Use of Animals in Research Teaching and Testing











































- Slides: 53

Use of Animals in Research, Teaching, and Testing Silvia I. La. Rue, B. S. , CPIA Director, IACUC Office of V. P. for Research

Goals: Understanding Animal Research Regulations � Public � Early Perspective of Animal Research History of Animal Protection in the US � Federal Regulations � The Institutional Animal Care and Use Committee (IACUC) � Protocol Review Process � Relationship between animal use protocols and grants/contracts

Why does animal research need to be regulated? � Protection of animal subjects. � Ensure safety and well-being of subjects and personnel involved in research. � Avoid unnecessary use of animals in research. � Garner public trust in the scientific research process. Wise use of resources. � Other ethical considerations.

Early History of Animal Protection Legislation in the US � 1641 Puritan’s Body of Liberties � 1828 First Sate anti-cruelty law passed in New York � 1873 First Federal legislation: “ 28 hour law” � 1958 Humane Methods of Slaughter Act passed � 1965 Disappearance of Pepper and publication of Sports Illustrated article � 1966 Life Magazine Article Published: “Concentration Camp for Dogs”

Pepper � In the summer of 1965, a female Dalmatian was stolen from the Lakavage family in Pennsylvania. � Her story changed America. � Seven days after Pepper’s death, Rep. Joe Resnick introduced a pet theft bill on the House floor.

Life Magazine - 1966

Guidelines and Regulations Pertaining to the Use of Animals � United States Department of Agriculture Animal Welfare Act (CFR 9) as amended � Public Health Service Policy on Humane Care and Use of Laboratory Animals � U. S. Govt. Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training � Guide for the Care and Use of Laboratory Animals � FDA (GLP studies), EPA, Endangered Species Act � Freedom of Information Act (FOIA)

PHS Policy on Humane Care and Use of Laboratory Animals Health Research Extension Act PL 99 -158 (1985) � Intended to ensure that PHS grantees and contractors care for and use animals humanely. � Implements and supports the US Govt. Principles. � Office of Laboratory Animal Welfare (OLAW) provides oversight. � Covers all live vertebrate animals. � “The Guide for the Care and Use of Laboratory Animals” must be followed. � Animal Welfare Assurance

PHS Policy: Assurance �A written description of animal care and use program. � Qualifications, authority and responsibility of the veterinarian. � List of members of the IACUC and procedures they will follow to fulfill reqts. of PHS Policy. � Summary of training programs in humane animal care and use. � PENALTY FOR NONCOMPLIANCE: Revocation of Assurance and loss of PHS support for entire institution.

PHS Policy Requires Compliance with AWRs and “The Guide” – n o ti i d E ally h ti g n i y a E n t a s sub ed – m l is tiona v e r d i n d a ” S T ” S S U D “M OUL “SH

Animal Welfare Act Code of Federal Regulations, Title 9, Subchapter A, Parts 1 -4 � Promulgated by US Dept. of Agriculture � All research facilities that use or intend to use live animals, as defined by the regulations, in research, testing and education must comply with the AWRs. � License and registration � Enforced by USDA APHIS, AC

AWRs Definition of Covered Species: Any warm-blooded animal used or intended for use in research, testing, or education except birds, rats of genus Rattus and mice of genus Mus bred for use in research; and horses and other farm animals used or intended for use in agricultural research and production.

AWRs � Subpart C – sets rules for research facilities and requires compliance with standards listed in Part 3 of the Act. � Title 9, Part 3 establishes minimum standards for animal husbandry, care, treatment, and transportation. � 1989 amendments set standards for exercise for dogs and psychological well-being for primates

AWRs � IACUC oversees humane care and use of regulated animals. � IACUC reviews protocols to ensure they comply with AWA requirements. � Animal use must consider alternative methods to replace use of animals; reduce number of animals needed; and refine procedures to minimize pain and distress (3 Rs of alternatives)

AWA requires search for alternatives � USDA Animal Care Policies #11 and 12 � Provide written narrative of methods used and sources consulted to determine availability of alternatives

