ChemistryWater Hot Spot Review Molecule of Life Molecule



![p. H and [H+] conversions Ex. p. H= 8 or [H+]= 1 x 10 p. H and [H+] conversions Ex. p. H= 8 or [H+]= 1 x 10](https://slidetodoc.com/presentation_image_h2/a5fc5e817f452cd4e6393103bb523ecf/image-18.jpg)
![Acids- Strong versus Weak Acid- donor of [H+] , increases [H+] in sol’n ●Strong Acids- Strong versus Weak Acid- donor of [H+] , increases [H+] in sol’n ●Strong](https://slidetodoc.com/presentation_image_h2/a5fc5e817f452cd4e6393103bb523ecf/image-21.jpg)
![Base- Strong versus Weak ● Base- acceptor of [H+] , dec. [H+] in sol’n Base- Strong versus Weak ● Base- acceptor of [H+] , dec. [H+] in sol’n](https://slidetodoc.com/presentation_image_h2/a5fc5e817f452cd4e6393103bb523ecf/image-22.jpg)

- Slides: 29

Chemistry/Water Hot Spot Review

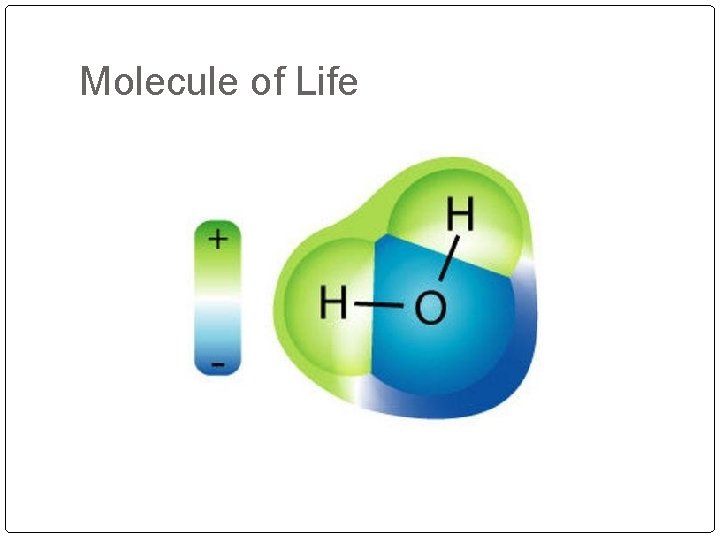
Molecule of Life

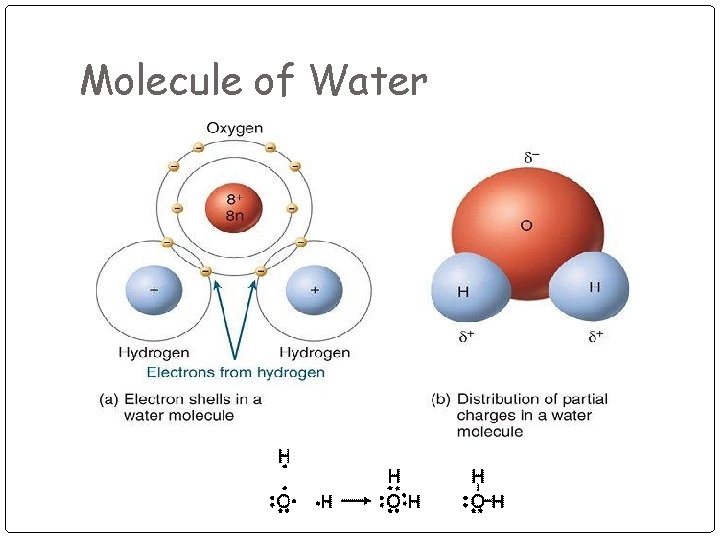
Molecule of Water

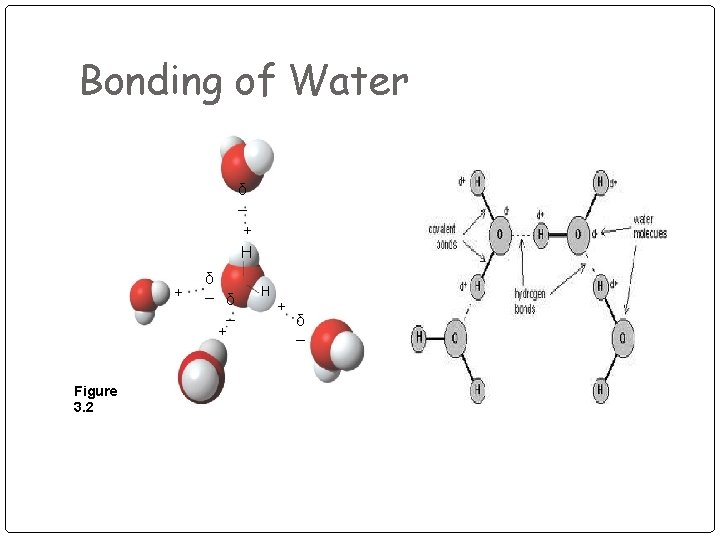
Bonding of Water δ – + H + Figure 3. 2 δ – + H + δ –

