Chemistry Notes A molecule consists of two or


















- Slides: 53

Chemistry Notes

• A molecule consists of two or more atoms of the same element, or different elements, that are chemically bonded together. • The smallest part of a pure substance (element or compound) • Ex. One molecule H 20 vs. a glass of water.

• Diatomic molecule - composed of two of the same atom • Examples: H 2, N 2, O 2, Cl 2, Br 2 • In the animation above, two nitrogen atoms (N + N = N 2) make one Nitrogen molecule.

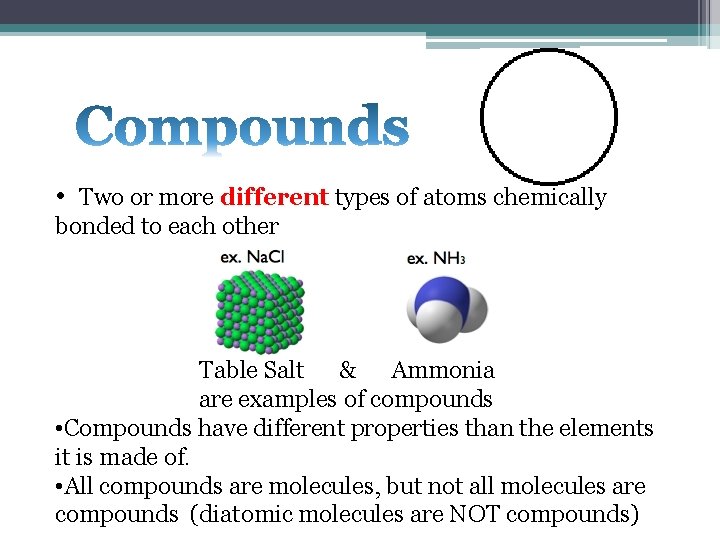
• Two or more different types of atoms chemically bonded to each other Table Salt & Ammonia are examples of compounds • Compounds have different properties than the elements it is made of. • All compounds are molecules, but not all molecules are compounds (diatomic molecules are NOT compounds)


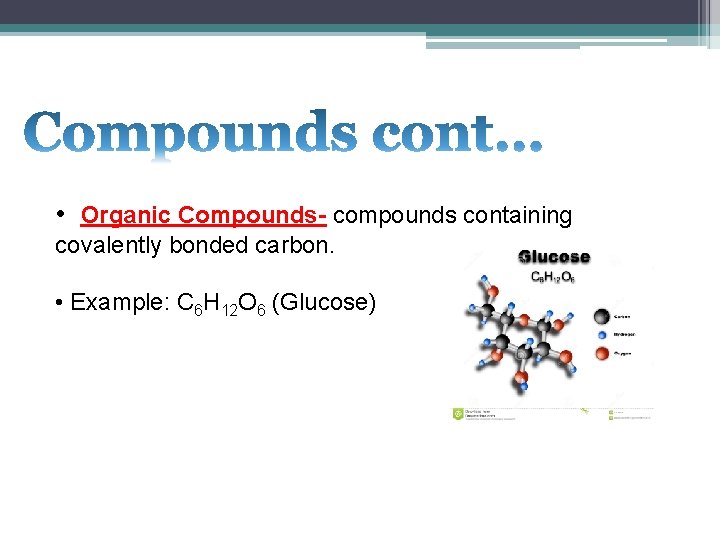
• Organic Compounds- compounds containing covalently bonded carbon. • Example: C 6 H 12 O 6 (Glucose)

• The rule of 8. • Valence electrons are the electrons in the outermost energy level. • You must know the number of valence electrons an atom has. • When atoms chemically combine they try to end up with a full outermost energy level or 8 valence electrons by either gaining or losing electrons. • To meet this need: ▫ Metals tend to lose (give away) electrons. ▫ Nonmetals tend to gain electrons.

• Nature seeks balance! Having a full outermost energy level is stable. • That’s why the Noble Gases do not react. They already have a full outer level.

• The group (column) represents the number of valence electrons in the outer shell. • The period (row) represents the number of energy levels in the atom.

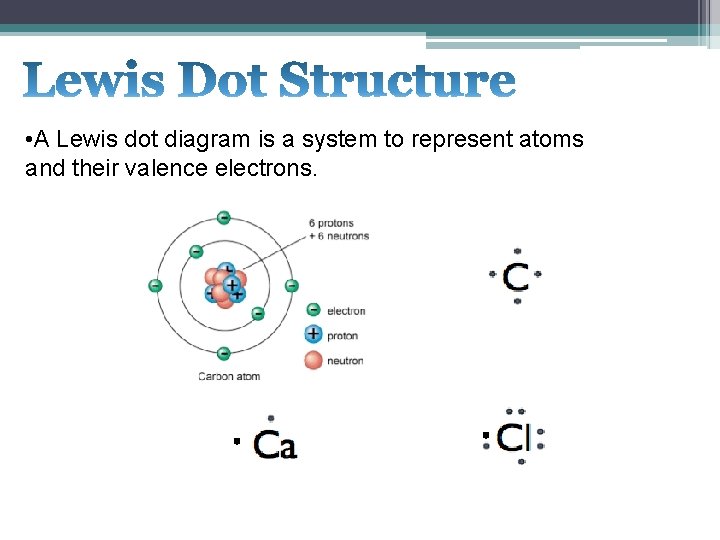
• A Lewis dot diagram is a system to represent atoms and their valence electrons.

