Real World Evidence and FDA My Studies David












- Slides: 17

Real World Evidence and FDA My. Studies David Martin, M. D. , M. P. H. Associate Director for RWE Analytics, Office of Medical Policy Center for Drug Evaluation and Research U. S. Food and Drug Administration ACT-IAC Health Innovation Day April 10, 2019

1 BIG DATA www. fda. gov 2

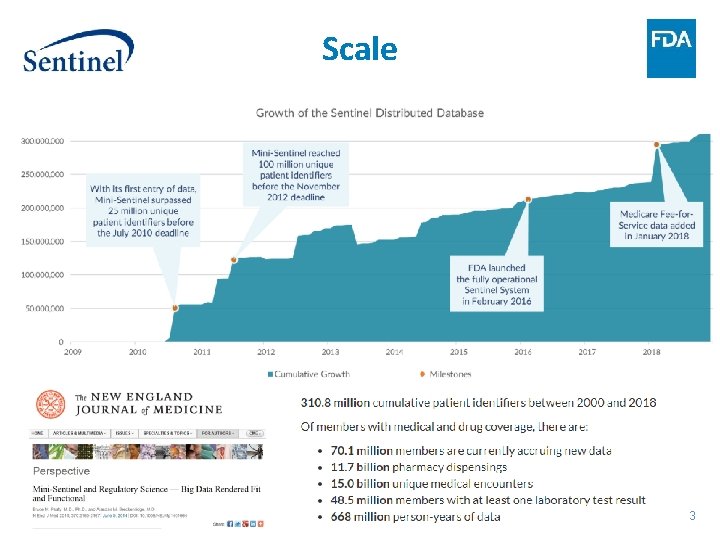
Scale 3

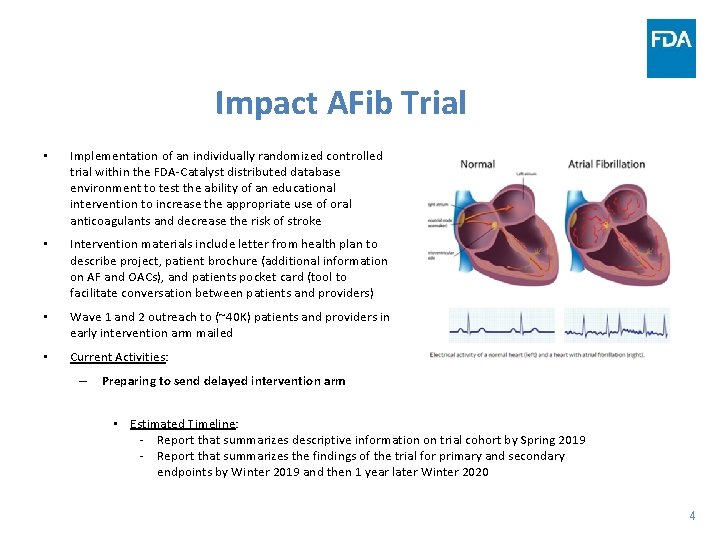
Impact AFib Trial • Implementation of an individually randomized controlled trial within the FDA-Catalyst distributed database environment to test the ability of an educational intervention to increase the appropriate use of oral anticoagulants and decrease the risk of stroke • Intervention materials include letter from health plan to describe project, patient brochure (additional information on AF and OACs), and patients pocket card (tool to facilitate conversation between patients and providers) • Wave 1 and 2 outreach to (~40 K) patients and providers in early intervention arm mailed • Current Activities: – Preparing to send delayed intervention arm • Estimated Timeline: - Report that summarizes descriptive information on trial cohort by Spring 2019 - Report that summarizes the findings of the trial for primary and secondary endpoints by Winter 2019 and then 1 year later Winter 2020 4

2 21 ST CENTURY CURES www. fda. gov 5

https: //www. fda. gov/downloads/Science. Research/Special. Topics/Real. World. Evidence/UCM 627769. pdf 6

Benchmark • Substantial evidence standard unchanged – Goal is to distinguish the effect of the drug from other influences such as spontaneous change in disease course, placebo effect, or bias – Common practices: • Probabilistic control of confounding through randomization • Blinding • Controlled/Standardized outcome assessment • Adjudication criteria • Audits 7