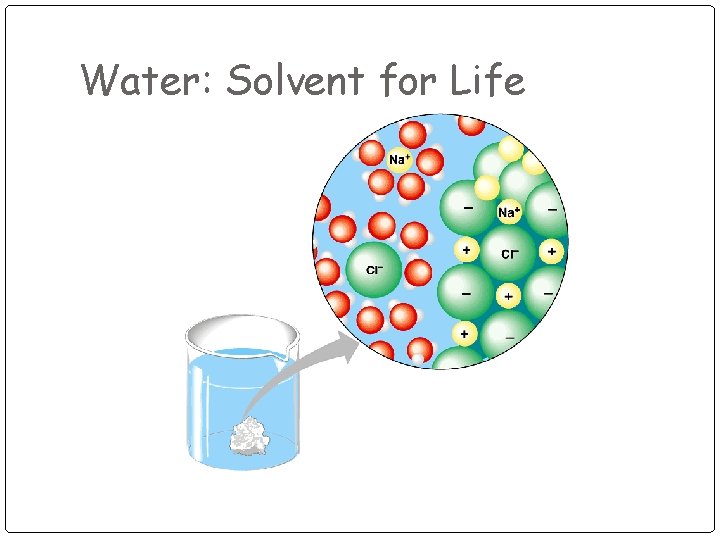
Water: Solvent for Life

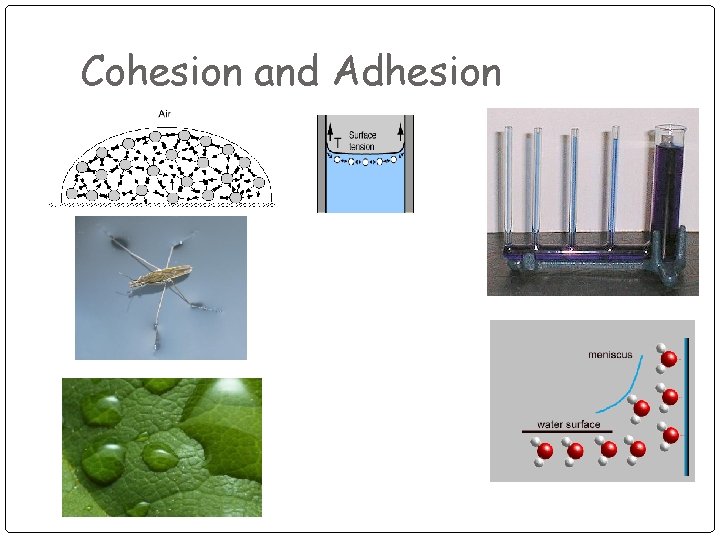
Cohesion and Adhesion

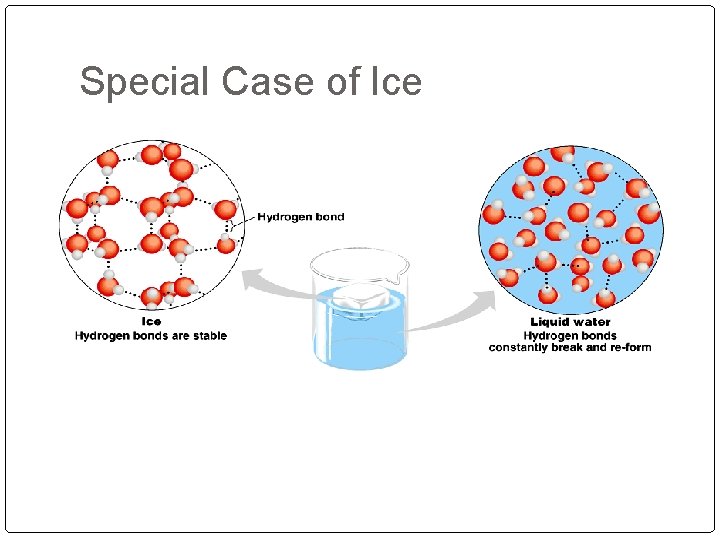
Special Case of Ice