• Drawing Lewis Dot Structures:

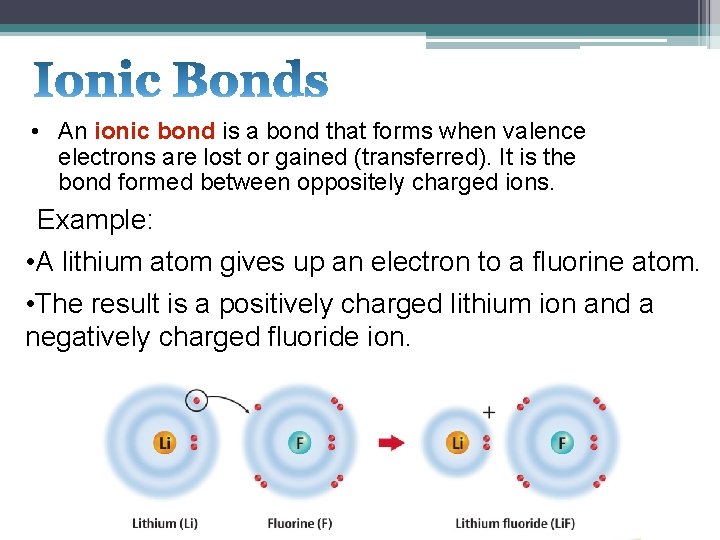
• An ionic bond is a bond that forms when valence electrons are lost or gained (transferred). It is the bond formed between oppositely charged ions. Example: • A lithium atom gives up an electron to a fluorine atom. • The result is a positively charged lithium ion and a negatively charged fluoride ion.

• Other ionic bond examples…….

• A metal bonded to a nonmetal (Groups 1 & 2 with Groups 6 & 7) • Conducts electricity when dissolved in water or melted. • High melting points. • Solids at room temperature.

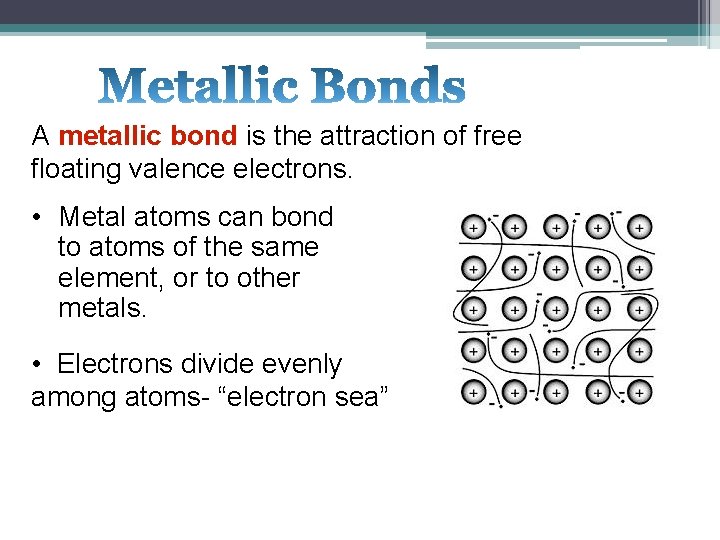
A metallic bond is the attraction of free floating valence electrons. • Metal atoms can bond to atoms of the same element, or to other metals. • Electrons divide evenly among atoms- “electron sea”

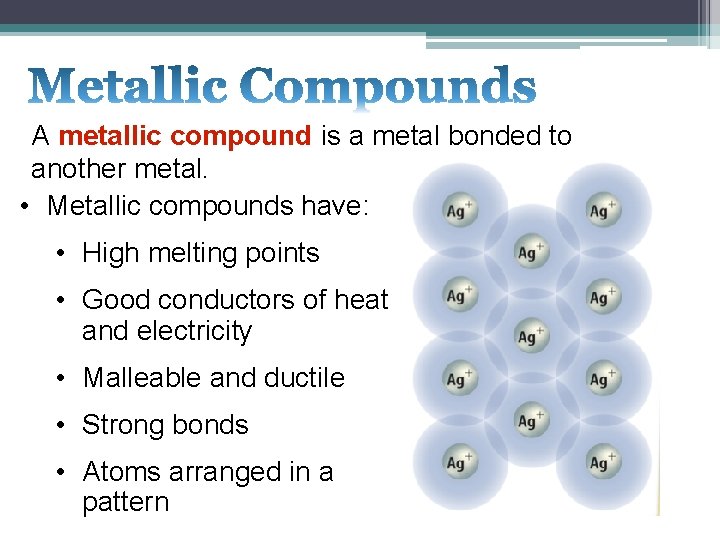
A metallic compound is a metal bonded to another metal. • Metallic compounds have: • High melting points • Good conductors of heat and electricity • Malleable and ductile • Strong bonds • Atoms arranged in a pattern

• A covalent bond is a chemical bond formed when atoms share valence electrons. Usually nonmetals with nonmetals. • Elements that are close together on the periodic table are more likely to share electrons in a covalent bond than to transfer electrons. • Hydrogen, Carbon, Nitrogen, and Oxygen usually form covalent bonds.