Animal Care Acronyms � IACUC - Institutional Animal Care and Use Committee � USDA - United States Department of Agriculture � AWA – Animal Welfare Act � AWR/AWAR – Animal Welfare Act Regulations � OLAW – Office of Laboratory Animal Welfare – (formerly called OPRR) � PHS – Public Health Service � NIH – National Institutes of Health

Animal Care Acronyms IO – Institutional Official * AV – Attending Veterinarian * HREA – Health Research Extension Act (PL-99 -1588) AAALAC, Intl. - Association for the Assessment and Accreditation of Laboratory Animal Care, International AVMA – American Veterinary Medical Assoc. USDA: APHIS AC – Animal & Plant Health Inspection Service, Animal Care

Inspection of Programs � IACUC inspects facilities and reviews program semi-annually (self-regulating) -inspection of animal facilities and laboratories -review of “program” – administrative oversight, occupational health and safety, infrastructure, training, etc. � USDA unannounced inspection by veterinary medical officer

What is an animal care and use program? AAALAC Evaluations; semi-annual inspections

Accreditation Program � AAALAC, Intl. = Assoc. for the Assessment and Accreditation of Laboratory Animal Care, Intl. � Voluntary accreditation program � Institutions apply for evaluation of their animal care and use program. � If accredited, a site visit occurs once every 3 years. � Most grant applications ask about AAALAC accreditation in the animal subjects section.

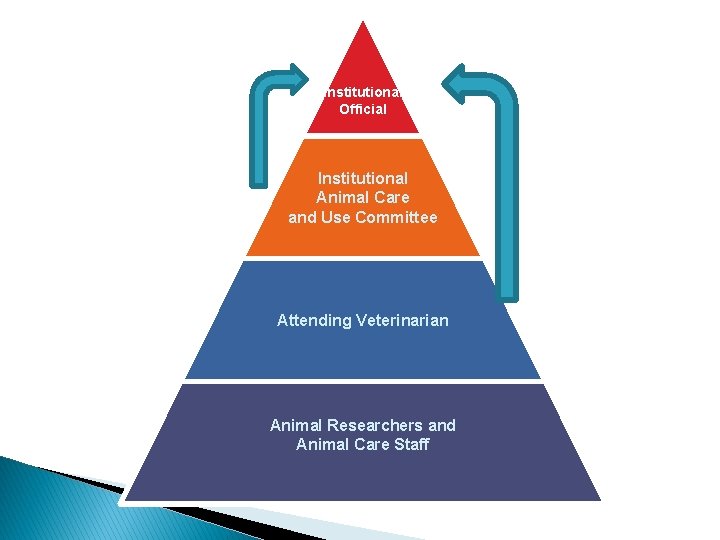
Institutional Official Institutional Animal Care and Use Committee Attending Veterinarian Animal Researchers and Animal Care Staff

Institutional Official � Bears ultimate responsibility for program. � Authority to allocate resources needed. � Receives semi-annual and non-compliance reports from IACUC. � Appoints members of the IACUC. � Sends reports to NIH OLAW. Melur K. “Ram” Ramasubramanian, Ph. D V. P. for Research

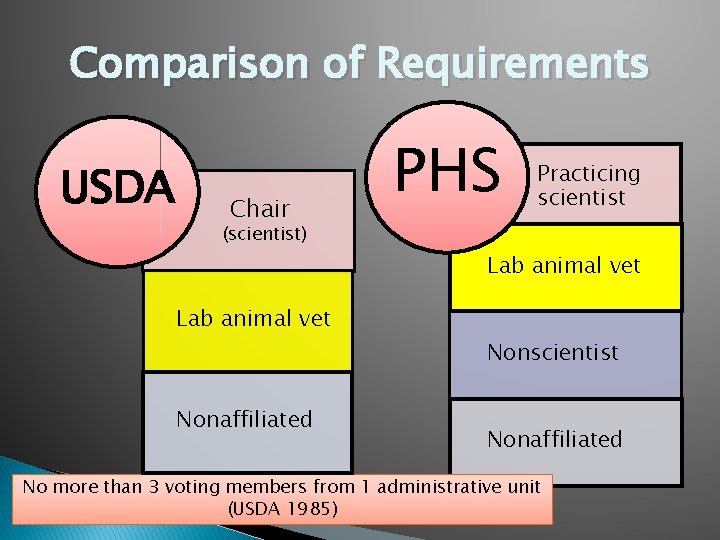
Committee Composition � Members appointed by CEO. � DVM trained in laboratory animal medicine. � Practicing scientist with experience in animal research. � Non-scientist and non-affiliated members. � No more than 3 from one reporting unit. � Alternate members.

