FDAS OFFICE OF REGULATORY AFFAIRS ALIGNS FOR THE










![Office of Human and Animal Food Operations Michael Rogers, Assistant Commissioner [acting] www. fda. Office of Human and Animal Food Operations Michael Rogers, Assistant Commissioner [acting] www. fda.](https://slidetodoc.com/presentation_image_h/0141a34908d84a2eb3e09dc46f59befe/image-24.jpg)












- Slides: 43

FDA’S OFFICE OF REGULATORY AFFAIRS ALIGNS FOR THE FUTURE www. fda. gov 1

and strengthen the “…Modernize FDA workforce to improve public health response. ” 2013 FDA Program Alignment Charge www. fda. gov 2

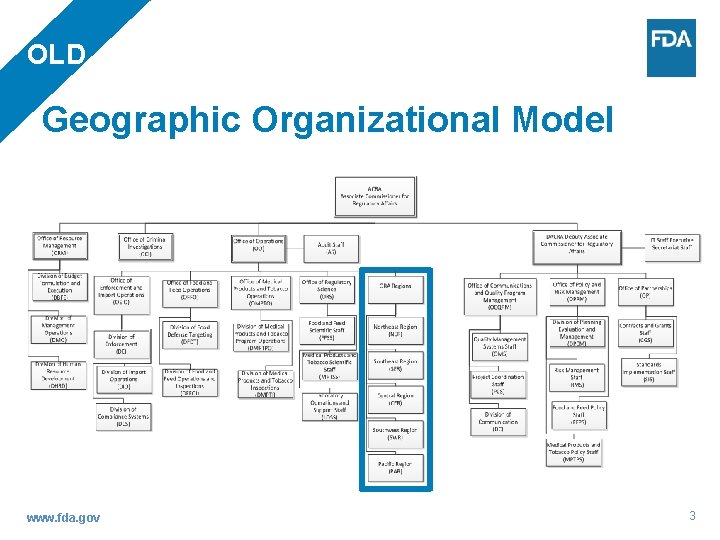
OLD Geographic Organizational Model www. fda. gov 3

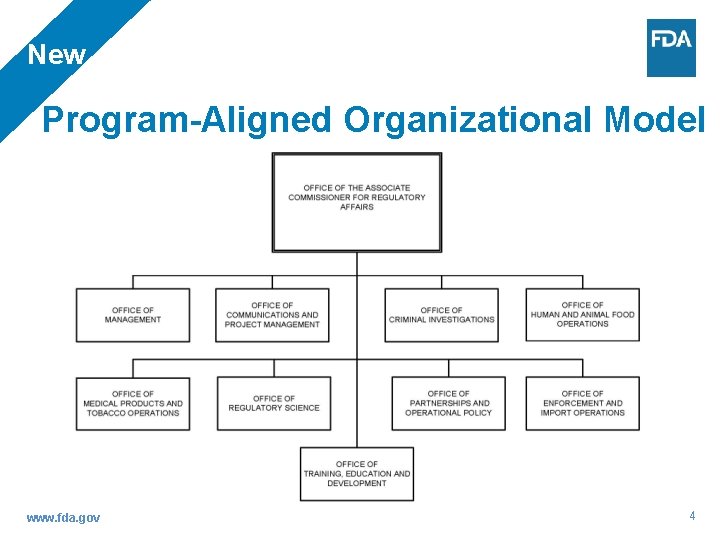
New Program-Aligned Organizational Model www. fda. gov 4

Import Operations Civil & Criminal Investigators Compliance Officers State Cooperative Programs – Milk, Retail, Shellfish 13 Laboratories Emergency Response Coordinators State Contracts, Grants & Agreements Recall Coordinators ORA at a Glance State Liaisons Consumer Complaint Coordinators Quality Systems Managers Official Establishment Inventory Coordinators Planning & Evaluation Analysts Operational Policy Analysts Communications Staff www. fda. gov Administrative & Mission Support Training, Education & Development Staff Disclosure & FOIA 5

