Molecules and Compounds Molecule A molecule is formed









- Slides: 30

Molecules and Compounds

Molecule A molecule is formed when two or more atoms join together chemically. Ex: O₂, Na. Cl, H₂O

Compounds A compound is a molecule that contains at least two different elements. Ex: Na. Cl, H₂O, NOTE: All compounds are molecules but not all molecules are compounds.

Compound or Molecule? CO₂ H₂ H₂SO₄ N₂

Water (H 2 O), carbon dioxide (CO 2) and methane (CH 4) are compounds because each is made from more than one element. For example, a single molecule of molecular hydrogen is made from two atoms of hydrogen (H₂) A single molecule of water is made from two atoms of hydrogen and one atom of oxygen (H 2 O)

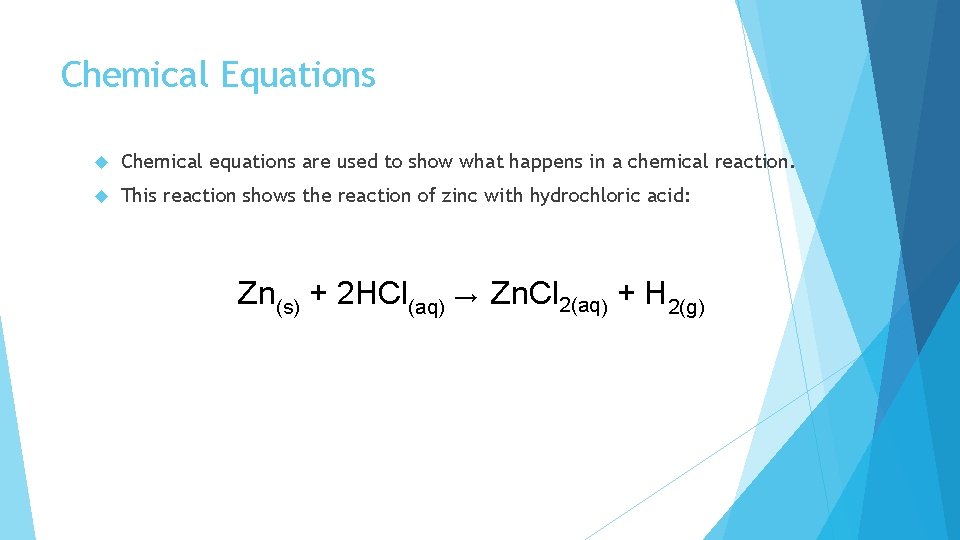
Chemical Equations Chemical equations are used to show what happens in a chemical reaction. This reaction shows the reaction of zinc with hydrochloric acid: Zn(s) + 2 HCl(aq) → Zn. Cl 2(aq) + H 2(g)

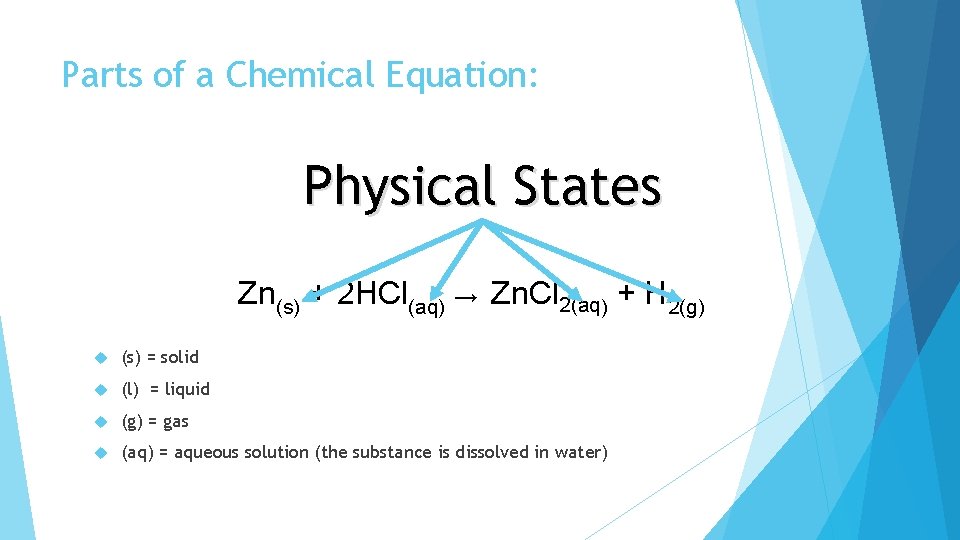
Parts of a Chemical Equation: Physical States Zn(s) + 2 HCl(aq) → Zn. Cl 2(aq) + H 2(g) (s) = solid (l) = liquid (g) = gas (aq) = aqueous solution (the substance is dissolved in water)

