FDA Regulation of Pharmaceuticals and Devices FDA Organizational

































- Slides: 44

FDA Regulation of Pharmaceuticals and Devices

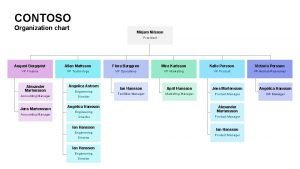
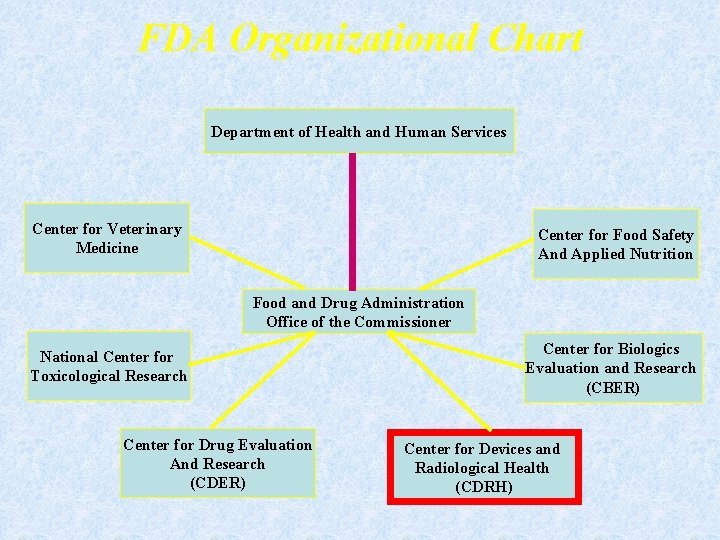
FDA Organizational Chart Department of Health and Human Services Center for Veterinary Medicine Center for Food Safety And Applied Nutrition Food and Drug Administration Office of the Commissioner National Center for Toxicological Research Center for Drug Evaluation And Research (CDER) Center for Biologics Evaluation and Research (CBER) Center for Devices and Radiological Health (CDRH)

CDRH Mission: Ensure medical devices are safe and effective via premarket and postmarket evaluation • guide manufacturers in product development • evaluate data submitted on device design, performance, and clinical use • authorize marketing of devices found safe and effective • ensure that claims are supported by valid scientific evidence • focus special emphasis on medical breakthrough devices (expedited review)

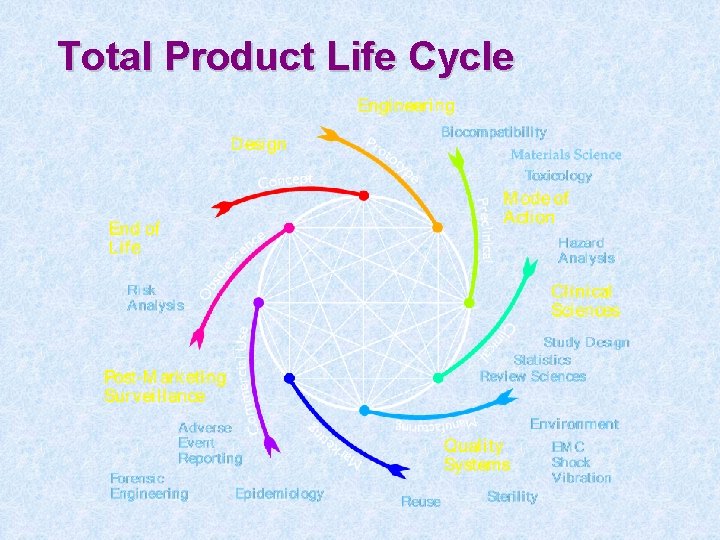
Total Product Life Cycle Coated Stents Brachytherapy

Total Product Life Cycle

FDA • FDA regulations found in Title 21, Code of Federal Regulations – 21 CFR • Regulate products • Coverage includes – but not limited to: – – – – Nonclinical studies Clinical studies Human Subject Protection Institutional Review Boards (IRBs) Manufacturing Labeling Post-market adverse event reporting

FDA • FDA regulations “speak” to: – Importers/exporters – Study sponsors – Nonclinical laboratory personnel – Clinical investigators – IRBs – Medical product users – hospitals, clinics, nursing homes, individual practitioners