Comparison of Requirements USDA Chair PHS Practicing scientist (scientist) Lab animal vet Nonscientist Nonaffiliated No more than 3 voting members from 1 administrative unit (USDA 1985)

Attending Veterinarian � Responsible for health and wellbeing of all laboratory animals used at institution. � Must have sufficient authority including access to animals and resources to manage program of veterinary care. � Oversees aspects of animal care and use to ensure program complies with the Guide.

IACUC Functions: General Overview � Reviews and approves protocols and modifications to protocols � Inspect facilities and reviews program semiannually and reports to I. O. � Reviews concerns about animal care and use � Suspends activities not in compliance with AWRs and PHS Policy � Recommendations to I. O. concerning animal care and use program, facilities, or personnel training.

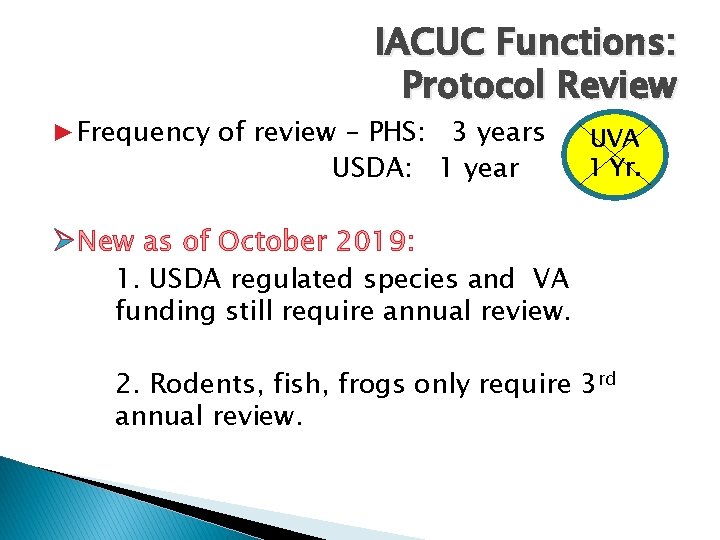
IACUC Functions: Protocol Review ▶Frequency of review – PHS: 3 years USDA: 1 year UVA 1 Yr. ØNew as of October 2019: 1. USDA regulated species and VA funding still require annual review. 2. Rodents, fish, frogs only require 3 rd annual review.

IACUC Functions: Protocol Review § Rationale & purpose of the proposed use of animals § Clear, concise & sequential description of the procedures. Must be easily understood by all members. § Consideration of alternatives to the use of animals or less invasive procedures § Justification of the species and the number to be used § Avoidance of unnecessary duplication

IACUC Functions: Protocol Review § Use of appropriate sedation, analgesia, anesthesia & method of euthanasia § Housing & husbandry description § Post-procedural care & consideration of humane endpoints § Training & experience of personnel § Appropriate use of hazardous materials in animal research

IACUC Functions: Training Researchers � Humane methods of animal maintenance and experimentation � Methods that limit use of animals or minimize distress � Proper use of anesthetics, analgesics, and tranquilizers � Methods for reporting deficiencies in care and treatment

Required Reports - AWRs � Annual report; assurance that standards of care and treatment followed; PI considered alternatives to painful procedures; facility adhering to regulations and have IACUC approval for all exceptions. � Locations where animals housed and used. � #s and species used by pain and distress categories. � Prompt notification of any suspended activity.