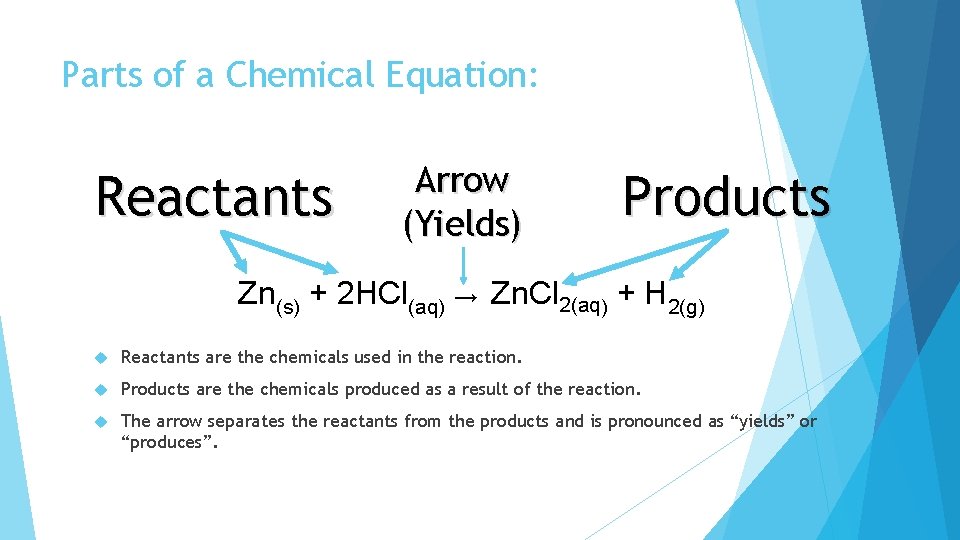
Parts of a Chemical Equation: Reactants Arrow (Yields) Products Zn(s) + 2 HCl(aq) → Zn. Cl 2(aq) + H 2(g) Reactants are the chemicals used in the reaction. Products are the chemicals produced as a result of the reaction. The arrow separates the reactants from the products and is pronounced as “yields” or “produces”.

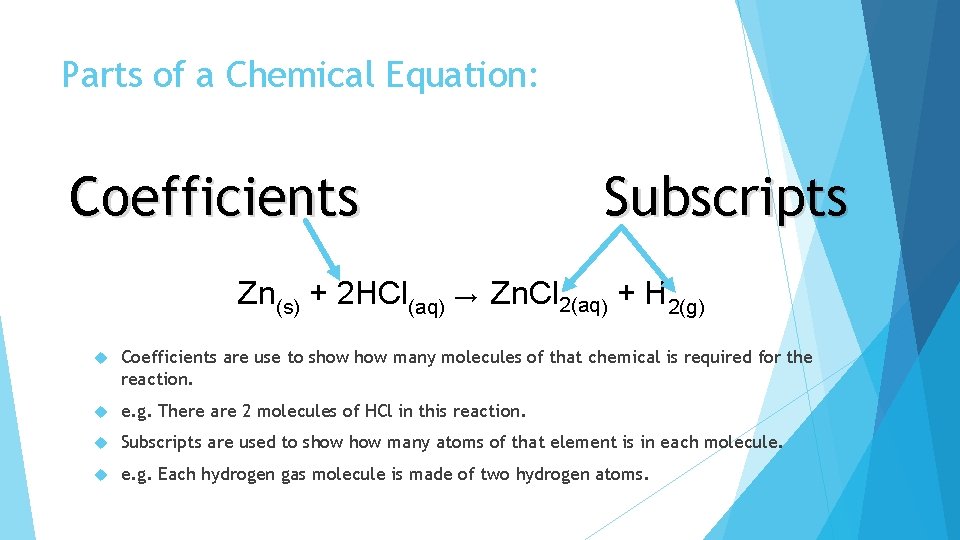
Parts of a Chemical Equation: Coefficients Subscripts Zn(s) + 2 HCl(aq) → Zn. Cl 2(aq) + H 2(g) Coefficients are use to show many molecules of that chemical is required for the reaction. e. g. There are 2 molecules of HCl in this reaction. Subscripts are used to show many atoms of that element is in each molecule. e. g. Each hydrogen gas molecule is made of two hydrogen atoms.