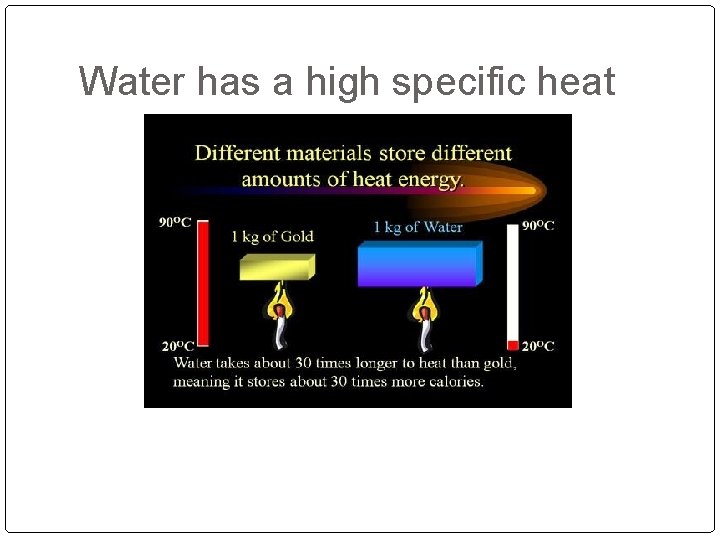
Water has a high specific heat

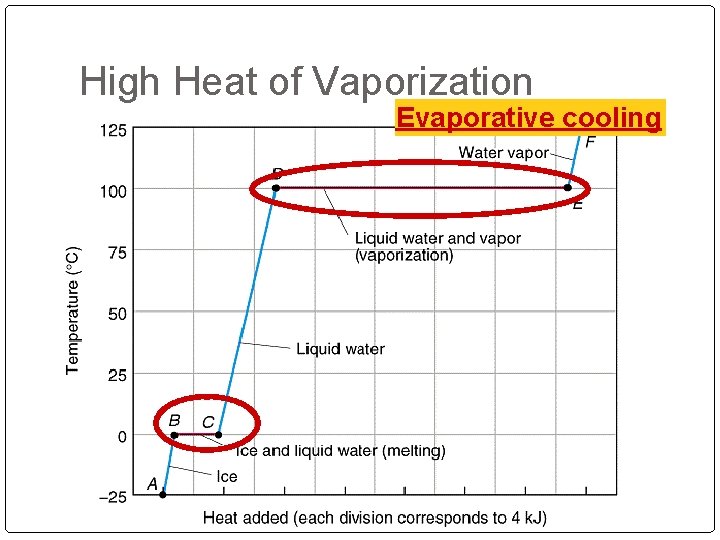
High Heat of Vaporization Evaporative cooling Organisms rely on heat of vaporization to remove body heat