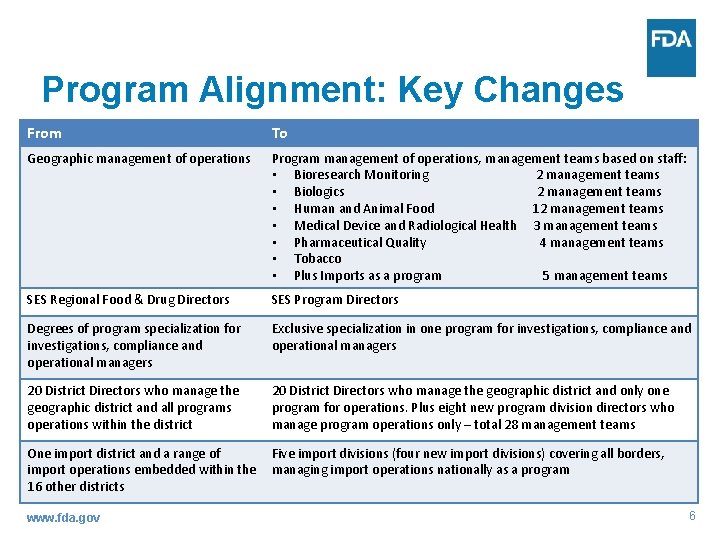
Program Alignment: Key Changes From To Geographic management of operations Program management of operations, management teams based on staff: • Bioresearch Monitoring 2 management teams • Biologics 2 management teams • Human and Animal Food 12 management teams • Medical Device and Radiological Health 3 management teams • Pharmaceutical Quality 4 management teams • Tobacco • Plus Imports as a program 5 management teams SES Regional Food & Drug Directors SES Program Directors Degrees of program specialization for investigations, compliance and operational managers Exclusive specialization in one program for investigations, compliance and operational managers 20 District Directors who manage the geographic district and all programs operations within the district 20 District Directors who manage the geographic district and only one program for operations. Plus eight new program division directors who manage program operations only – total 28 management teams One import district and a range of import operations embedded within the 16 other districts Five import divisions (four new import divisions) covering all borders, managing import operations nationally as a program www. fda. gov 6

Program Alignment: Key Changes From To 13 labs reporting into the regions National Lab Management - 13 labs reporting into ORA’s Office of Regulatory Science with three additional directors managing separate program operations Division of Human Resource Development within the Office of Resource Management Office of Training, Education and Development State Cooperative Programs decentralized across five regions – shellfish, milk, retail Office of State Cooperative Programs under the Human and Animal Food Operations, as a single national program(s) Functions based in geography: consumer Retain certain functions based in geography: complaint coordinators, state liaisons, emergency consumer complaint coordinators, state liaisons, response coordinators emergency response coordinators Functions decentralized local reporting: Freedom of Information (FOI) staff, industrial hygienists (IH), and administrative staff www. fda. gov Staff remain embedded locally, but report into a single office: FOI staff report into Division of Information Disclosure; IHs report into the Office of Regulatory Science; administrative staff report into the Office of Management 7

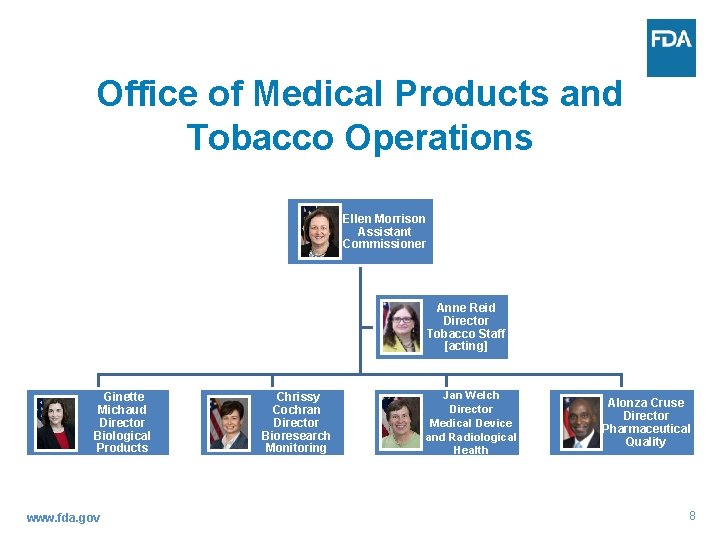
Office of Medical Products and Tobacco Operations Ellen Morrison Assistant Commissioner Anne Reid Director Tobacco Staff [acting] Ginette Michaud Director Biological Products www. fda. gov Chrissy Cochran Director Bioresearch Monitoring Jan Welch Director Medical Device and Radiological Health Alonza Cruse Director Pharmaceutical Quality 8

Office of Biological Products Operations Ginette Michaud, MD Director Biological Products www. fda. gov 9

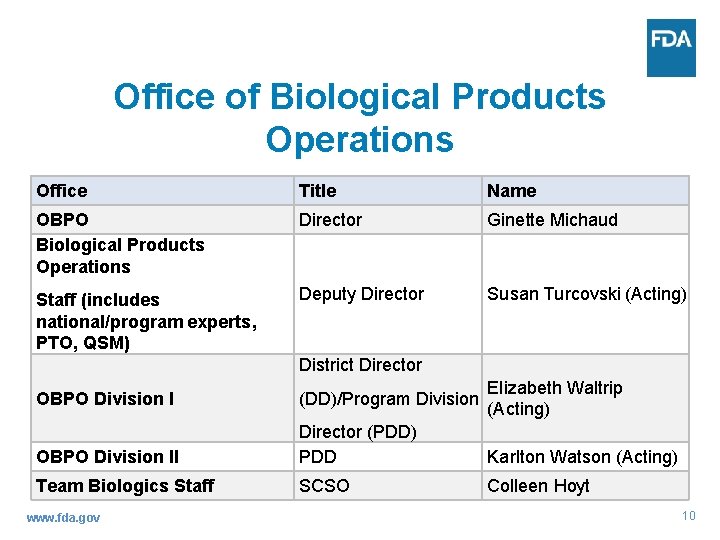
Office of Biological Products Operations Office Title Name OBPO Biological Products Operations Director Ginette Michaud Staff (includes national/program experts, PTO, QSM) Deputy Director Susan Turcovski (Acting) District Director Elizabeth Waltrip (Acting) OBPO Division I (DD)/Program Division OBPO Division II Director (PDD) PDD Karlton Watson (Acting) Team Biologics Staff SCSO Colleen Hoyt www. fda. gov 10