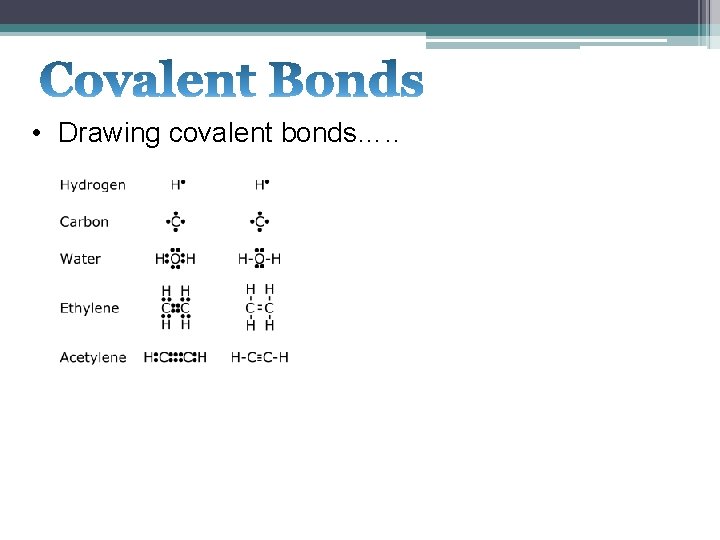
• Drawing covalent bonds…. .

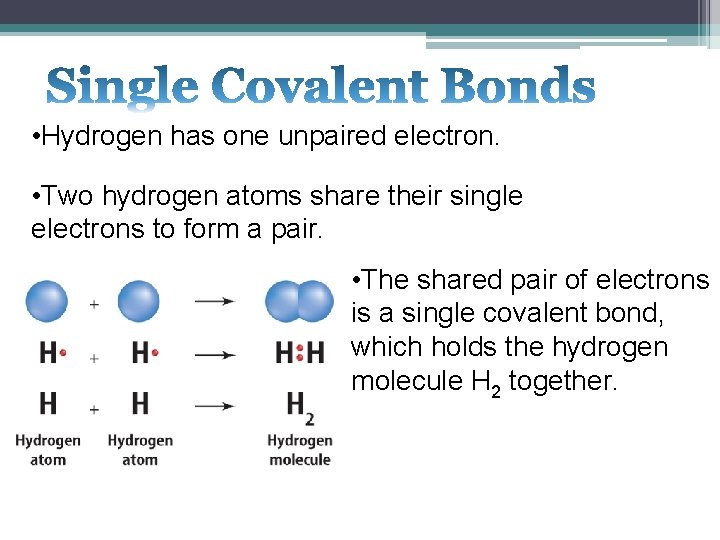
• Hydrogen has one unpaired electron. • Two hydrogen atoms share their single electrons to form a pair. • The shared pair of electrons is a single covalent bond, which holds the hydrogen molecule H 2 together.

Drawing single covalent bonds….

• Some atoms may form stronger bonds by sharing more than one pair of electrons. • A double bond has two pairs of shared electrons and is stronger than a single bond. • A triple bond has three pairs of shared electrons and is stronger than a double bond.

Drawing double & triple bonds….

• Drawing covalent bonds…. .

Hydrogen, Oxygen, Nitrogen, and Carbon make up 98% of all living things

• Every Hydrogen atom has 1 line connecting it to other atoms. • Every Oxygen atom has 2 lines connecting it to other atoms. • Every Nitrogen atom has 3 lines connecting it to other atoms. • Every Carbon atom has 4 lines connecting it to other atoms.

• Do not conduct electricity in water. • More flammable than ionic compounds. • Softer than ionic compounds. • Low melting and boiling points. • Usually do not dissolve in water (not soluble)

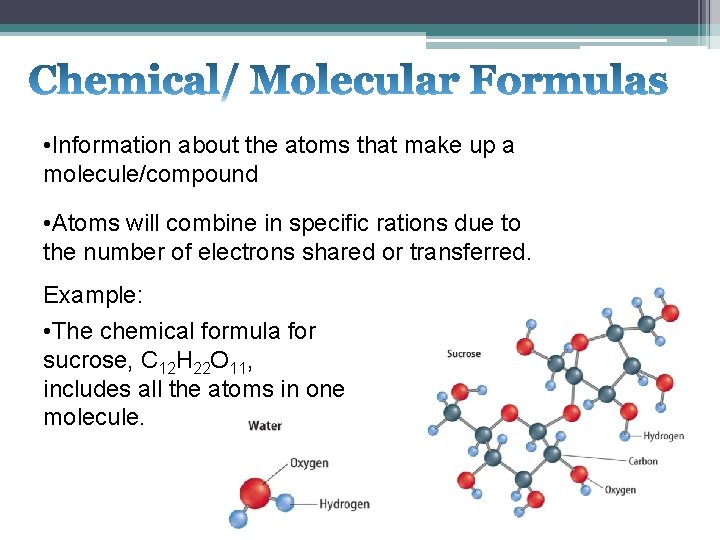
• Information about the atoms that make up a molecule/compound • Atoms will combine in specific rations due to the number of electrons shared or transferred. Example: • The chemical formula for sucrose, C 12 H 22 O 11, includes all the atoms in one molecule.