Pharmaceuticals versus devices • Pharmaceuticals (drugs and biologics) are covered by different FDA regulations from those covering devices, though some regulations are shared • Many differences result from differences among the products themselves

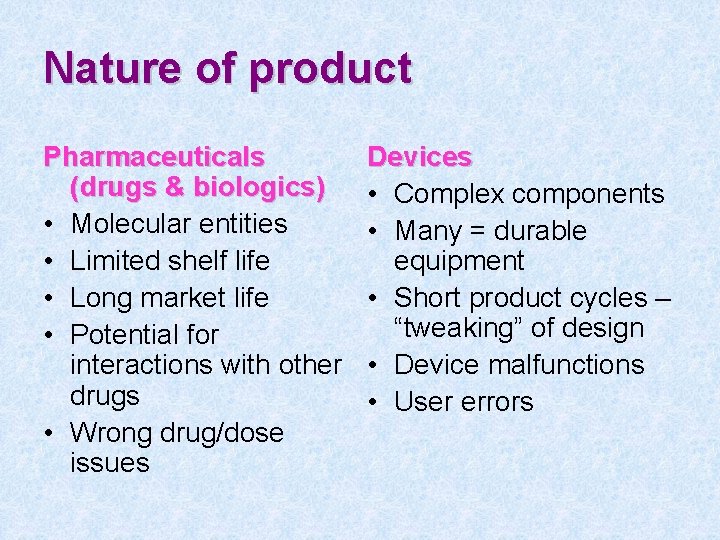
Nature of product Pharmaceuticals (drugs & biologics) • Molecular entities • Limited shelf life • Long market life • Potential for interactions with other drugs • Wrong drug/dose issues Devices • Complex components • Many = durable equipment • Short product cycles – “tweaking” of design • Device malfunctions • User errors

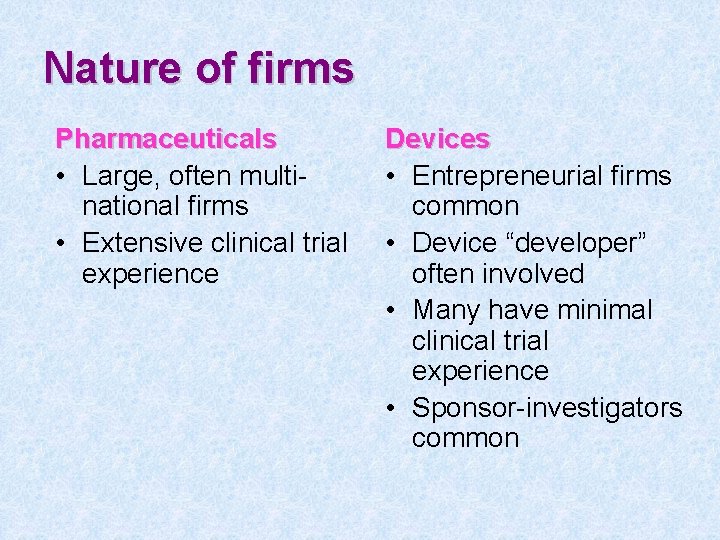
Nature of firms Pharmaceuticals • Large, often multinational firms • Extensive clinical trial experience Devices • Entrepreneurial firms common • Device “developer” often involved • Many have minimal clinical trial experience • Sponsor-investigators common