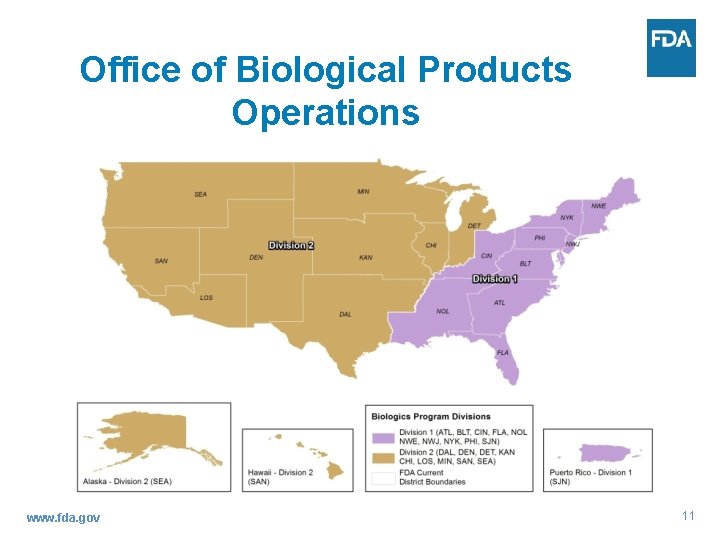
Office of Biological Products Operations www. fda. gov 11

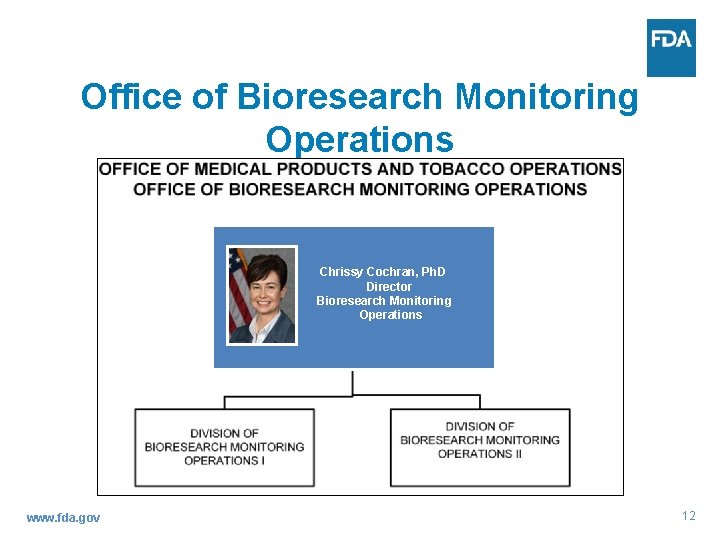
Office of Bioresearch Monitoring Operations Chrissy Cochran, Ph. D Director Bioresearch Monitoring Operations www. fda. gov 12

Office of Bioresearch Monitoring Operations Office Title Name OBIMO Director Chrissy Cochran OBIMO Deputy Director District Director (DD)/Program Division Director (PDD) PDD David Glasgow (Acting) OBIMO/Division II www. fda. gov Anne Johnson Eric Pittman 13

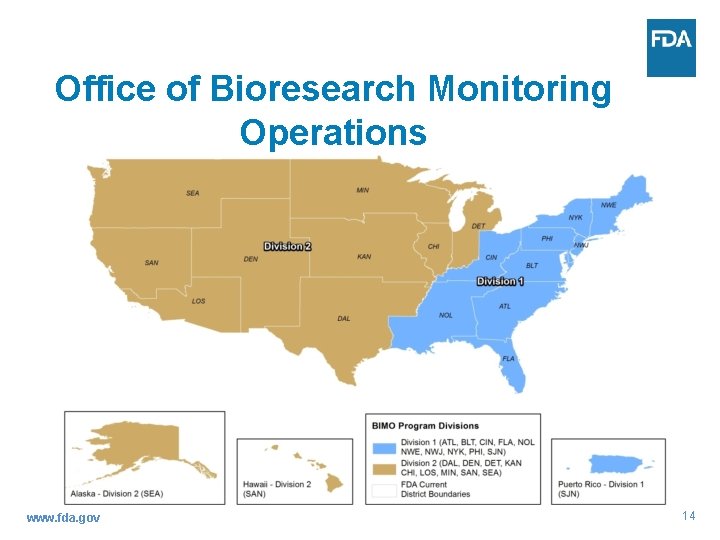
Office of Bioresearch Monitoring Operations www. fda. gov 14

