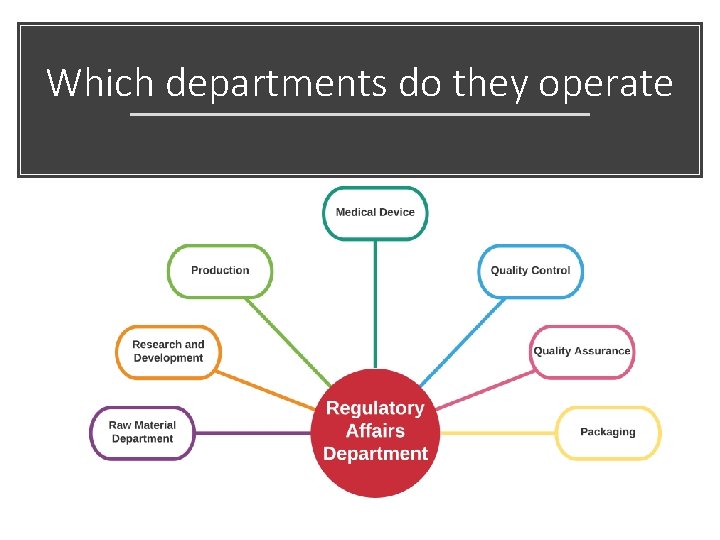
Which departments do they operate

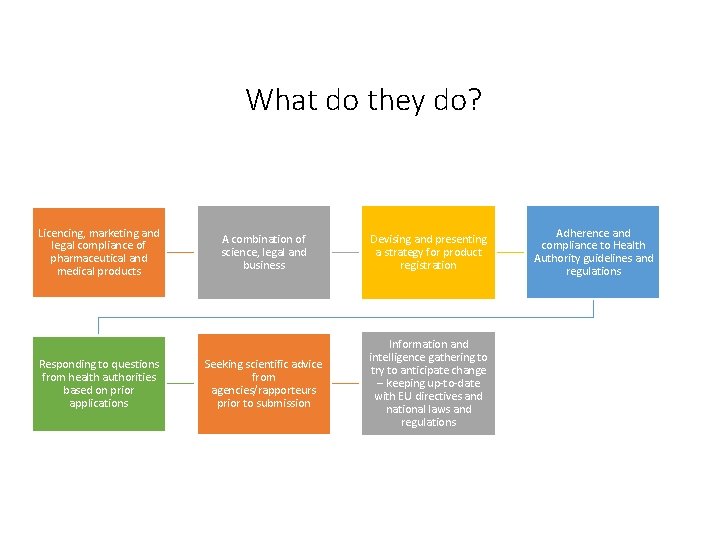
What do they do? Licencing, marketing and legal compliance of pharmaceutical and medical products Responding to questions from health authorities based on prior applications A combination of science, legal and business Devising and presenting a strategy for product registration Seeking scientific advice from agencies/rapporteurs prior to submission Information and intelligence gathering to try to anticipate change – keeping up-to-date with EU directives and national laws and regulations Adherence and compliance to Health Authority guidelines and regulations

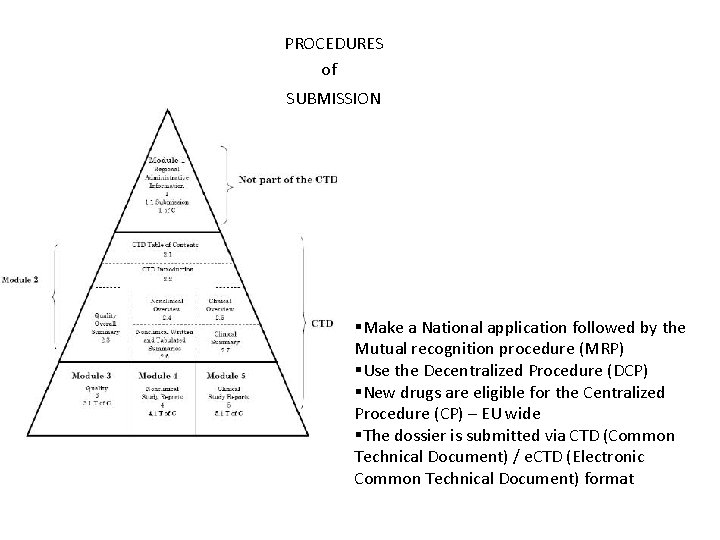
PROCEDURES of SUBMISSION §Make a National application followed by the Mutual recognition procedure (MRP) §Use the Decentralized Procedure (DCP) §New drugs are eligible for the Centralized Procedure (CP) – EU wide §The dossier is submitted via CTD (Common Technical Document) / e. CTD (Electronic Common Technical Document) format

Control

CV EXAMPLE

§ § § Career Development As someone progresses through a career in Reg they would be expected to learn about lifecycle management and start to combine the technical aspects of RA with project management and increased strategic thinking – as a guide this would usually take around 5/8 years’ of solid regulatory grounding. After a further 5 years’ an individual can expect to move there focus to a more tactical level; integrating their RA knowledge into strategic planning and business-critical activities. At the very highest level RA professionals are leaders and mentors – able to effectively communicate with regulators and internal / external policy makers – it is essential that you have these in spades at this level as it is unlikely you will reach this point if you don’t. • Title • Regulatory Associate • Senior Regulatory Associate £ 28, 000 - £ 45, 000 • Associate Regulatory Manager £ 40, 000 - £ 60, 000 • Regulatory Affairs Manager £ 50, 000 - £ 75, 000 + car + LTIP • Senior Regulatory Affairs Manager £ 65, 000 - £ 80, 000 + car + LTIP • Associate Director Regulatory Affairs £ 68, 000 – £ 90, 000 + car + LTIP • Director Regulatory Affairs £ 80, 000 - £ 115, 000 + car + LTIP • Senior Director Regulatory Affairs £ 120, 000 - £ 155, 000 + car + LTIP SALARY £ 20, 000£ 30, 000

THANK YOU • Juan Jose Muñoz