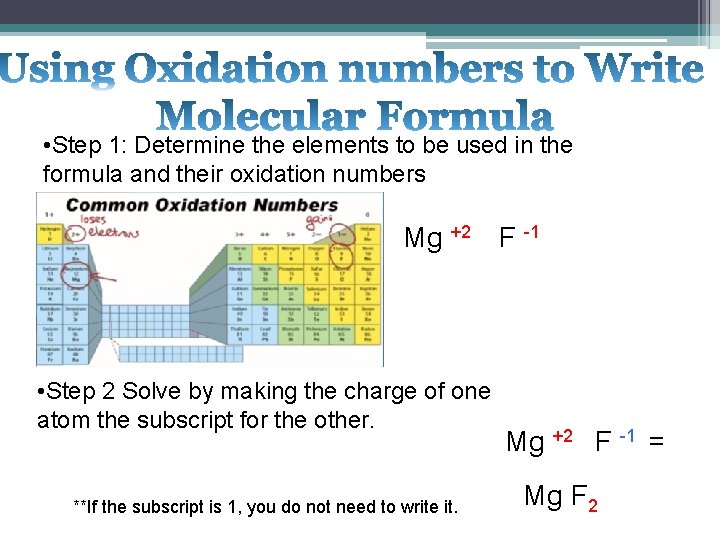
• Step 1: Determine the elements to be used in the formula and their oxidation numbers Mg +2 • Step 2 Solve by making the charge of one atom the subscript for the other. **If the subscript is 1, you do not need to write it. F -1 Mg +2 F -1 = Mg F 2

• Try it: • Sodium Chloride • Calcium Fluoride • Carbon Tetrachloride • Aluminum Oxide • Magnesium Oxide • Carbon Dioxide **If the subscript is 1, you do not need to write it.

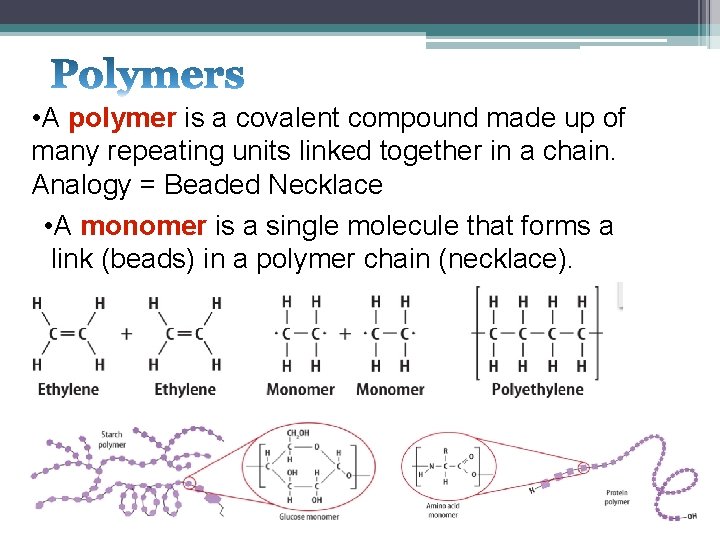
• A polymer is a covalent compound made up of many repeating units linked together in a chain. Analogy = Beaded Necklace • A monomer is a single molecule that forms a link (beads) in a polymer chain (necklace).

• Examples of Polymers: • • • Carbohydrates Plastics Proteins DNA Rubber

• Acids are substances that release a positively charged hydrogen ion, H+, in water. The strength of the acid depends on the concentration of H+ ions. • Acids are also used in making many products, such as fertilizers, detergent, and cleaners. • Acids are important in several body processes, including breaking down food in the stomach. Acid Properties • Can neutralize a base • Sour • Turn blue litmus paper red • Feel like water • Conduct electricity • p. H between 1 -6 • React with metals

• Bases are substances that produce negatively charged hydroxide ions (OH-) when dissolved in water. • Common bases include baking soda and cleaning agents. Base Properties • Feel slippery • Dissolve fats, oils, and grease • Bitter taste • Neutralize acids to produce salt and water • Turn red litmus paper blue • Conduct electricity • Have a p. H between 8 -14 � Acids and Bases