Office of Medical Device and Radiological Health Operations Jan Welch Director Medical Device and Radiological Health www. fda. gov 15

Office of Medical Device and Radiological Health Operations Office Title Name OMDRHO Director Jan Welch OMDRHO Deputy Director Vacant OMDRHO Division I District Director (DD)/Program Division Director (PDD) Joseph S. Matrisciano Jr OMDRHO Division II PDD Blake Bevill OMDRHO Division III PDD Kelly Sheppard (Acting) OMDRHO/Foreign Medical Staff Director Devices and Radiological Health Inspection Staff www. fda. gov Dorothy Lee 16

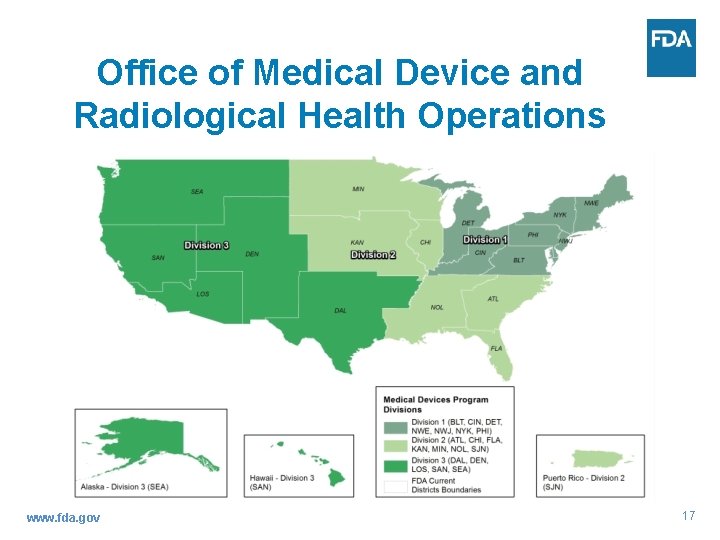
Office of Medical Device and Radiological Health Operations www. fda. gov 17

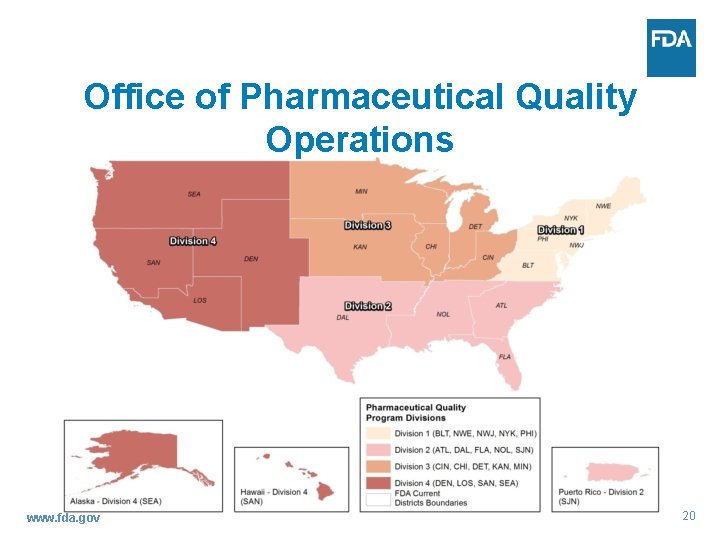
Office of Pharmaceutical Quality Operations Alonza Cruse Director Pharmaceutical Quality Operations www. fda. gov 18

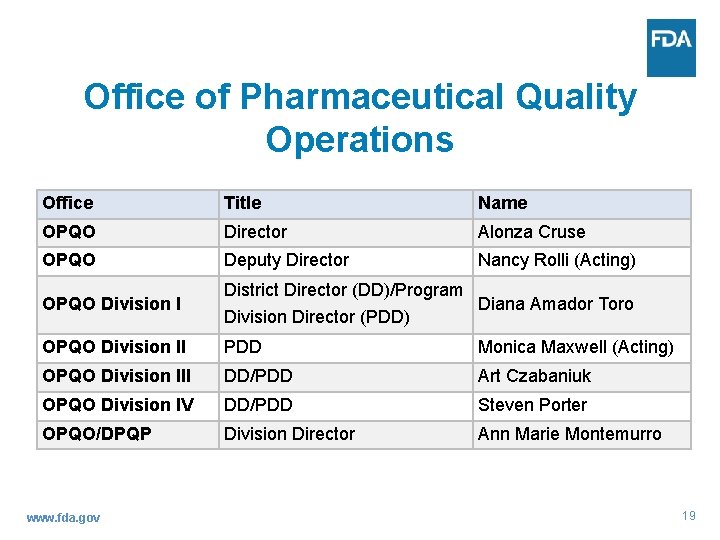
Office of Pharmaceutical Quality Operations Office Title Name OPQO Director Alonza Cruse OPQO Deputy Director Nancy Rolli (Acting) OPQO Division I District Director (DD)/Program Diana Amador Toro Division Director (PDD) OPQO Division II PDD Monica Maxwell (Acting) OPQO Division III DD/PDD Art Czabaniuk OPQO Division IV DD/PDD Steven Porter OPQO/DPQP Division Director Ann Marie Montemurro www. fda. gov 19