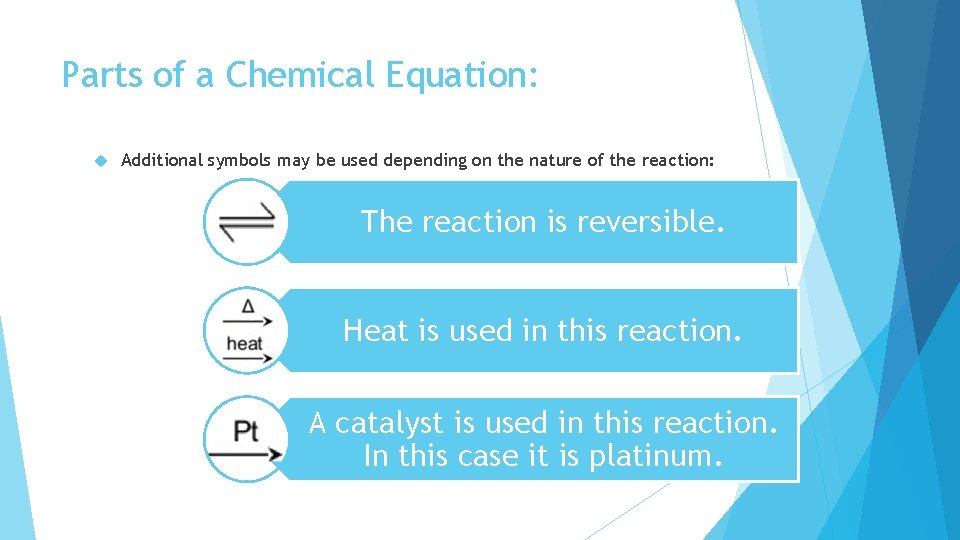
Parts of a Chemical Equation: Additional symbols may be used depending on the nature of the reaction: The reaction is reversible. Heat is used in this reaction. A catalyst is used in this reaction. In this case it is platinum.

Chemical Bonds For compounds or molecules to form, bonds must be created between them A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges known as electrons

Chemical Bonds There are two different types of chemical bonds, ionic bonds and covalent bonds Covalent bonds are formed when atoms share electrons and are generally stronger Ionic bonds are formed when atoms give their electrons to other atoms and are generally weaker

Structural Formulas When atoms bond together to form molecules or compounds, they take on a structure We can represent this as a structural formula which shows us how many atoms make up the compound and the bonds between them

