Quality Compliance In FDAregulated Industries Good Documentation Practices



















- Slides: 36

Quality & Compliance In FDA-regulated Industries Good Documentation Practices

Good Documentation Practice • What are they? • Standards by which documents are created and maintained. • Not codified by FDA, but are considered c. GMP. • While not law: • Authorities will inspect against these guidelines and c. GMP expectations. • Make observations if not in place. 2

Good Documentation Practice • Why are they needed? • To standardize the documentation practices throughout the industry. • To increase and assure the accuracy, quality and integrity of data, documents and records. 3

Good Documentation Practice • Who needs to implement them? • EVERYONE! Cleaning Logs Training Records • ALL POSITIONS! Correspondence 4

Good Documentation Practice • What are the standards? • Creation of New Documents • Review and Approval of Documents • Completion of Documents - Data Entry • Reproduction of Documents - Copying • Maintenance of Documents • Modification of Documents - Change 5

Good Documentation Practice • What are the standards? • Creation of New Documents: • Contemporaneous (Timely) • Typed (Not Handwritten) • Checked for Accuracy (Error-free) • Formatted for Trend Evaluation • Adequate space for data entries • For SOPs, include: • Process Flow Diagram • RACI Matrix (Responsible, Accountable, Consulted, Informed)6

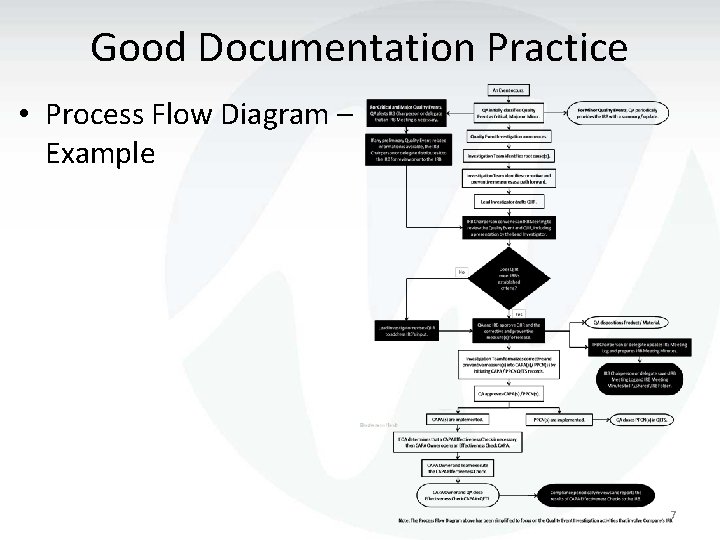
Good Documentation Practice • Process Flow Diagram – Example 7

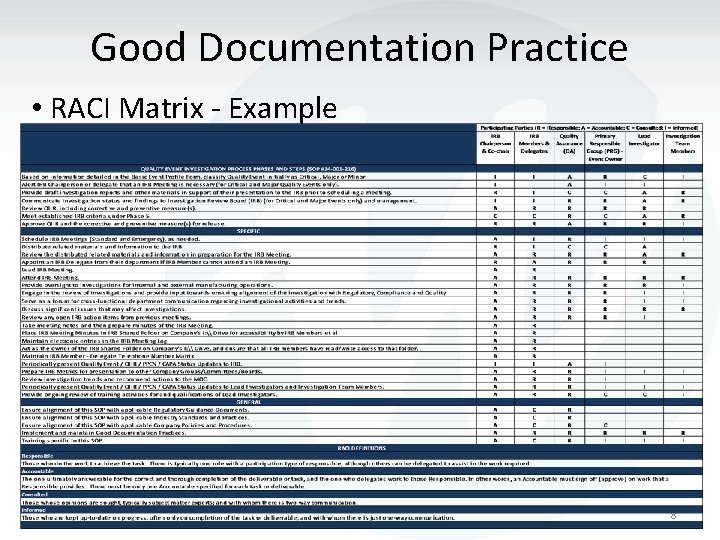
Good Documentation Practice • RACI Matrix - Example 8