Office of Pharmaceutical Quality Operations www. fda. gov 20

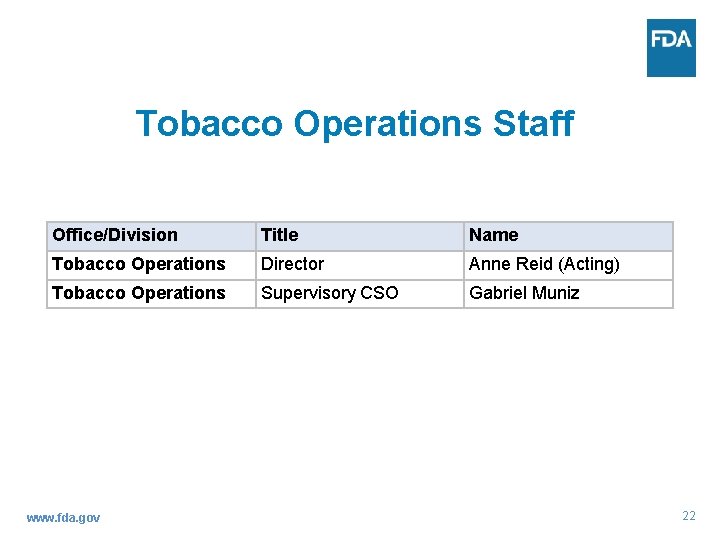
Tobacco Operations Staff Anne Reid Director Tobacco Operations Staff (Acting) www. fda. gov 21

Tobacco Operations Staff Office/Division Title Name Tobacco Operations Director Anne Reid (Acting) Tobacco Operations Supervisory CSO Gabriel Muniz www. fda. gov 22

Tobacco Operations Staff www. fda. gov 23
![Office of Human and Animal Food Operations Michael Rogers Assistant Commissioner acting www fda Office of Human and Animal Food Operations Michael Rogers, Assistant Commissioner [acting] www. fda.](https://slidetodoc.com/presentation_image_h/0141a34908d84a2eb3e09dc46f59befe/image-24.jpg)
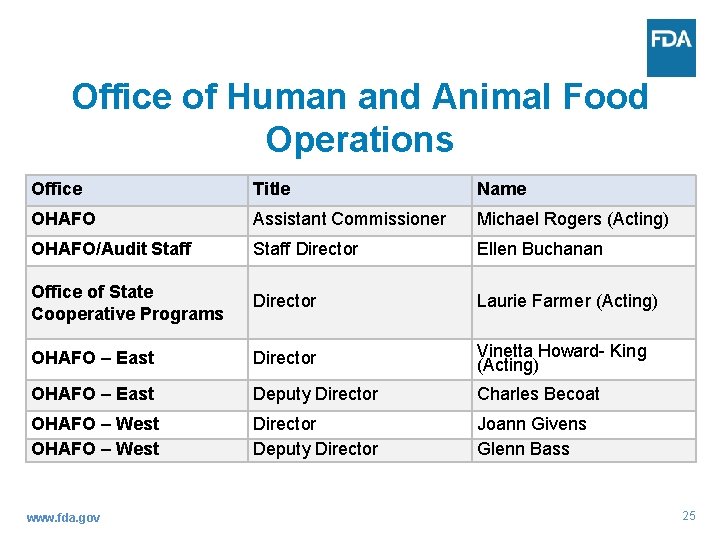
Office of Human and Animal Food Operations Michael Rogers, Assistant Commissioner [acting] www. fda. gov Ellen Buchanan, Director Audit Staff Laurie Farmer, Director Office of State Cooperative Programs Vinetta Howard King, East Director [acting] Joann Givens, West Director 24

Office of Human and Animal Food Operations Office Title Name OHAFO Assistant Commissioner Michael Rogers (Acting) OHAFO/Audit Staff Director Ellen Buchanan Office of State Cooperative Programs Director Laurie Farmer (Acting) OHAFO – East Director Vinetta Howard- King (Acting) OHAFO – East Deputy Director Charles Becoat OHAFO – West Director Deputy Director Joann Givens Glenn Bass www. fda. gov 25

Office of Human and Animal Food Operations www. fda. gov 26

Offices of… Melinda Plaisier Associate Commissioner for Regulatory Affairs Doug Stearn Director Office of Enforcement and Import Operations www. fda. gov Paul Norris Director Office of Regulatory Science 27

Office of Enforcement and Import Operations Doug Stearn, JD Director Enforcement and Import Operations www. fda. gov 28