Structural Formulas Example: H Hydrogen Gas H

Structural Formulas Example: H Hydrogen Chloride Cl

Structural Formulas Example: Na Sodium Chloride Cl

Structural Formulas Example: Water O H H

Structural Formulas Example: Ammonia H N H H

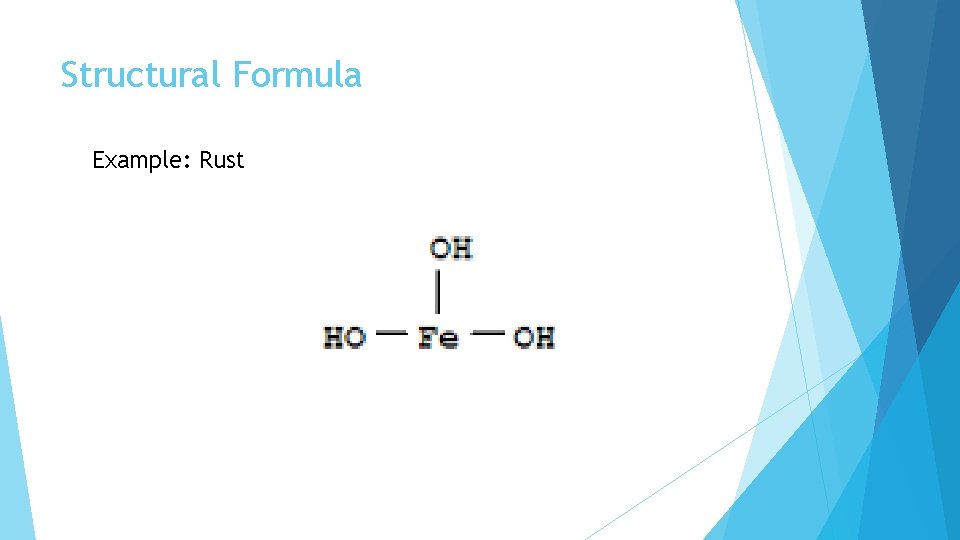
Structural Formula Example: Rust

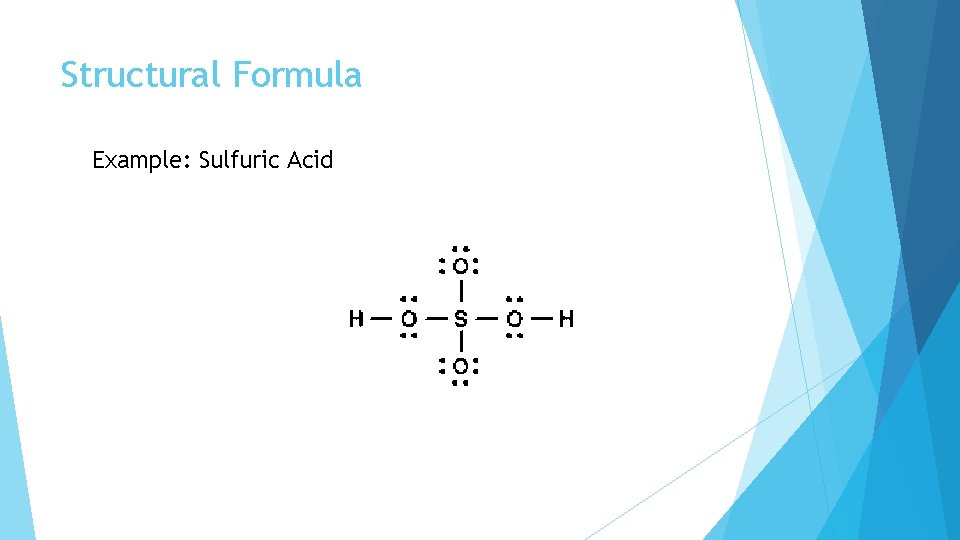
Structural Formula Example: Sulfuric Acid

Condensed Formulas We can write our structural formulas as molecular formulas that will still show the structure We call these condensed formulas

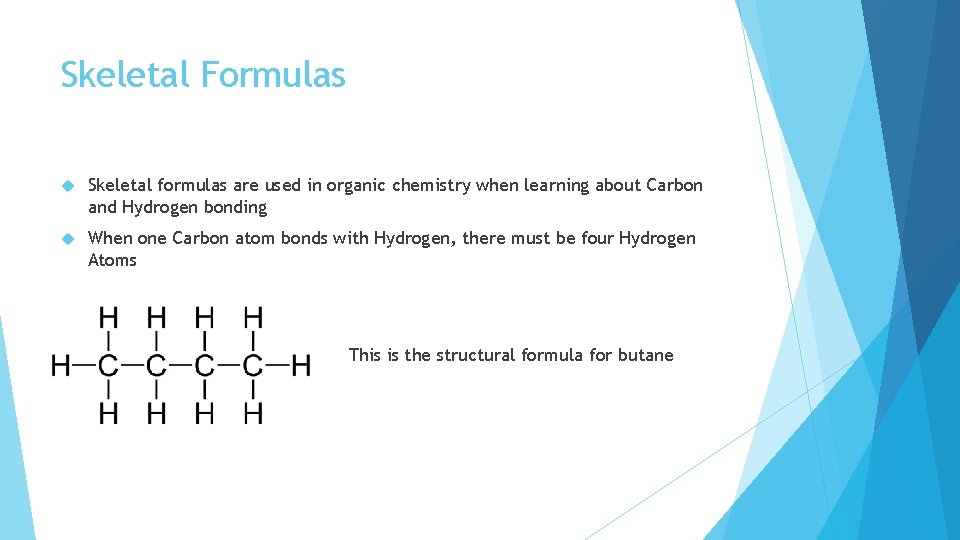
Skeletal Formulas Skeletal formulas are used in organic chemistry when learning about Carbon and Hydrogen bonding When one Carbon atom bonds with Hydrogen, there must be four Hydrogen Atoms