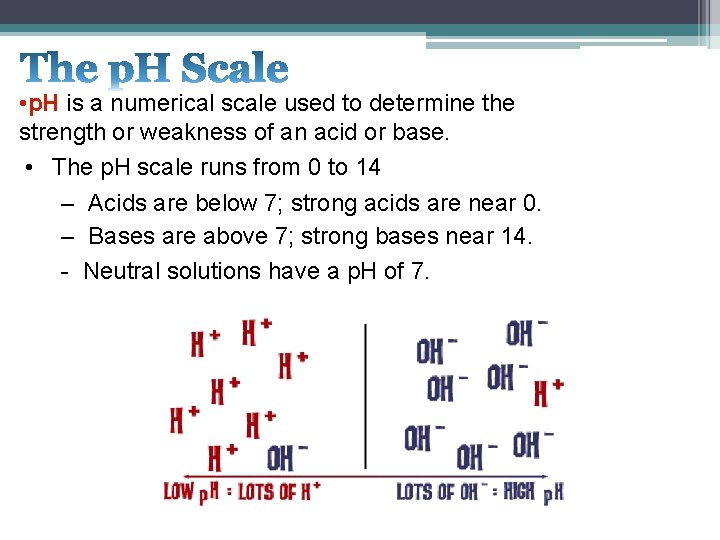
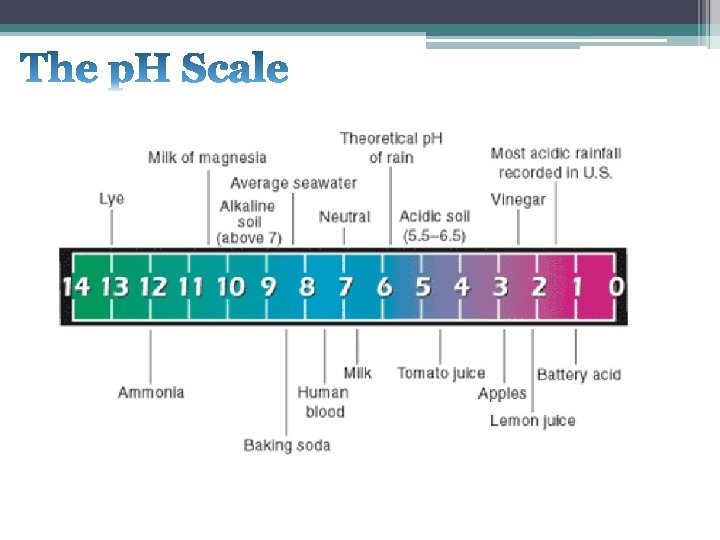
• p. H is a numerical scale used to determine the strength or weakness of an acid or base. • The p. H scale runs from 0 to 14 – Acids are below 7; strong acids are near 0. – Bases are above 7; strong bases near 14. - Neutral solutions have a p. H of 7.

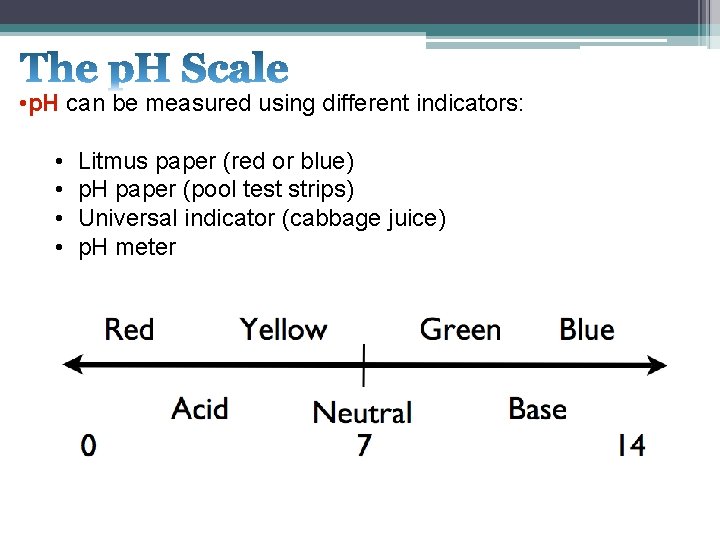
• p. H can be measured using different indicators: • • Litmus paper (red or blue) p. H paper (pool test strips) Universal indicator (cabbage juice) p. H meter


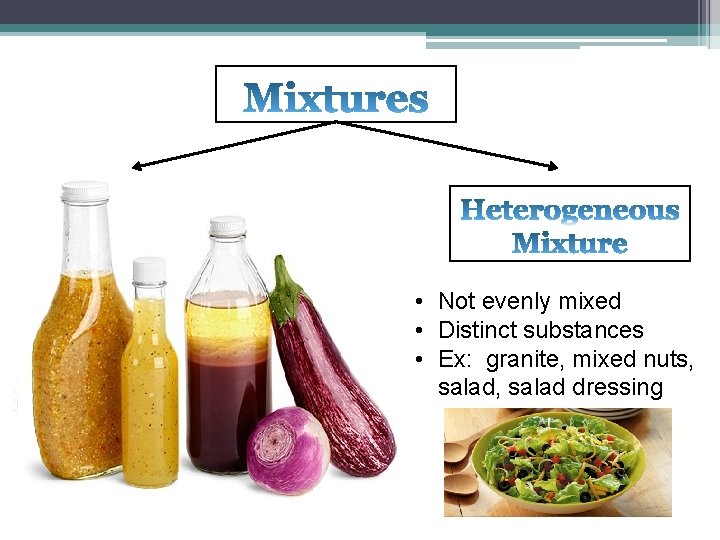
• A mixture is a combination of two or more substances that can be separated by physical means (i. e. filtering, evaporation, magnet, etc. ) • NO CHEMICAL BOND • Solutions are also mixtures. • Solutions are groups of molecules that are mixed up in a completely even distribution.

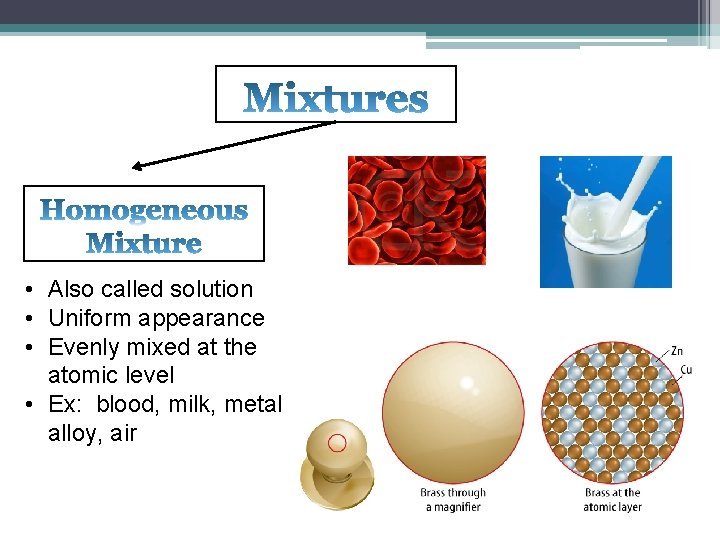
• Also called solution • Uniform appearance • Evenly mixed at the atomic level • Ex: blood, milk, metal alloy, air