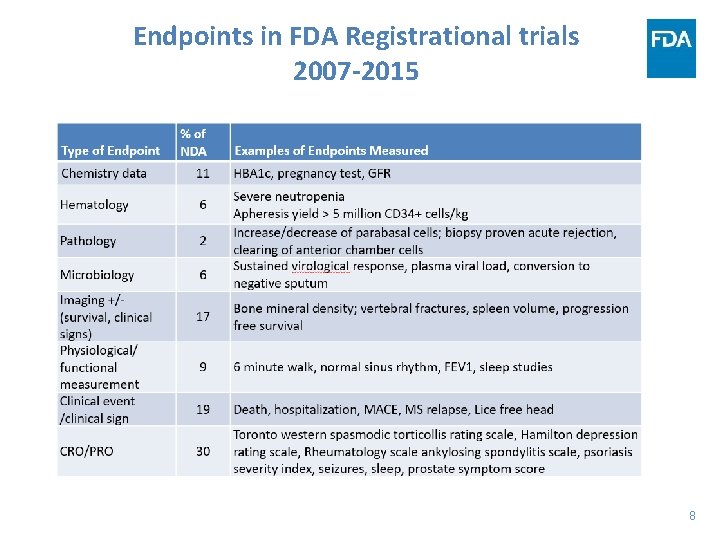
Endpoints in FDA Registrational trials 2007 -2015 8

3 FDA MYSTUDIES www. fda. gov 9

FDA My Studies • Mobile App – Standard frameworks - Research. Kit (i. OS), Research. Stack (Android) – Gateway capability • • Web-based configuration portal Secure Storage Environment – 21 CFR Part 11 and FISMA complaint – Partitioned for distributed research • • • One private sector research organization has successfully re-purposed the app in a test environment. App integral to two new demonstration projects FDA SBIA webinar scheduled for May 9 10

Limit JIA trial • Randomized real world trial in patients with Limited Juvenile Idiopathic Arthritis (<=4 joints affected and no uveitis) – Six month course of subcutaneous Abatacept (T cell co-stimulation inhibitor) plus usual care with NSAIDs and intra-articular glucocorticoids vs. usual care alone – Outcome: extension to more than 4 joints, new uveitis, and/or need for treatment with systemic medication at 18 months • FDA-Catalyst is aligning with the trial by providing support from the My Studies App – – First use of FDA-Catalyst to support a pediatric trial Potential support for the Childhood Arthritis & Rheumatology Research Alliance (CARRA) Registry Collection of primary outcome (uveitis) from ophthalmology appointments - Configured Collection of adherence information/adverse events for study drug with “drug diary” – Configuration stage 11

SPARC registry • SPARC Inflammatory Bowel Disease cohort within the IBD Plexus research exchange platform – Provider based recruitment of individuals >18 years of age with a confirmed IBD diagnosis from academic and community sites • FDA-Catalyst is aligning registry by providing support from the My Studies App – Configuration stage • Registry responses will be included in the PCORI Comparative Effectiveness of Biologic or Small Molecule Therapies in Inflammatory Bowel Disease study (prospective cohort for patient reported outcomes) 12

CDERMedical. Policy-Real. World. Evidence@fda. hhs. gov


Establishing Effectiveness • Section 505 (d) of the Food Drug and Cosmetic Act requires adequate and well controlled investigations, including clinical investigations, on the basis of which it could fairly and responsibly be concluded by such experts that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the labeling or proposed labeling thereof. 15

Regulatory Considerations 16

FDA My. Studies: now open source • • • https: //www. fda. gov/News. Events/Newsroom/FDAIn. Brief/ucm 625228. htm https: //www. fda. gov/Drugs/Science. Research/ucm 624785. htm https: //github. com/Pop. Med. Net-Team/FDA-My-Studies-Mobile-Application-System 17
 Real world evidence
Real world evidence Application of polynomials in daily life
Application of polynomials in daily life Difference between class and individual evidence
Difference between class and individual evidence Real world vs digital world
Real world vs digital world World of forms
World of forms Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies What is primary sources
What is primary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Why is fiber considered class evidence
Why is fiber considered class evidence Class vs individual evidence
Class vs individual evidence Individual vs class evidence
Individual vs class evidence Red herring fallacy
Red herring fallacy Fda v brown and williamson
Fda v brown and williamson Direct evidence
Direct evidence World studies themes
World studies themes
































