Unique Device Identification UDI Implementation and Adoption Leslie




- Slides: 7

Unique Device Identification (UDI): Implementation and Adoption Leslie M Tompkins, Ph. D FDA, Center for Devices and Radiological Health UDI Lead, Standards and Vocabularies HL 7 Working Group Meeting, Phoenix May 2014

Basics of the UDI Rule • 21 CFR 801. 20 – …the label of every medical device shall bear a unique device identifier (UDI)… • 21 CFR 830. 300 – …the labeler of a device must provide the information required … for each model or version required to bear a unique device identifier (UDI)… 2

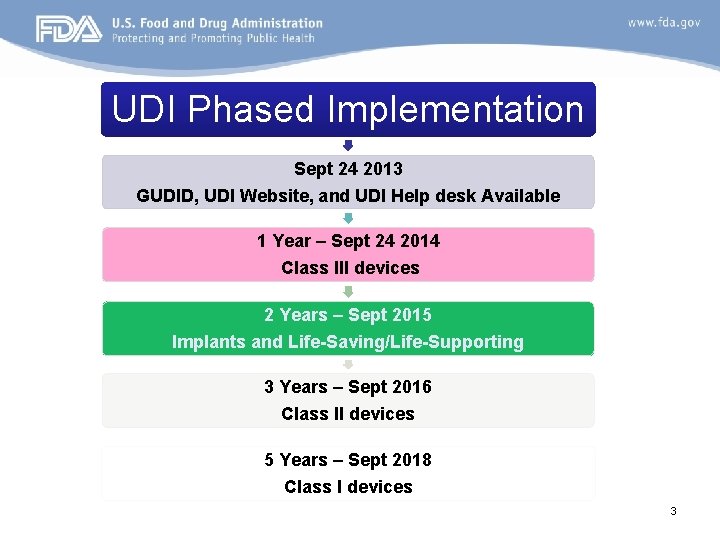
UDI Phased Implementation Sept 24 2013 GUDID, UDI Website, and UDI Help desk Available 1 Year – Sept 24 2014 Class III devices 2 Years – Sept 2015 Implants and Life-Saving/Life-Supporting 3 Years – Sept 2016 Class II devices 5 Years – Sept 2018 Class I devices 3

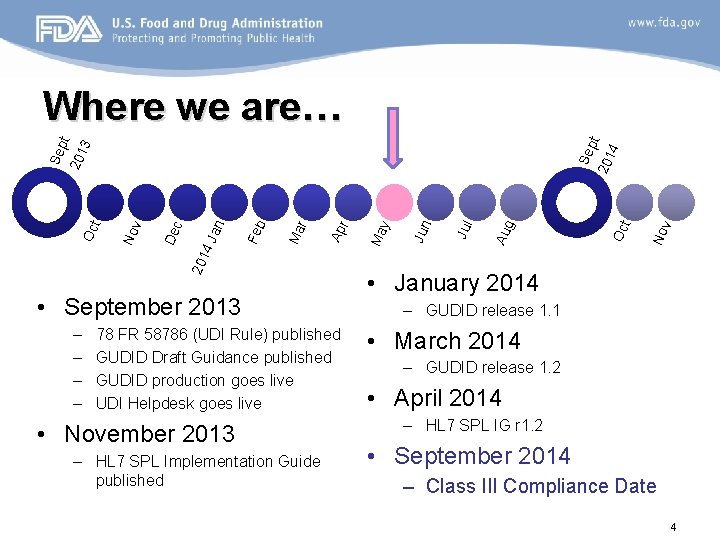
• September 2013 – – 78 FR 58786 (UDI Rule) published GUDID Draft Guidance published GUDID production goes live UDI Helpdesk goes live • November 2013 – HL 7 SPL Implementation Guide published 4 No v t Oc g Au Jul Jun y Ma r Ap r Ma Feb Jan 201 4 De c No v Oc t 201 Se pt 201 3 Where we are… • January 2014 – GUDID release 1. 1 • March 2014 – GUDID release 1. 2 • April 2014 – HL 7 SPL IG r 1. 2 • September 2014 – Class III Compliance Date 4

Where are we… • Helpdesk • GUDID – >1400 questions received – Answered: • 711 Regulatory • 599 Technical • 15 Data Quality • www. fda. gov/udi – 80 records submitted (unpublished state) – Dozens of draft records • Accounts – 71 production – 32 pre-production (SPL testing) – 10 Third-party 5

Adoption of UDI into Health IT • Harmonization of UDI representation across HL 7 documents and messages • NPRM 2015 Certification Criteria for EHRs • PROPOSED: Meaningful Use, Stage 3 • …should record the FDA Unique Device Identifier (UDI) when patients have devices implanted for each newly implanted device. 6

UDI Resources UDI website - www. fda. gov/UDI – UDI Help Desk – Sign up for UDI alerts – GUDID Draft Guidance – Appendix B – Vocabulary – Appendix C - UDI Specifications by Issuing Agency Strengthening our National System for Medical Device Postmarket Surveillance http: //www. fda. gov/downloads/Medical. Devices/Safety/CDRHPostmarket. Sur veillance/UCM 348845. pdf HL 7 UDI Task Force http: //hl 7 tsc. org/wiki/index. php? title=2013 -11 -21_TSC_UDI_Task_Force 7