• Also called solution • Uniform appearance • Evenly mixed at the atomic level • Ex: blood, milk, metal alloy, air • Not evenly mixed • Distinct substances • Ex: granite, mixed nuts, salad dressing

Physical properties can be observed without changing the substance. Ex: phase of matter, color, taste, odor, shape, texture, density, melting point, boiling point, hardness, conductivity, malleability


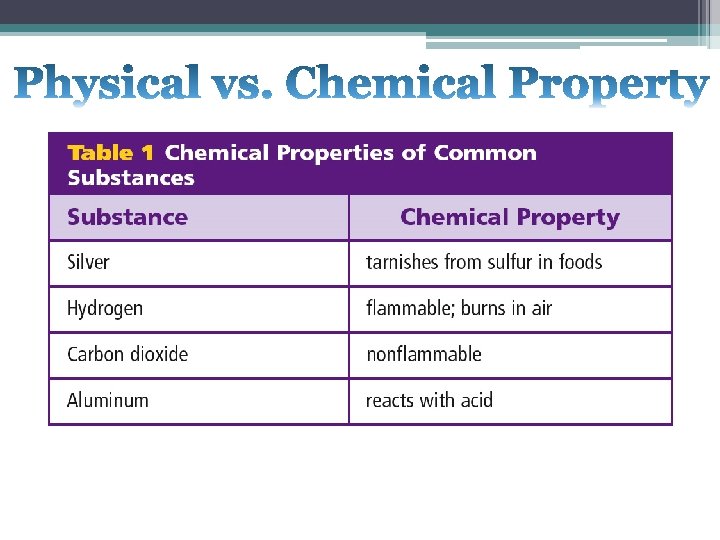
A chemical property is the ability or inability of a substance to combine with or change into one or more new substances (how a substance reacts with other substances). • Examples of chemical properties include burning or rusting. • Some substances do not react.


• Physical change: What the substance is made of does not change. Physical changes are reversible. • Ex) Changing from one state to another Other Examples: Cutting a lawn, breaking glass, Melting ice

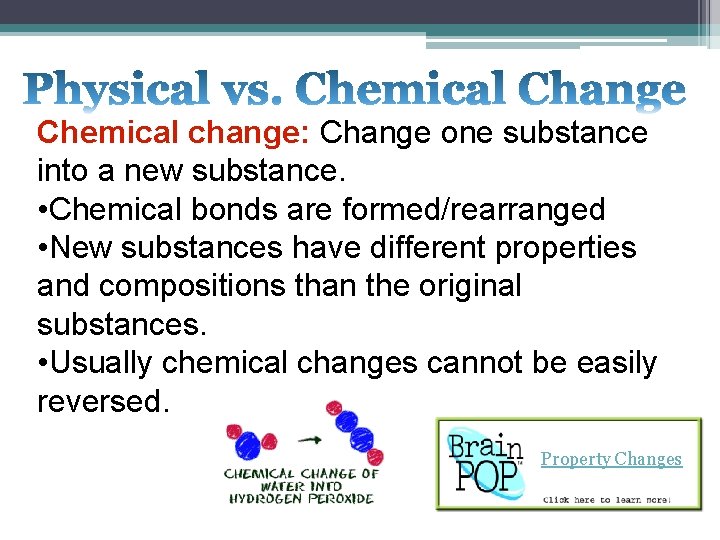
Chemical change: Change one substance into a new substance. • Chemical bonds are formed/rearranged • New substances have different properties and compositions than the original substances. • Usually chemical changes cannot be easily reversed. Property Changes

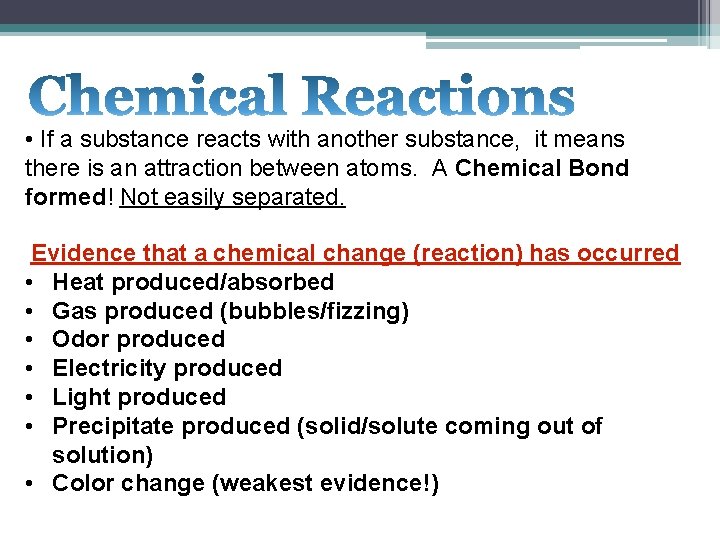
• If a substance reacts with another substance, it means there is an attraction between atoms. A Chemical Bond formed! Not easily separated. Evidence that a chemical change (reaction) has occurred • Heat produced/absorbed • Gas produced (bubbles/fizzing) • Odor produced • Electricity produced • Light produced • Precipitate produced (solid/solute coming out of solution) • Color change (weakest evidence!)