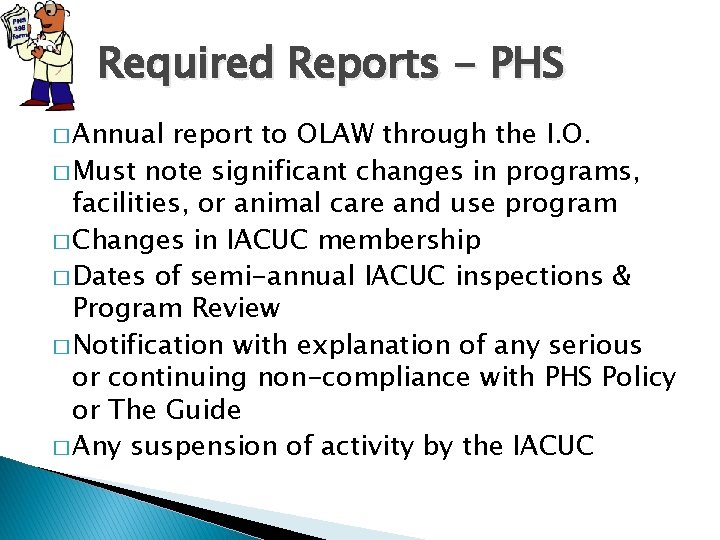
Required Reports - PHS � Annual report to OLAW through the I. O. � Must note significant changes in programs, facilities, or animal care and use program � Changes in IACUC membership � Dates of semi-annual IACUC inspections & Program Review � Notification with explanation of any serious or continuing non-compliance with PHS Policy or The Guide � Any suspension of activity by the IACUC

Grant Applications � Vertebrate Animal Section 1. Proposed use of the animals 2. Justification for use of animals, choice of species, and numbers to be used 3. Description of veterinary care 4. Description of procedures for minimizing pain and distress 5. Description of and rationale for method of euthanasia

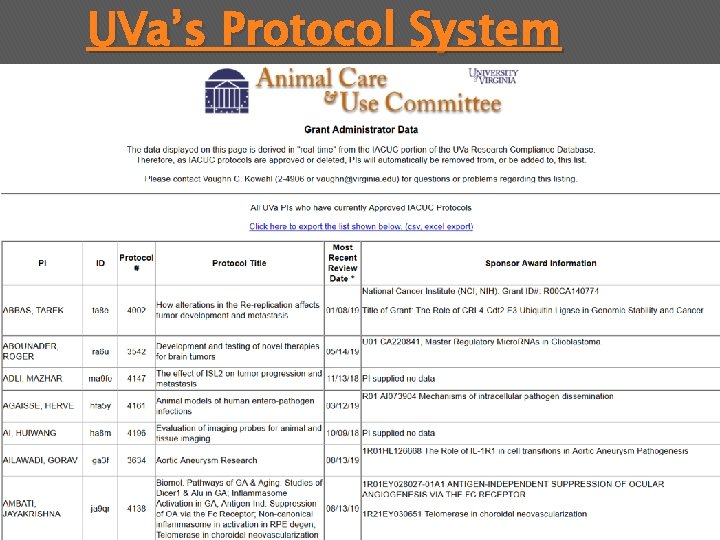
UVa’s Protocol System

Is the IACUC required to review the grant application? � PHS Policy and NIH grants policy statement require that institution verify, before award, that IACUC has reviewed and approved components related to care and use of animals. � NOT an explicit requirement that IACUC conduct side-by-side comparisons. � Institutional responsibility for ensuring that the application/proposal and IACUC protocol are congruent.