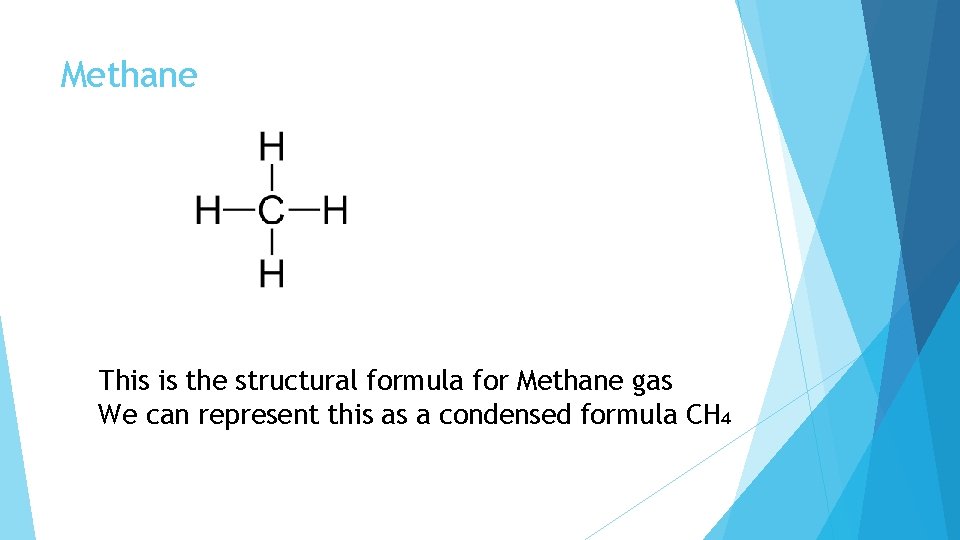
Methane This is the structural formula for Methane gas We can represent this as a condensed formula CH₄

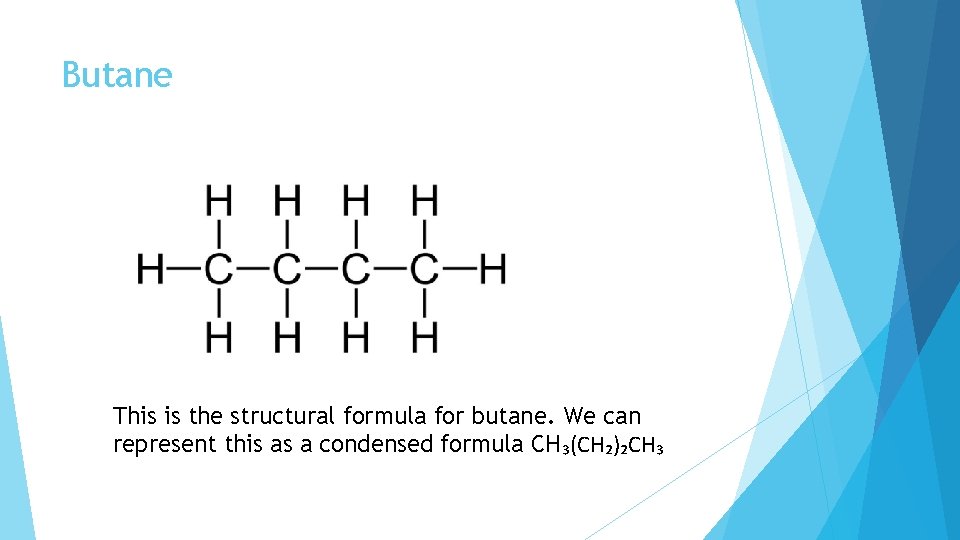
Butane This is the structural formula for butane. We can represent this as a condensed formula CH₃(CH₂)₂CH₃