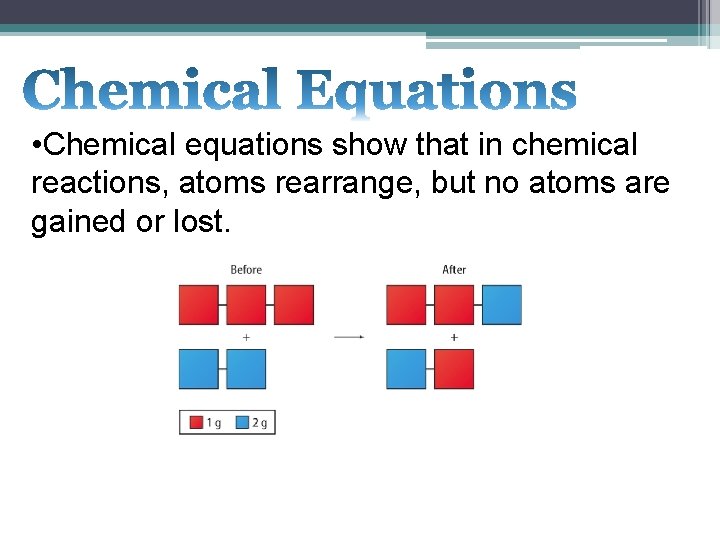
• Chemical equations show that in chemical reactions, atoms rearrange, but no atoms are gained or lost.

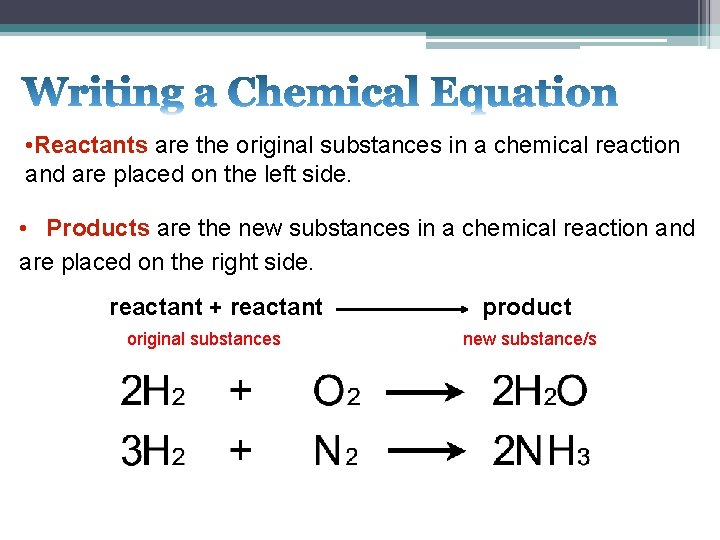
• Reactants are the original substances in a chemical reaction and are placed on the left side. • Products are the new substances in a chemical reaction and are placed on the right side. reactant + reactant original substances product new substance/s

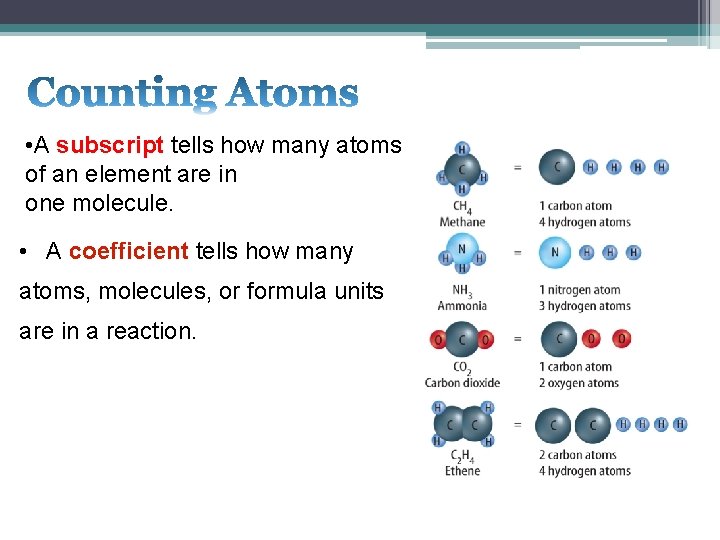
• A subscript tells how many atoms of an element are in one molecule. • A coefficient tells how many atoms, molecules, or formula units are in a reaction.