Good Documentation Practice • What are the standards? • Review and Approval of Documents • Reviewed by appropriate and authorized personnel • Approved by appropriate and authorized personnel • Signed • Dated 9

Good Documentation Practice • What are the standards? • Completion of Documents - Data Entry – The Dos) • Make entries in permanent/indelible Ink (NOT RED) • Make entries Concise, Accurate and Legible • Enter data in English (unless otherwise directed) • For Critical Entries, have a second authorized person independently verify activity/entry • Number the pages to be added: • Actual Page # followed by Total Page # • Follow company’s format for Date and Time: • Format cannot have numerous different meanings 10

Good Documentation Practice • What are the standards? • Completion of Documents - Data Entry – The Dos) • Follow company’s format for Initials and Signatures • Signatures and initials are serve as an employee’s confirmation that the activity performed was per the appropriate SOP or Work Instruction. • Signatures and initials have company-specific, legal, and/or ethical ramifications. 11

Good Documentation Practice • What are the standards? • Completion of Documents - Data Entry – The Dos) • For incorrect data entries: • Use a single line-out • Leave original date/entry legible • Sign or initial • Date • At the time that the correction is made • Include justification of the correction • Concise and Accurate 12

Good Documentation Practice • What are the standards? • Completion of Documents – Data Entry – The Don’ts • Make entries on the reverse side of the document • Unless a note is written on the front page • Leave spaces blank • If unused, cross-out or enter "N/A" (or similar text) • Use pencils! • Entries must be in indelible ink • Use ditto marks (‘’) • Use continuation lines • Use signature stamps 13

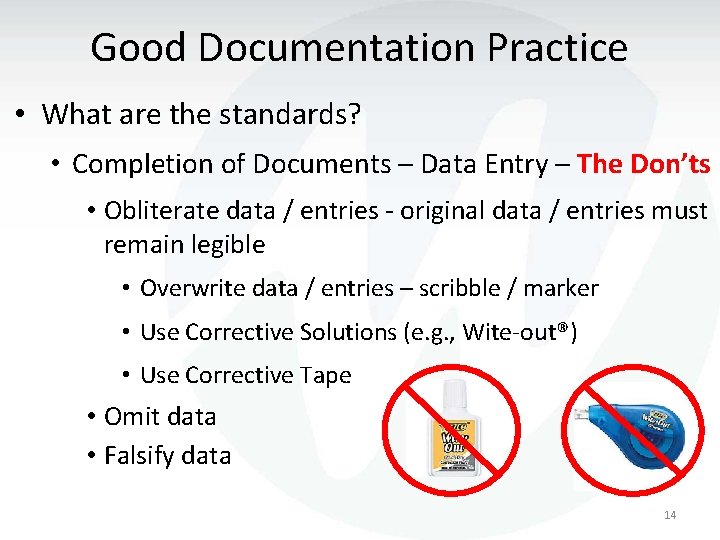
Good Documentation Practice • What are the standards? • Completion of Documents – Data Entry – The Don’ts • Obliterate data / entries - original data / entries must remain legible • Overwrite data / entries – scribble / marker • Use Corrective Solutions (e. g. , Wite-out®) • Use Corrective Tape • Omit data • Falsify data 14

Good Documentation Practice • What are the standards? • Completion of Documents – Data Entry – The Don’ts • Back date • Use current date and include an explanation of the delay • Destroy or remove pages – obscures data that were present • To invalidate a page, use a single line cross-out, then initial and date it. 15

Good Documentation Practice • What are the standards? • Completion of Documents – Data Entry – The Don’ts • Transcribe Data – obscures original data • Data Transcription may prove helpful if original is of poor quality writing or is physically damaged • If used, should be clearly marked as a transcription and the original retained. • Use Scrap Paper or Post-it® Notes to record raw data • Temporary, non-official record 16

Good Documentation Practice • What are the standards? • Completion of Documents – Data Entry – The Don’ts • Use asterisks (*) where there is insufficient space for a fully notated hand change • Use a number near the correction, and record the same number along with a notation where this is sufficient space. • Reduces the risk that an additional change made by another person will not use the same mark. • Sign / initial for another person • Can sign of behalf of the other person, but must identify this along with an explanation. 17

