Clinical Research Definition Clinical research is research that










- Slides: 11

Clinical Research

Definition: § Clinical research is research that directly involves a particular person or group of people, or that uses materials from humans, such as their behaviour or samples of their tissue. A clinical trial is one type of clinical research that follows a predefined plan or protocol.

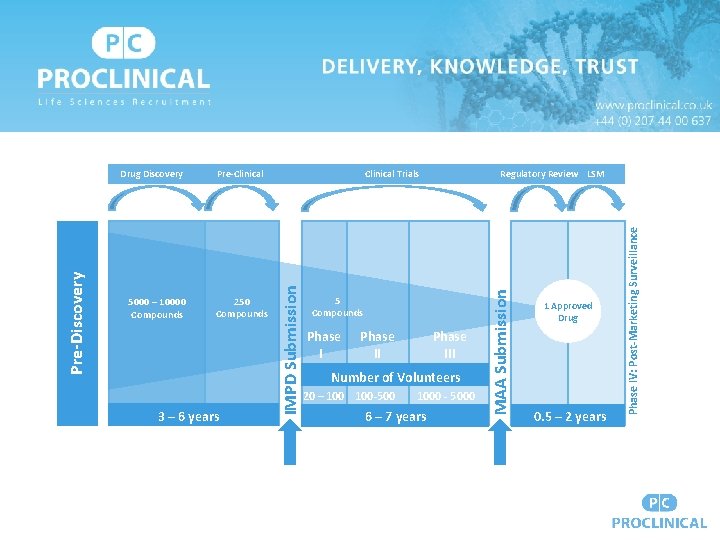
3 – 6 years Regulatory Review LSM 5 Compounds Phase III Number of Volunteers 20 – 100 Drug Development Lifecycle 100 -500 1000 - 5000 6 – 7 years 1 Approved Drug 0. 5 – 2 years Phase IV: Post-Marketing Surveillance 250 Compounds Clinical Trials MAA Submission 5000 – 10000 Compounds Pre-Clinical IMPD Submission Pre-Discovery Drug Discovery

Clinical Trials: Phase I • 20 – 100 healthy volunteers • Approx. 1. 5 years • To determine safety, tolerability, pharmacokinetics (how absorbed/metabolised and eliminated? ), side effects? • Conducted in an inpatient clinical trial clinic and closely monitored • Often 1 - 5% of the dose used in animal testing • Dose ranging, placebo controlled, randomised and double-blinded • Companies; Richmond Pharmacology, Eli Lily, Ono

Clinical Trials: Phase II • • • 2 years 100 – 500 volunteers Test effectiveness in treating targeted disease / medical disorder Determining dose that minimises adverse events Continuing safety Companies; Ono, AZ, Roche, PPD

Clinical Trials: Phase III • 3. 5 years, longest and most costly trial • 1000 – 5000 volunteers, statistically significant data about safety, efficacy and benefit / risk • Multiple sites, randomised, use of placebo • ID adverse drug reactions & determine dose for more diverse population • Plans for full scale production & manufacture • Companies; Takeda, Pfizer, Novo Nordisk, Amgen

Clinical Trials: Phase IV & Manufacturing • Post-Marketing Surveillance Trial, the license has already been granted • Monitoring of: • • Side effects Safety Long term risks and benefits How well it works • Periodic reporting to the authorities • Manufacturing – formulation, scale up and production of the medicine. • • May be requested by sponsor for competition / new market discovery purposes Companies; GSK (Consumer Healthcare), Biogen, Gilead

3 – 6 years Regulatory Review LSM 5 Compounds Phase III Number of Volunteers 20 – 100 -500 1000 - 5000 6 – 7 years 1 Approved Drug 0. 5 – 2 years Phase IV: Post-Marketing Surveillance 250 Compounds Clinical Trials MAA Submission 5000 – 10000 Compounds Pre-Clinical IMPD Submission Pre-Discovery Drug Discovery

Types of Roles § Clinical Trial Administrator (CTA), Clinical Trial Coordinator (CTC), Clinical Admin Associate § Clinical Research Associate (CRA), Clinical Research Scientist § Clinical Study Manager, Global Study Manager, Clinical Project Manager, Clinical Operations Lead, Clinical Country Lead § Associate Clinical Director, Clinical Project Director, Senior Study Management Director § Senior Director, Vice President (VP)

Questions?

 Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block xoang nhĩ là gì
Block xoang nhĩ là gì Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Importance of biostatistics
Importance of biostatistics Socra clinical research
Socra clinical research