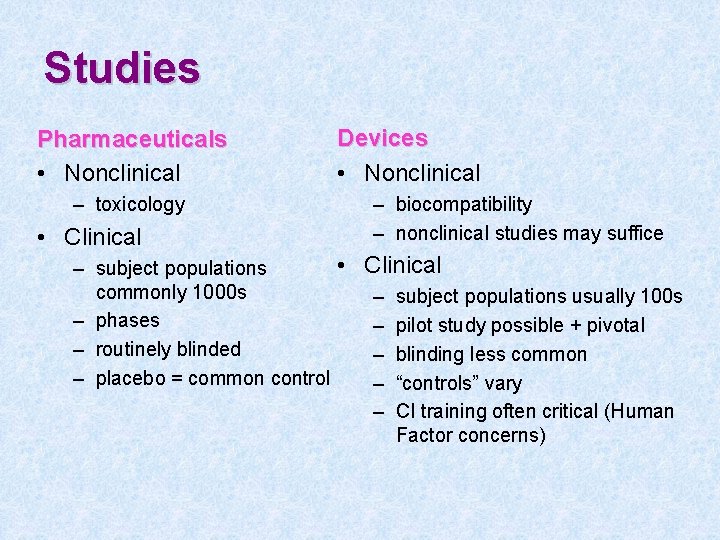
Studies Pharmaceuticals • Nonclinical – toxicology • Clinical Devices • Nonclinical – biocompatibility – nonclinical studies may suffice • Clinical – subject populations commonly 1000 s – subject populations usually 100 s – phases – pilot study possible + pivotal – routinely blinded – blinding less common – placebo = common control – “controls” vary – CI training often critical (Human Factor concerns)

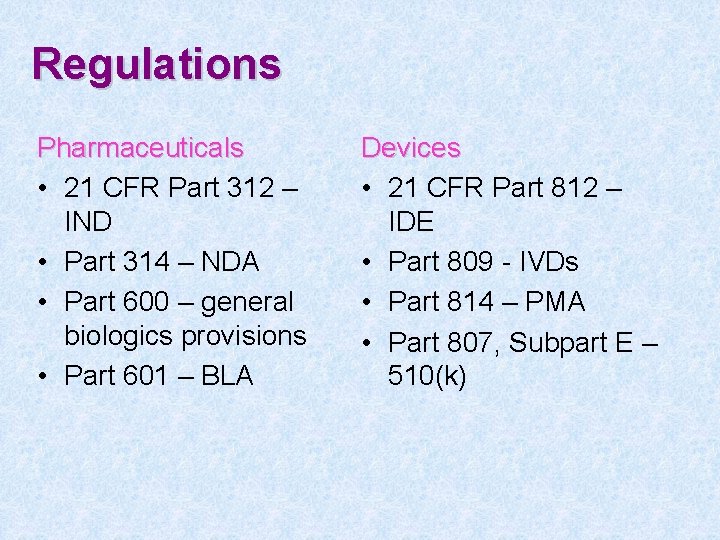
Regulations Pharmaceuticals • 21 CFR Part 312 – IND • Part 314 – NDA • Part 600 – general biologics provisions • Part 601 – BLA Devices • 21 CFR Part 812 – IDE • Part 809 - IVDs • Part 814 – PMA • Part 807, Subpart E – 510(k)

Clinical Investigators -1 • Common responsibilities across products: – Personally conduct or supervise the study – Ensure site study team is properly trained – Follow FDA regulations regarding HSP, including obtaining and maintaining IRB approval and obtaining subject informed consent – Follow the approved investigational plan/protocol

Clinical Investigators -2 • CI responsibilities (cont. ): – Maintain adequate, complete, and accurate study records – Submit all required reports (e. g. , IND safety reports, study progress reports) – Maintain control of the investigational product

Sponsors -1 • Common responsibilities across products: – Obtain FDA approval, where necessary, before study initiation – Manufacture and label investigational products appropriately – Initiate, withhold, or discontinue clinical trials as required – Refrain from commercialization of investigational products – Maintain control of the investigational product

Sponsors -2 • Sponsor responsibilities (cont): – Select qualified investigators and disseminate appropriate information to them – Select qualified monitors and ensure the study is adequately monitored – Evaluate and report adverse experiences – Maintain adequate records – Submit progress and final reports