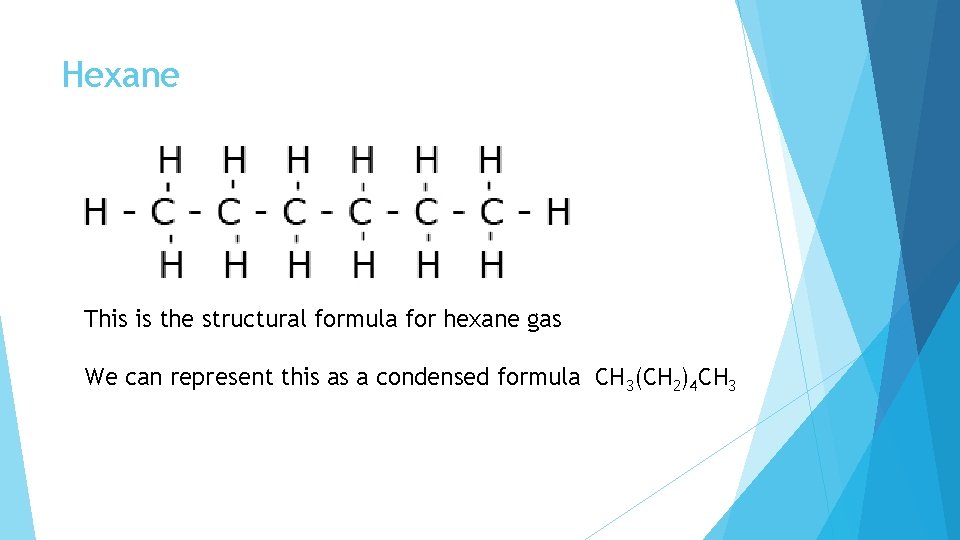
Hexane This is the structural formula for hexane gas We can represent this as a condensed formula CH 3(CH 2)4 CH 3

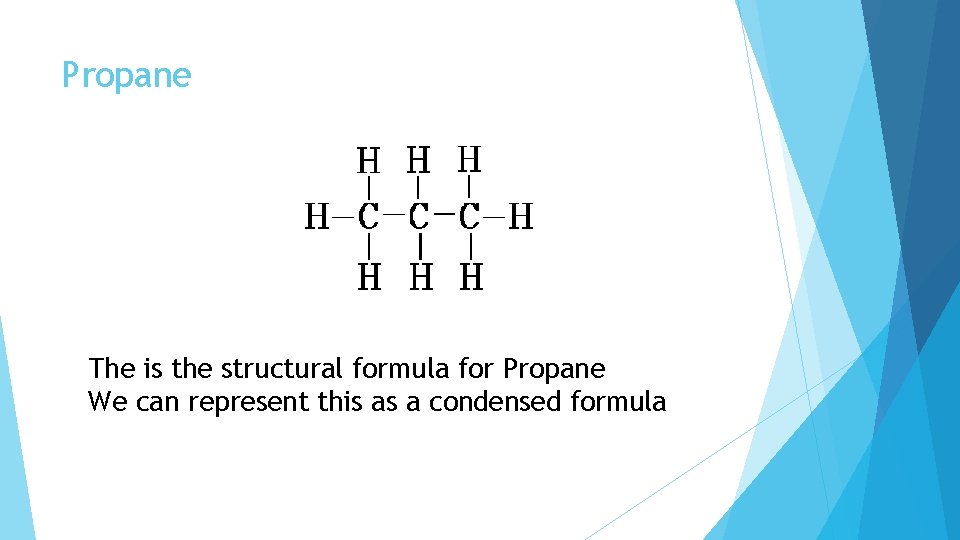
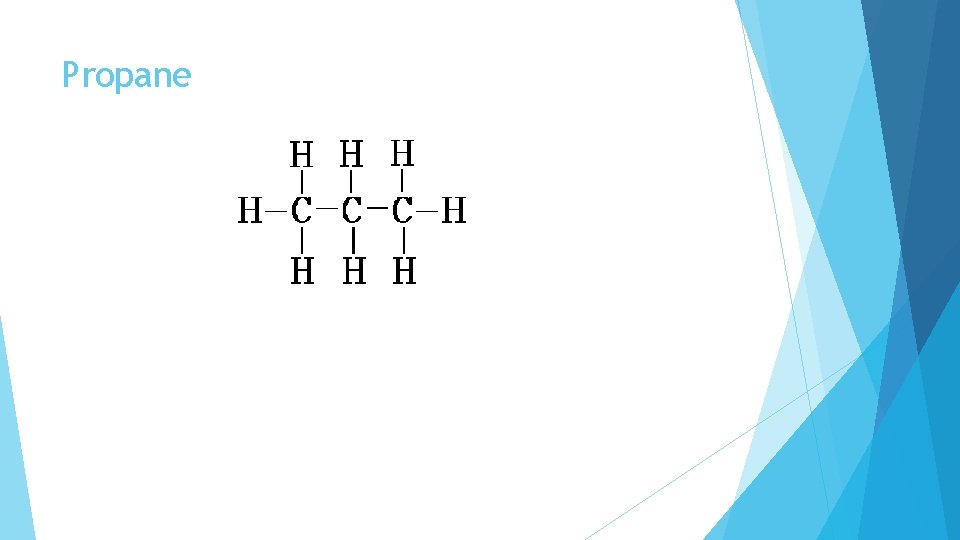
Propane The is the structural formula for Propane We can represent this as a condensed formula