Mr. Anderson Water Review http: //www. youtube. com/watch? v=DVCYl. ST 6 m. YQ&feature=rel ated

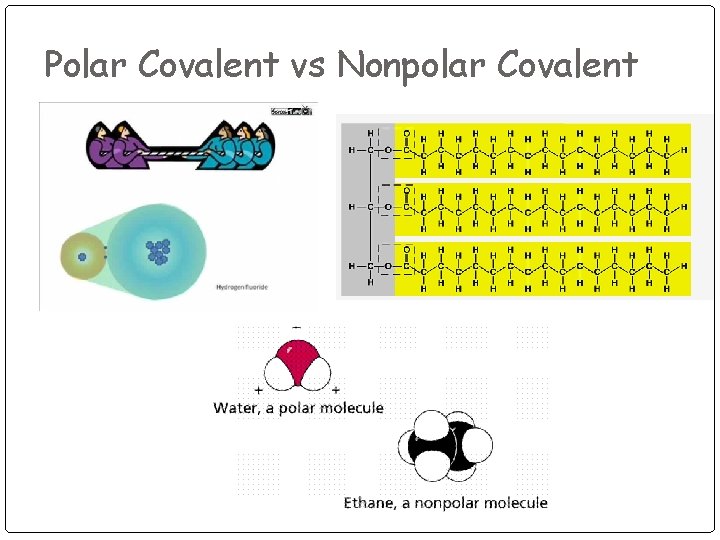
Polar Covalent vs Nonpolar Covalent

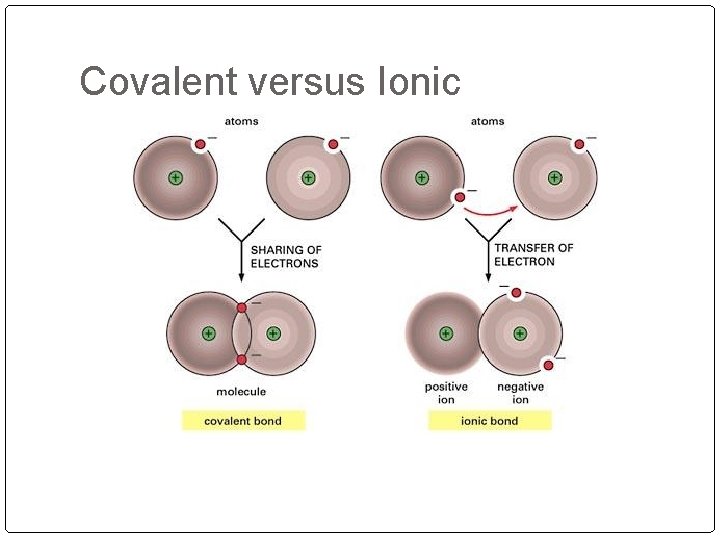
Covalent versus Ionic

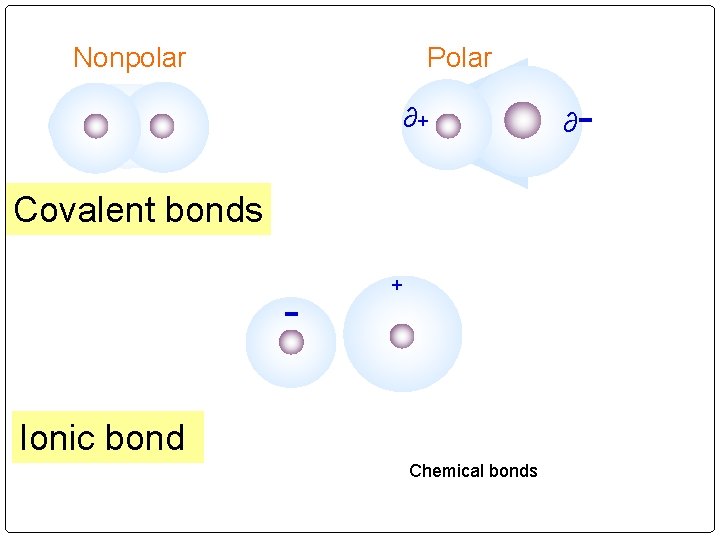
Nonpolar Polar ∂+ Covalent bonds - + Ionic bond Chemical bonds ∂ -