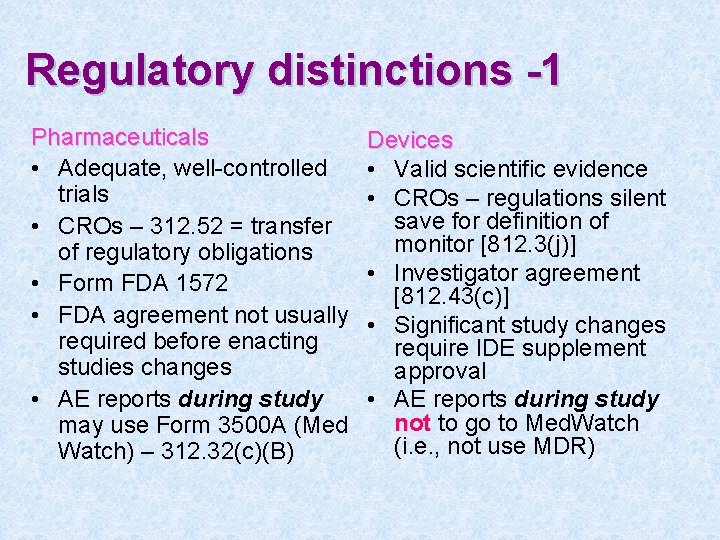
Regulatory distinctions -1 Pharmaceuticals • Adequate, well-controlled trials • CROs – 312. 52 = transfer of regulatory obligations • Form FDA 1572 • FDA agreement not usually required before enacting studies changes • AE reports during study may use Form 3500 A (Med Watch) – 312. 32(c)(B) Devices • Valid scientific evidence • CROs – regulations silent save for definition of monitor [812. 3(j)] • Investigator agreement [812. 43(c)] • Significant study changes require IDE supplement approval • AE reports during study not to go to Med. Watch (i. e. , not use MDR)

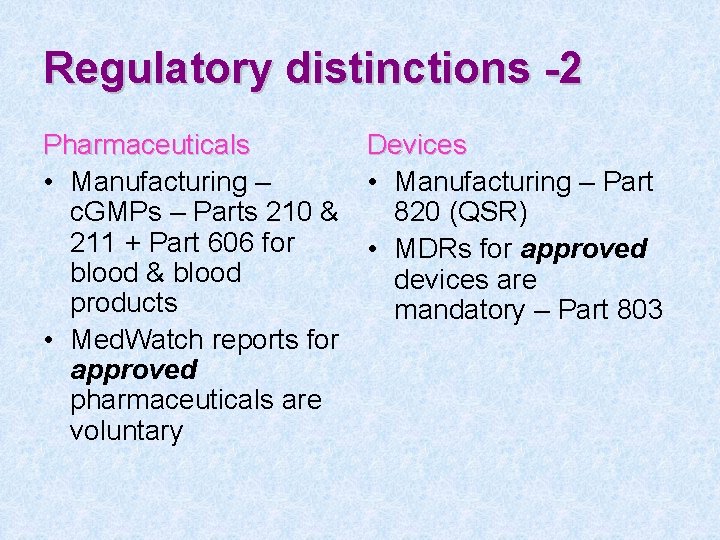
Regulatory distinctions -2 Pharmaceuticals Devices • Manufacturing – Part c. GMPs – Parts 210 & 820 (QSR) 211 + Part 606 for • MDRs for approved blood & blood devices are products mandatory – Part 803 • Med. Watch reports for approved pharmaceuticals are voluntary

Additional Device Distinctions -1 • Classes of Devices – risk-based determination – 21 CFR 860 – classification procedures – 21 CFR 862 through 892 – specific device classifications by product type

Additional Device Distinctions -2 • Cleared devices – 510(k) – 21 CFR 807, subpart E – Premarket Notification Procedures – “substantially equivalent” • Approved devices – 21 CFR Part 814 – PMA, PDP, HDE – Safety and effectiveness – PMA & PDP – Safety – HDE

Additional Device Distinctions -3 • Significant risk/non-significant risk studies • Exempt studies/in vitro diagnostics (IVDs) • Protocol changes and 5 -day notices

Significant Risk (SR) • Regulatory definition (21 CFR 812. 3(m)) – device that presents potential for serious risk to health, safety, or welfare of a subject, particularly if it • Is intended as an implant • Is purported or represented for use in supporting or sustaining life • Is for a use of substantial importance in diagnosing, curing, mitigating, or treating disease, or otherwise preventing impairment of human health

Non-Significant Risk (NSR) • • Decision based on use of device in study Sponsor makes initial assessment IRB makes determination FDA can disagree If NSR study, no IDE application to FDA Informed consent required Abbreviated requirements apply (21 CFR 812. 2(b)) • Considered to have an IDE