Skeletal Formulas Skeletal formulas are used in organic chemistry when learning about Carbon and Hydrogen bonding When one Carbon atom bonds with Hydrogen, there must be four Hydrogen Atoms This is the structural formula for butane

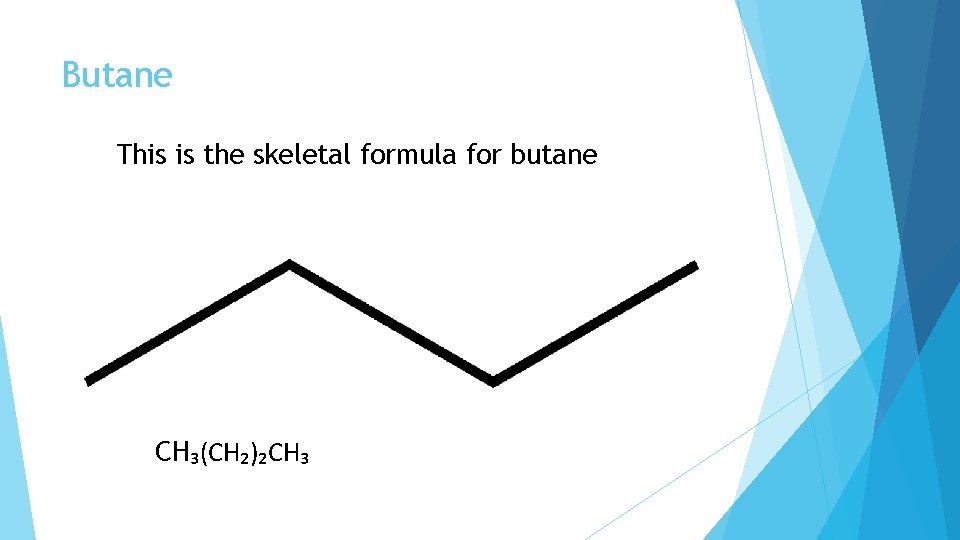
Butane This is the skeletal formula for butane CH₃(CH₂)₂CH₃

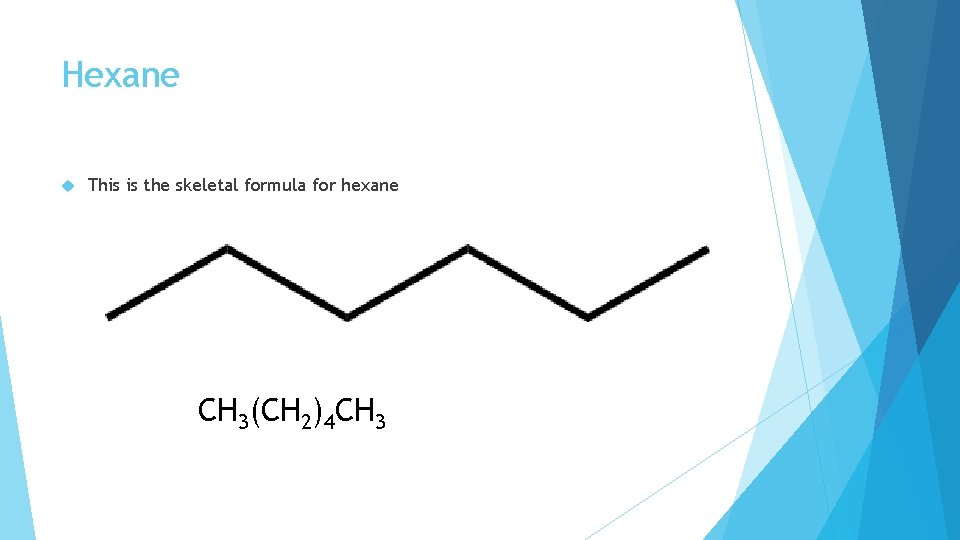
Hexane This is the skeletal formula for hexane CH 3(CH 2)4 CH 3

Propane