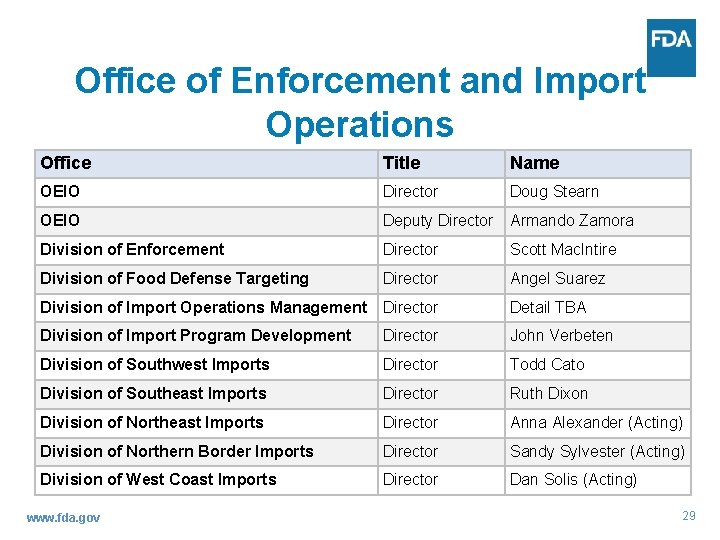
Office of Enforcement and Import Operations Office Title Name OEIO Director Doug Stearn OEIO Deputy Director Armando Zamora Division of Enforcement Director Scott Mac. Intire Division of Food Defense Targeting Director Angel Suarez Division of Import Operations Management Director Detail TBA Division of Import Program Development Director John Verbeten Division of Southwest Imports Director Todd Cato Division of Southeast Imports Director Ruth Dixon Division of Northeast Imports Director Anna Alexander (Acting) Division of Northern Border Imports Director Sandy Sylvester (Acting) Division of West Coast Imports Director Dan Solis (Acting) www. fda. gov 29

Office of Enforcement and Import Operations www. fda. gov 30

Office of Regulatory Science Dr. Paul Norris Director Office of Regulatory Science www. fda. gov 31

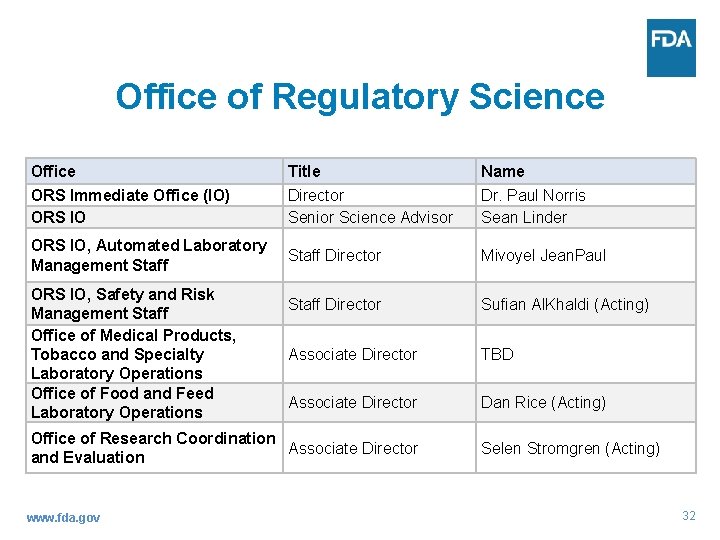
Office of Regulatory Science Office ORS Immediate Office (IO) ORS IO Title Director Senior Science Advisor Name Dr. Paul Norris Sean Linder ORS IO, Automated Laboratory Management Staff Director Mivoyel Jean. Paul Staff Director Sufian Al. Khaldi (Acting) Associate Director TBD Associate Director Dan Rice (Acting) ORS IO, Safety and Risk Management Staff Office of Medical Products, Tobacco and Specialty Laboratory Operations Office of Food and Feed Laboratory Operations Office of Research Coordination Associate Director and Evaluation www. fda. gov Selen Stromgren (Acting) 32

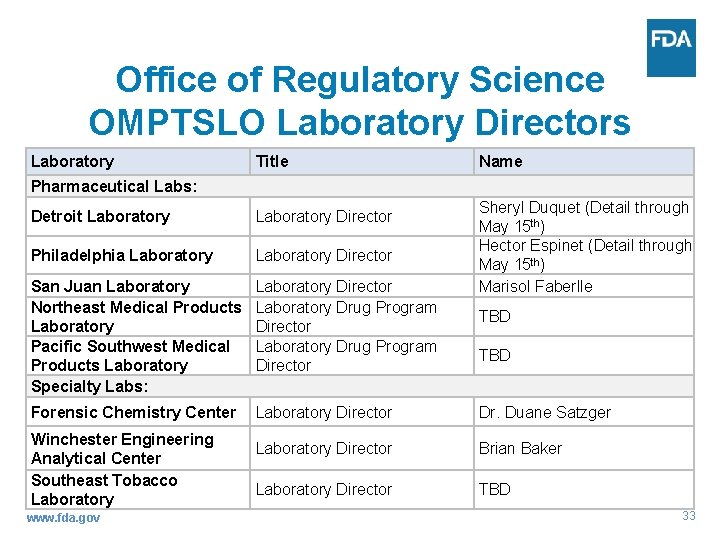
Office of Regulatory Science OMPTSLO Laboratory Directors Laboratory Title Name Pharmaceutical Labs: Sheryl Duquet (Detail through May 15 th) Hector Espinet (Detail through May 15 th) Marisol Faberlle Detroit Laboratory Director Philadelphia Laboratory Director San Juan Laboratory Northeast Medical Products Laboratory Pacific Southwest Medical Products Laboratory Specialty Labs: Laboratory Director Laboratory Drug Program Director Forensic Chemistry Center Laboratory Director Dr. Duane Satzger Laboratory Director Brian Baker Laboratory Director TBD Winchester Engineering Analytical Center Southeast Tobacco Laboratory www. fda. gov TBD 33