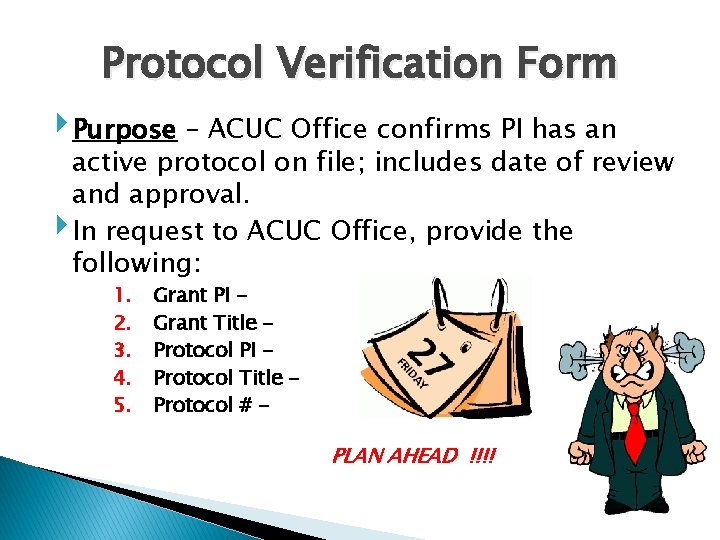
Protocol Verification Form ‣Purpose – ACUC Office confirms PI has an ‣ active protocol on file; includes date of review and approval. In request to ACUC Office, provide the following: 1. 2. 3. 4. 5. Grant PI Grant Title Protocol PI Protocol Title Protocol # - PLAN AHEAD !!!!


Post Award Change in Scope obtain prior approval from NIH awarding office for change in direction, type of research or training, or other significant change in aims, objectives, or purposes of approved project. NIH must be notified in advance and approve. � Must � If these include a significant change in animal use, the IACUC must review and approve.

Contracts/Subcontracts Outside the University �Follow the money! � What if the animal research is performed in another country? OLAW lists all domestic and foreign Assured Institutions. � The grantee always retains primary responsibility for ensuring compliance with PHS Policy (and AWA if applicable).

Animal Welfare Assurance for Foreign Institutions �A collaborating foreign institution must obtain its own PHS Assurance.

Inter-Institutional Collaborations In cases of collaboration involving animal use, “the participating institutions should have a formal written understanding [e. g. a contract, memorandum of understanding (MOU), or agreement (IIA)] that addresses the responsibility for offsite animal care and use, animal ownership, and IACUC review and oversight. ” Guide p. 15

Inter-Institutional Assurance �Used by US institutions that receive PHS funds through a grant or contract award when the institution has neither its own animal care & use program facilities to house animals, nor an IACUC, and will conduct the animal activity at an Assured institution (aka “performance site”). �They are asking to use our “license” (PHS Assurance) to do their work. �Form must be signed by IO and IACUC Chair & approved by OLAW. �Provide: IIA Form, copy of grant/contract & animal work, UVA PI’s name & protocol #.


Reducing Administrative Burden for Researchers

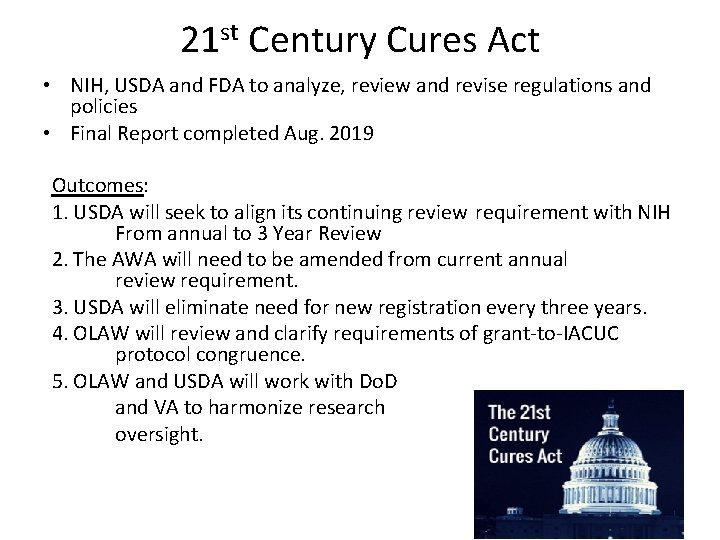
21 st Century Cures Act • NIH, USDA and FDA to analyze, review and revise regulations and policies • Final Report completed Aug. 2019 Outcomes: 1. USDA will seek to align its continuing review requirement with NIH From annual to 3 Year Review 2. The AWA will need to be amended from current annual review requirement. 3. USDA will eliminate need for new registration every three years. 4. OLAW will review and clarify requirements of grant-to-IACUC protocol congruence. 5. OLAW and USDA will work with Do. D and VA to harmonize research oversight.