In Vitro Diagnostics (IVDs) • SR/NSR/exempt studies • Exempt if: • labeled according to 21 CFR 809. 10 • noninvasive sampling or no significant risk • does not introduce energy into a subject • not used as the diagnostic for determination of treatment

Significant Risk IVD Studies • If study involves invasive sampling that presents a significant risk • If results from use of an investigational IVD will determine treatment, could inaccurate results: - be life-threatening - result in permanent functional impairment - result in permanent structural damage - necessitate medical or surgical intervention to prevent impairment or damage

Clinical Investigators • Compliance inspection program covers study specific inspections and audits of CIs (physicians, veterinarians, others) conducting clinical trials on human and veterinary products • Usually preannounced • Inspection includes an interview with the clinical investigator and pertinent study staff + an in-depth study/data audit – to validate study findings and verify compliance with regulations

Most Common CI Deficiencies • • Failure to follow the investigational plan Protocol deviations Inadequate recordkeeping Inadequate accountability for the investigational product • Inadequate subject protection – including informed consent issues

Administrative/regulatory options • Untitled or Warning letter • Initiation of disqualification procedures • Sharing information with Office of Criminal Investigations (OCI) for pursuit of prosecution • Recommendation for rejection of site/study data

Institutional Review Boards (IRBs) • Board, committee, or other group formally designated by an institution to – review – approve the initiation of – conduct periodic review of research involving human subjects • Primary purpose of review = ensure protection of rights, safety, and welfare of the human subjects

Applicable regulations • 21 CFR Part 50 – Protection of Human Subjects – contains informed consent requirements • 21 CFR Part 56 – Institutional Review Boards – includes specifics of IRB’s makeup and duties

IRB Inspections • Compliance program provides for regularly scheduled inspections to verify compliance with regulations • Objective is protection of human subjects rather than data validation • Inspections – usually preannounced – consist of • interviews with responsible IRB staff • in-depth review of SOPs, files, and records • review of active studies to assess IRB operations

Most common IRB deficiencies • • Inadequate initial and/or continuing review Inadequate SOPs Inadequate membership rosters Inadequate meeting minutes Specific to devices – lack of or incorrect SR/NSR determination

Administrative/regulatory options • Untitled or Warning letter • Restriction of functions – prohibiting increase of subject population in on -going FDA-regulated studies – prohibiting review of new FDA-regulated studies • Initiation of disqualification procedures

Sponsors/CROs/Monitors • Compliance program – covers parties responsible for initiating and overseeing research and for submitting research results to FDA – lists sponsor responsibilities • Inspections – usually preannounced – consist of interviews and audits of study records – objective is to both evaluate compliance with regulations and validate data – commonly assigned for NDAs for new molecular entities (NMEs) and for PMAs

Most common S/M deficiencies • Inadequate monitoring • Failure to bring investigators into compliance • Inadequate accountability for the investigational product

Administrative/regulatory options • Untitled or Warning letter • Invocation of the Application Integrity Policy (AIP) • Refusal to accept site or study data • Denial of NDA/BLA/PMA • Sharing information with Office of Criminal Investigations (OCI) for pursuit of prosecution

Bioequivalence (BEQ) studies • Primarily support – Abbreviated drug applications (ANDA) for generic drugs – Applications for new form or formulation of marketed drugs • Compliance program – Provides for inspection of both clinical facilities and analytical laboratories involved with BEQ studies – Focuses on inspecting • • • New facilities Previously violative sites Suspicious data Non-conventional studies Studies pivotal to NDA decision-making

Resources - 1 • GCP website – http: //www. fda. gov/oc/gcp/ – Links include • pertinent regulations and guidance • FDA contacts • related sites with HSP/GCP information • Recent documents of interest relate to – – – Data monitoring committees Use of a centralized IRB AE reporting CI supervisory responsibilities Computerized systems in clinical trials

Resources - 2 • GCP queries e-mail account (about 1, 200 queries answered per year) – gcp. questions@fda. hhs. gov • Previous answers captured – http: //www. fda. gov/oc/gcp/redacted. Emails/default. htm • Listserve – via GCP website – notice of updates on FDA’s GCP/HSP activities • Site maintained by Good Clinical Practice Program (GCPP)