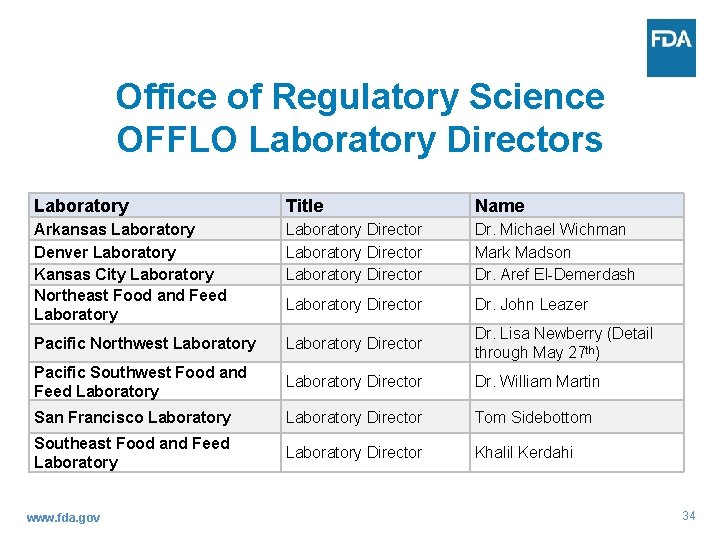
Office of Regulatory Science OFFLO Laboratory Directors Laboratory Title Name Arkansas Laboratory Denver Laboratory Kansas City Laboratory Northeast Food and Feed Laboratory Director Dr. Michael Wichman Mark Madson Dr. Aref El-Demerdash Laboratory Director Dr. John Leazer Pacific Northwest Laboratory Director Dr. Lisa Newberry (Detail through May 27 th) Pacific Southwest Food and Feed Laboratory Director Dr. William Martin San Francisco Laboratory Director Tom Sidebottom Southeast Food and Feed Laboratory Director Khalil Kerdahi www. fda. gov 34

Office of Partnerships Barbara Cassens, Director Office of Partnerships www. fda. gov 35

Office of Partnerships Office/Division Title Name Office of Partnerships/Immediate Office Director Barbara Cassens Office of Partnerships/Immediate Office Deputy Office Director Alan Tart Office of Partnerships/Immediate Office/Partnership Services Group Acting Supervisor Matthew Avis (acting) Office of Partnerships/Division of Integration Division Director Detail TBA Office of Partnerships/Division of Standards Implementation Division Director Timothy Weigner (acting) Office of Partnerships/Division of Partnership Investments and Agreements Division Director Abram Brown III (acting) www. fda. gov 36

Resources and Contacts www. FDA. gov/ORA • ORA Organization Charts and Boundary Maps • Fact Sheets – ORA’s New Structure – Operational Offices • Investigations Operations Manual Contacts • State and local inquiries – District Director – State Liaison • General Inquiries – engageora@fda. hhs. gov • Partnerships – OP-ORA@fda. hhs. gov – Headquarters and District Contact Information www. fda. gov 37

ORA Ombudsman • Informally and impartially addresses concerns, complaints, and disputes between ORA and external parties: – Industry – Federal, state, territory, and tribal government entities – Public • Contact: Jessica Zeller, JD, MA Ombudsman PUBLIC HEALTH PARTNERSHIPS: www. fda. gov – 513 -679 -2777 or 240 -535 -6021 – ORAOmbudsman@fda. hhs. gov Enhancing ORA operations by serving as an objective, neutral resource to improve communication channels, resolve disputes, and foster positive relationships with internal and external stakeholders. 38