Good Documentation Practice • What are the standards? • Reproduction of Documents – Copying • Clear and Legible • No Errors are introduced • Copy must remain identical to the original • Watermarks are sometimes used to identify original document from copies. T F A DR 18

Good Documentation Practice • What are the standards? • Maintenance of Documents • Periodically Reviewed to Keep Current • Securely Retained • Change Control Program • Controlled Availability for Appropriate Duration • Electronic Document Management Systems (EDMS) must be Validated • Electronic Records are Backed-up and Archived 19

Good Documentation Practice • What are the standards? • Modification of Documents – Change Handwritten modifications – signed/ dated Altered text is not to be obscured Justification for alteration must be noted Controls to prevent use of superseded documents Versions can only be modified by authorized personnel Access to electronic versions must be controlled by password or other means • A history (audit trail) must be maintained of changes and deletions to electronic versions • • • 20

Good Documentation Practice • Data • Significant Figures: All non-zero digits are significant, all zeros to the left of a nonzero digit are not significant, and a decimal point has no bearing on what numbers are significant. • The number of significant figures in the final result is determined by the significant figures of the original numbers. 21

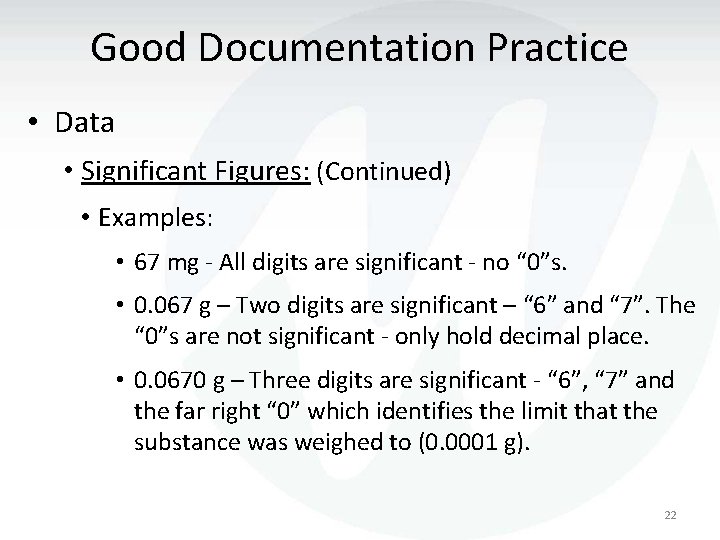
Good Documentation Practice • Data • Significant Figures: (Continued) • Examples: • 67 mg - All digits are significant - no “ 0”s. • 0. 067 g – Two digits are significant – “ 6” and “ 7”. The “ 0”s are not significant - only hold decimal place. • 0. 0670 g – Three digits are significant - “ 6”, “ 7” and the far right “ 0” which identifies the limit that the substance was weighed to (0. 0001 g). 22

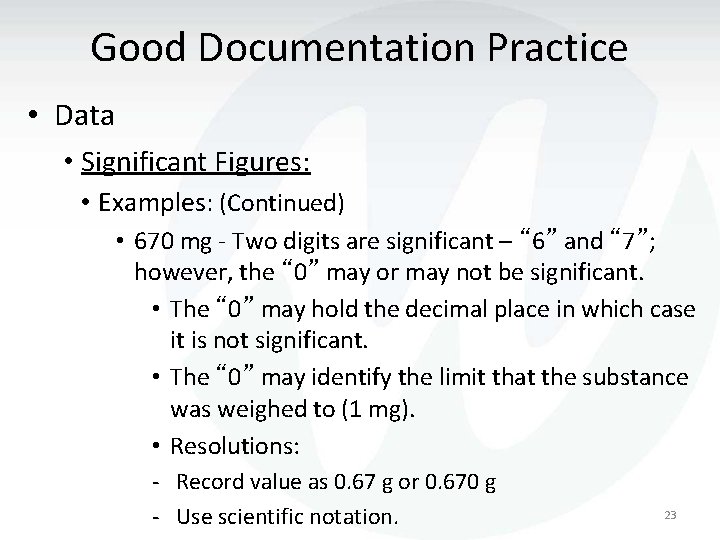
Good Documentation Practice • Data • Significant Figures: • Examples: (Continued) • 670 mg - Two digits are significant – “ 6” and “ 7”; however, the “ 0” may or may not be significant. • The “ 0” may hold the decimal place in which case it is not significant. • The “ 0” may identify the limit that the substance was weighed to (1 mg). • Resolutions: - Record value as 0. 67 g or 0. 670 g - Use scientific notation. 23