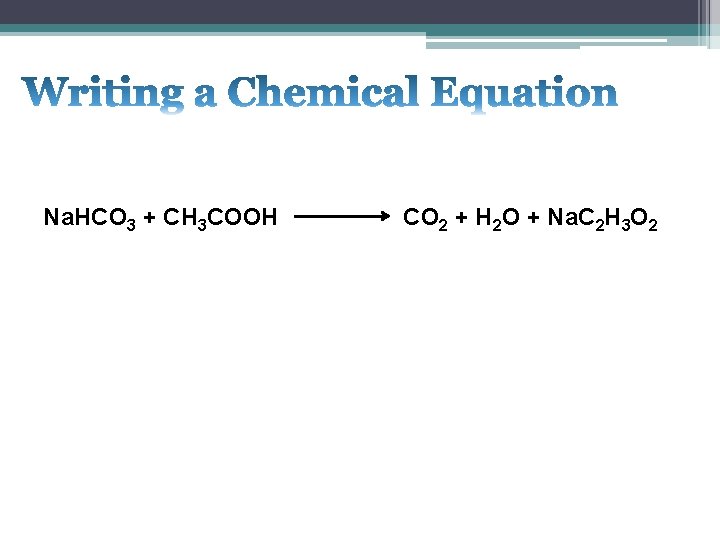
Na. HCO 3 + CH 3 COOH CO 2 + H 2 O + Na. C 2 H 3 O 2

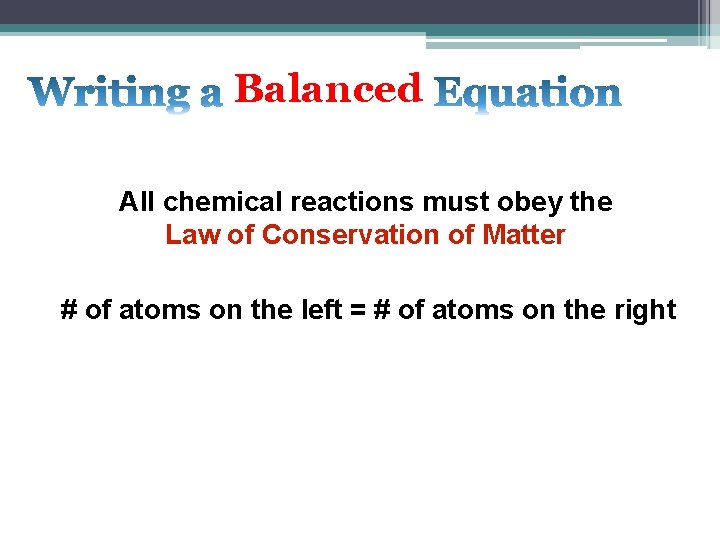
Balanced All chemical reactions must obey the Law of Conservation of Matter # of atoms on the left = # of atoms on the right

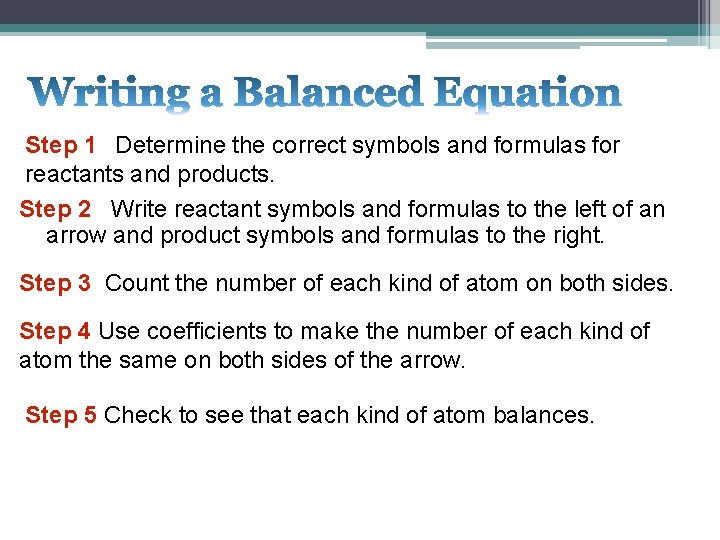
Step 1 Determine the correct symbols and formulas for reactants and products. Step 2 Write reactant symbols and formulas to the left of an arrow and product symbols and formulas to the right. Step 3 Count the number of each kind of atom on both sides. Step 4 Use coefficients to make the number of each kind of atom the same on both sides of the arrow. Step 5 Check to see that each kind of atom balances.

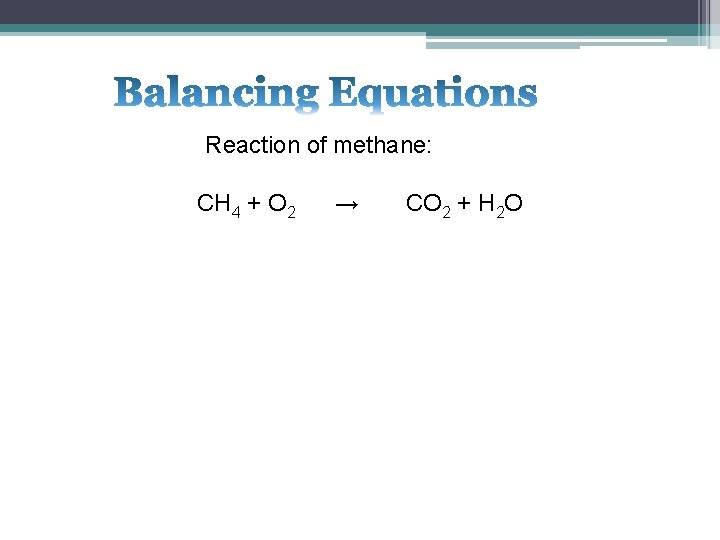
Reaction of methane: CH 4 + O 2 → CO 2 + H 2 O