ACUC Home Page Lower Section


Goal of regulations and oversight • Use of animals is essential to the advancement of knowledge in science and medicine. • This is done in a manner that ensures safe, humane, and judicious use of animals.

Result of regulations and oversight

ge a s es e h t to ic l b pu M ific t n ie c s e h t y to t i e n g u a m s s com Me ry o t la u g re He he t rm ess o f in c s p l pro

ACUC OFFICE 924 -0405 ACUC@VIRGINIA. EDU P. O. BOX 800720 COBB HALL, ROOM B 001

 Is a horse a producer consumer or decomposer
Is a horse a producer consumer or decomposer G h patel college of engineering and technology
G h patel college of engineering and technology Carnivore
Carnivore Pros and cons animal testing
Pros and cons animal testing Https://a-z-animals.com/
Https://a-z-animals.com/ Microteaching meaning
Microteaching meaning Computer aided teaching ppt
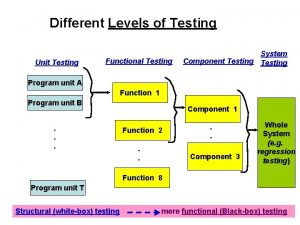
Computer aided teaching ppt Positive and negative testing
Positive and negative testing Static testing and dynamic testing
Static testing and dynamic testing Domain closure in software testing
Domain closure in software testing Motivational overview in software testing
Motivational overview in software testing Data flow testing strategies in software testing
Data flow testing strategies in software testing Anuj magazine
Anuj magazine Neighborhood integration testing
Neighborhood integration testing Language testing
Language testing Control structure testing in software testing
Control structure testing in software testing Decision table testing in software testing
Decision table testing in software testing Decision table technique
Decision table technique Jenis-jenis black box testing
Jenis-jenis black box testing Black-box testing disebut juga sebagai behavioral testing
Black-box testing disebut juga sebagai behavioral testing Decision tables testing
Decision tables testing Rigorous testing in software testing
Rigorous testing in software testing Testing blindness in software testing
Testing blindness in software testing Component testing is a black box testing
Component testing is a black box testing Software testing domains
Software testing domains Teaching as a vocation mission and profession
Teaching as a vocation mission and profession Why did orwell use animals instead of people
Why did orwell use animals instead of people A mushroom and a humpback whale are alike because both are
A mushroom and a humpback whale are alike because both are Animal testing for medical research pros and cons
Animal testing for medical research pros and cons Making best use of teaching assistants
Making best use of teaching assistants Direct method is also known as
Direct method is also known as Use case testing
Use case testing Yesterday
Yesterday Hypothesis sample
Hypothesis sample Westmoreland labs
Westmoreland labs Korea automobile testing & research institute
Korea automobile testing & research institute Korea automobile testing & research institute
Korea automobile testing & research institute Ultra testing & research laboratory
Ultra testing & research laboratory Meaning and scope of research
Meaning and scope of research Contrast applied research and basic research
Contrast applied research and basic research Longitudinal research and cross sectional research
Longitudinal research and cross sectional research Example of applied research
Example of applied research Conclusive quantitative research
Conclusive quantitative research Practical research nature of inquiry and research
Practical research nature of inquiry and research Identification and formulation of research problem
Identification and formulation of research problem Research report vs research proposal
Research report vs research proposal Method procedure example
Method procedure example Research appendices example
Research appendices example What is research gap meaning
What is research gap meaning Research instrument in experimental research
Research instrument in experimental research Define casual comparative
Define casual comparative Instrumentation in research methodology
Instrumentation in research methodology Bryman bell
Bryman bell Define reseach
Define reseach