THANK YOU Questions and Discussion www. fda. gov 39

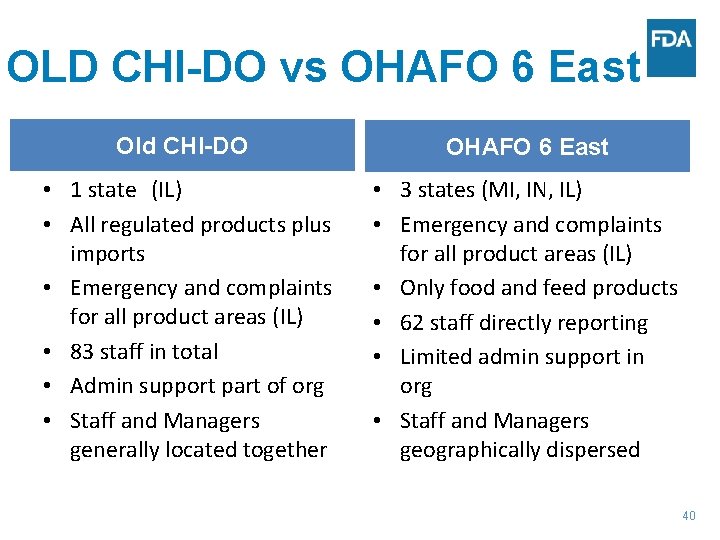
OLD CHI-DO vs OHAFO 6 East Old CHI-DO OHAFO 6 East • 1 state (IL) • All regulated products plus imports • Emergency and complaints for all product areas (IL) • 83 staff in total • Admin support part of org • Staff and Managers generally located together • 3 states (MI, IN, IL) • Emergency and complaints for all product areas (IL) • Only food and feed products • 62 staff directly reporting • Limited admin support in org • Staff and Managers geographically dispersed 40

OHAFO 6 E Contact • William Weissinger, PDD, – 550 W Jackson Blvd, Suite 1500, Chicago IL 60661 (312) 596 4202 – William. Weissinger@fda. hhs. gov • 483 Responses - Kelli Wilkinson, DCB, – 300 River Place, 5 th Floor, Detroit MI 48207 – (313) 393 8120 Kelli. Wilkinson@fda. hhs. gov • Recalls orahafeast 6 recalls@fda. hhs. gov – (313) 393 -8118 41

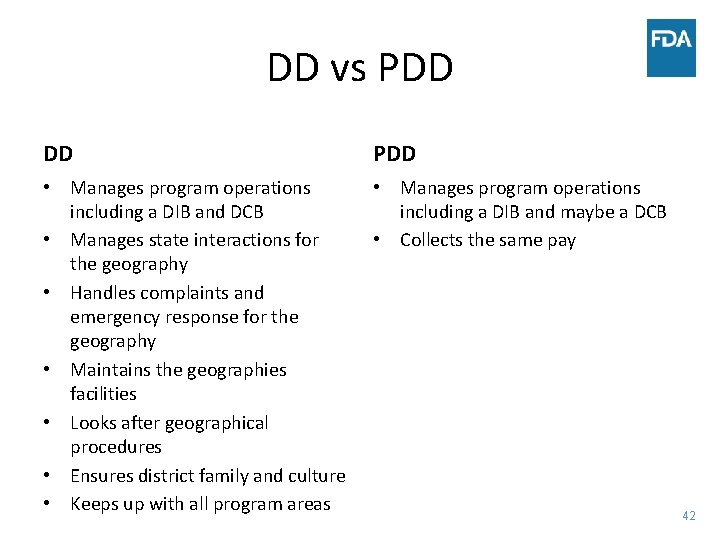
DD vs PDD DD PDD • Manages program operations including a DIB and DCB • Manages state interactions for the geography • Handles complaints and emergency response for the geography • Maintains the geographies facilities • Looks after geographical procedures • Ensures district family and culture • Keeps up with all program areas • Manages program operations including a DIB and maybe a DCB • Collects the same pay 42

 Michigan department of licensing and regulatory affairs
Michigan department of licensing and regulatory affairs Regulatory affairs kpi
Regulatory affairs kpi Post approval regulatory affairs
Post approval regulatory affairs By default, excel _____ aligns text entries.
By default, excel _____ aligns text entries. Student affairs office
Student affairs office Hms foundation funds
Hms foundation funds Hhs office of population affairs
Hhs office of population affairs Va form 10-250
Va form 10-250 Densitet vatten
Densitet vatten Elektronik för barn
Elektronik för barn Tack för att ni har lyssnat
Tack för att ni har lyssnat Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Novell typiska drag
Novell typiska drag Luftstrupen för medicinare
Luftstrupen för medicinare Frgar
Frgar Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Indikation för kejsarsnitt på moderns önskan
Indikation för kejsarsnitt på moderns önskan Multiplikation med decimaltal uppgifter
Multiplikation med decimaltal uppgifter Autokratiskt ledarskap
Autokratiskt ledarskap Toppslätskivling dos
Toppslätskivling dos Borra hål för knoppar
Borra hål för knoppar Redogör för vad psykologi är
Redogör för vad psykologi är Gumman cirkel
Gumman cirkel Bris för vuxna
Bris för vuxna En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Mat för idrottare
Mat för idrottare Ledarskapsteorier
Ledarskapsteorier Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Datorkunskap för nybörjare
Datorkunskap för nybörjare Fredsgudinnan
Fredsgudinnan Rita perspektiv
Rita perspektiv Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Kanaans land
Kanaans land Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Claes martinsson
Claes martinsson Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Dikt form
Dikt form Personlig tidbok
Personlig tidbok Datumr
Datumr Matematisk modellering eksempel
Matematisk modellering eksempel Orubbliga rättigheter
Orubbliga rättigheter Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Vishnuiter
Vishnuiter