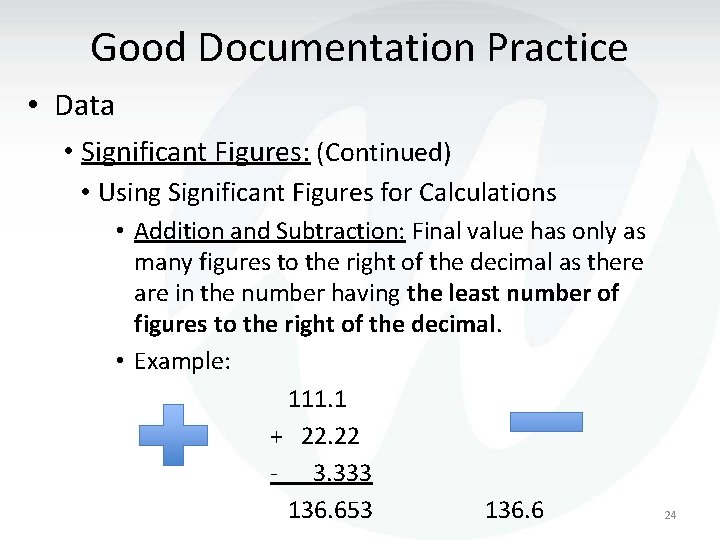
Good Documentation Practice • Data • Significant Figures: (Continued) • Using Significant Figures for Calculations • Addition and Subtraction: Final value has only as many figures to the right of the decimal as there are in the number having the least number of figures to the right of the decimal. • Example: 111. 1 + 22. 22 - 3. 333 136. 653 136. 6 24

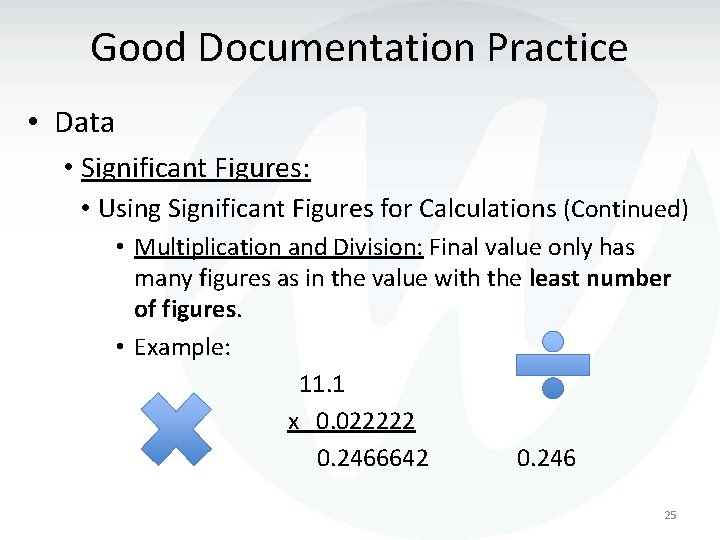
Good Documentation Practice • Data • Significant Figures: • Using Significant Figures for Calculations (Continued) • Multiplication and Division: Final value only has many figures as in the value with the least number of figures. • Example: 11. 1 x 0. 022222 0. 2466642 0. 246 25