Acronyms -1 • • 510(k) – premarket notification AE – adverse event (or effect) AIP – Application Integrity Policy BEQ – bioequivalence BIMO – Bioresearch Monitoring BLA – biologics license application CBER – Center for Biologics Evaluation and Research • CDER – Center for Drug Evaluation and Research

Acronyms -2 • CDRH – Center for Devices and Radiological Health • CFR – Code of Federal Regulations • CI – clinical investigator • c. GMPs – current good manufacturing practices • CRO – contract research organization • DBM – Division of Bioresearch Monitoring • DSI – Division of Scientific Investigations • DQ – disqualification

Acronyms -3 • EIR – establishment inspection report • FDAMA – Food and Drug Administration Modernization Act (1997) • GCP – Good Clinical Practice • GCPP – Good Clinical Practice Program • HDE – humanitarian device exemption • HSP – human subject protection • HQ – headquarters • IDE – investigational device exemption • IND – investigational new drug

Acronyms -4 • • • IRB – institutional review board IVD – in vitro diagnostic MDR – medical device report NAI – no action indicated NDA – new drug application NME – new molecular entity NSR – non-significant risk OAI – official action indicated OHRP – Office of Human Research Protections

Acronyms -5 • OIVD – Office of In Vitro Diagnostic Device Evaluation and Safety • ORA – Office of Regulatory Affairs • PDP – product development protocol • PMA – premarket approval • QSR – quality system regulation • SOPs – standard operating procedures • SR – significant risk • VAI – voluntary action indicated
 Michael marcarelli
Michael marcarelli Irbmakeup
Irbmakeup Sbir framework
Sbir framework Nikita atal
Nikita atal Contoso pharmaceuticals powerpoint
Contoso pharmaceuticals powerpoint Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals powerpoint
Contoso pharmaceuticals powerpoint August bergqvist
August bergqvist Ambalal sarabhai products list
Ambalal sarabhai products list Vddi pharmaceuticals
Vddi pharmaceuticals Samy shanmugam
Samy shanmugam Newbury pharmaceuticals keskustelu
Newbury pharmaceuticals keskustelu Contoso pharmaceuticals
Contoso pharmaceuticals Mccabes pharmacy discount code
Mccabes pharmacy discount code Lavet pharmaceuticals
Lavet pharmaceuticals Vertex pharmaceuticals canada
Vertex pharmaceuticals canada Phony pharmaceuticals speech
Phony pharmaceuticals speech Levolta pharmaceuticals
Levolta pharmaceuticals Tamper resistant packaging definition
Tamper resistant packaging definition Report pharma fraud
Report pharma fraud Contoso ltd
Contoso ltd Vddi pharmaceuticals
Vddi pharmaceuticals What are devices in literature
What are devices in literature Output and input devices of computer
Output and input devices of computer Fda v brown and williamson
Fda v brown and williamson Thermoreceptor
Thermoreceptor Section 4 gene regulation and mutation
Section 4 gene regulation and mutation Table tennis regulations
Table tennis regulations Thermoregulation
Thermoregulation How there is recyclability and self regulation in nature
How there is recyclability and self regulation in nature Regulation of glycolysis and gluconeogenesis
Regulation of glycolysis and gluconeogenesis 5 roles of an nco
5 roles of an nco Regulation of recruitment and placement activities
Regulation of recruitment and placement activities Chapter 12 section 4 gene regulation and mutations
Chapter 12 section 4 gene regulation and mutations Table tennis rules and regulation
Table tennis rules and regulation Regulation and inspection of social care wales
Regulation and inspection of social care wales Government regulation and the legal environment of business
Government regulation and the legal environment of business Hines hill train crash
Hines hill train crash Michigan department of licensing and regulatory affairs
Michigan department of licensing and regulatory affairs Licensing and regulation division
Licensing and regulation division Osha hazard and ghs training regulation cfr 1910
Osha hazard and ghs training regulation cfr 1910 Ground water management and regulation scheme
Ground water management and regulation scheme Blood bank regulation under drugs and cosmetics act
Blood bank regulation under drugs and cosmetics act