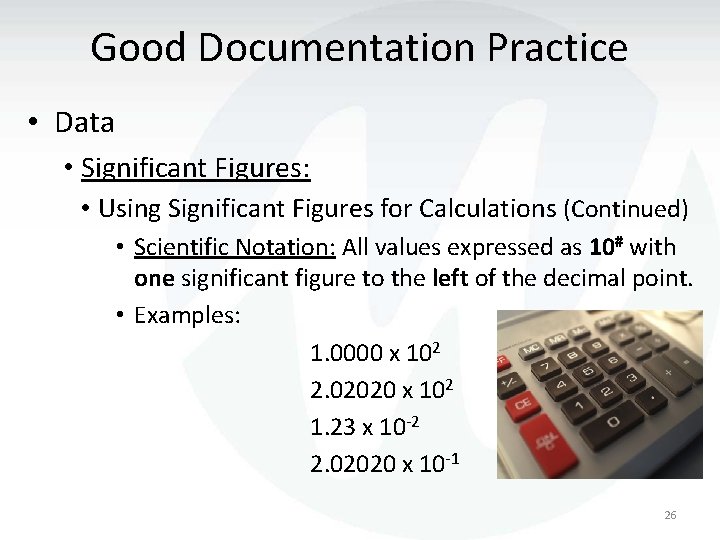
Good Documentation Practice • Data • Significant Figures: • Using Significant Figures for Calculations (Continued) • Scientific Notation: All values expressed as 10# with one significant figure to the left of the decimal point. • Examples: 1. 0000 x 102 2. 02020 x 102 1. 23 x 10 -2 2. 02020 x 10 -1 26

Good Documentation Practice • Data (Continued) • Rounding-off: Process by which one reduces the number of figures in a value. 27

Good Documentation Practice • Data • Rounding-off: (Continued) • Focus on the digit to the right of the last place in the limit expression. • If this digit is < 5, it is eliminated and the preceding digit is unchanged. • If the digit is > 5, it is eliminated and the preceding digit is increased by one. • If this digit = 5, the 5 is eliminated and the preceding digit is increased by one. • For calculations, do not round values until final result is calculated. 28

Good Documentation Practice • Data (Continued) • Truncation: Reduces the number of figures in a value by deleting the unnecessary figures. • No other change is made to the remaining number. • Unlike Rounding-off, only the last several digits in a numerical string are dropped. 29

Good Documentation Practice • Data • Truncation: (Continued) • Consider the significance of digits to the final result. • Do not truncate values until the final result is calculated. • Example: Values not truncated prior to calculation: 456. 789 x 0. 1234 = 56. 36 Values truncated prior to calculation: 456 x 0. 1 = 45. 6 30

CONFIDENTIALITY • What’s the Big Deal? • Company’s Code of Ethics • Adopted by companies to enable employees to: • Understand difference between 'right' and 'wrong' • Apply understanding to their business decisions. • Implies documents at three (3) levels: • Codes of Business Ethics • Codes of Conduct for Employees • Codes of Professional Practice 31

CONFIDENTIALITY • What’s the Big Deal? • Codes of Professional Practice (Continued) • Honesty • Integrity • Transparency • Accountability • Confidentiality • Objectivity • Respectfulness • Obedience to the Law 32

CONFIDENTIALITY • What’s the Big Deal? • Codes of Professional Practice (Continued) • Confidentiality - "confidential information" • Trade Secret: • A formula, practice, process, design, instrument, pattern or compilation of information • Not generally known or reasonably ascertainable • Require reasonable measures to protect the information • Enables a company to obtain an economic advantage over competitors 33

CONFIDENTIALITY • What’s the Big Deal? • Trade Secret (Continued) • Impact: • Intellectual Property: • Copyright • Patent • Trademark • Time To Market • Competitive Advantage • MONEY! 34

Summary • GDPs are considered c. GMP. • All employees must follow GDPs. • Original data and entries must not be obliterated; must remain legible. • Follow the GDP Dos and Don’ts. • Always use the current date. • Know what you are signing/initialing. • Follow company policies/SOPs on significant figures, rounding-off and truncating values. • Protect your company’s trade secrets by maintain confidentiality. 35

END 36