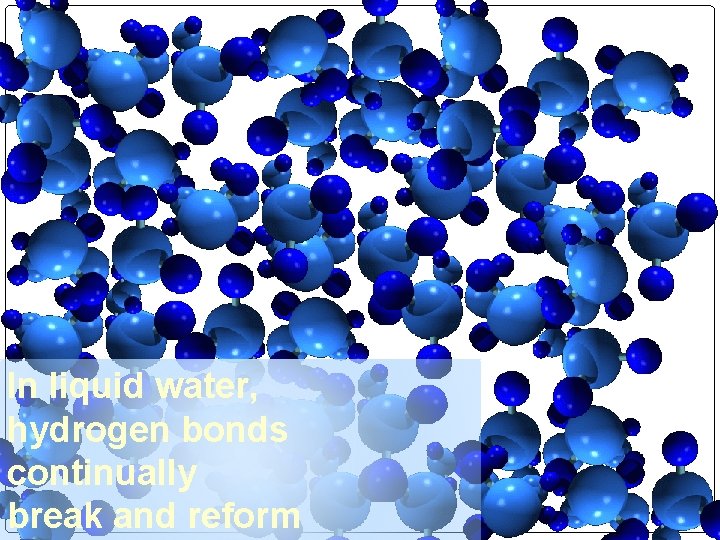
In liquid water, hydrogen bonds continually break and reform

Mr. Anderson p. H Review http: //www. youtube. com/watch? v=V 4 S 1 Kl. Jd. Mb. E&feature=BFa&list=PL 432856 91048 DAD 00


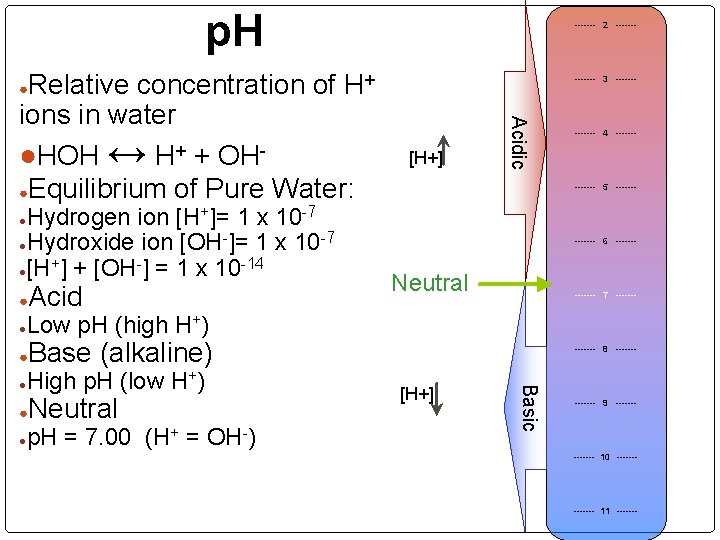
p. H Relative concentration of H+ ions in water ●HOH ↔ H+ + OH●Equilibrium of Pure Water: ------- 2 ------- 3 ------- ● Acidic Hydrogen ion [H+]= 1 x 10 -7 -7 ●Hydroxide ion [OH ]= 1 x 10 + -14 ●[H ] + [OH ] = 1 x 10 [H+] ------- 4 ------- 5 ------- ● ● Acid ------- 6 ------- Neutral ------- 7 ------- Low p. H (high H+) ● ● Base (alkaline) ● Neutral p. H = 7. 00 (H+ = OH-) [H+] Basic High p. H (low H+) ● ------- 8 ------- 9 ------- ● ------- 10 ------- 11 -------
![p H and H conversions Ex p H 8 or H 1 x 10 p. H and [H+] conversions Ex. p. H= 8 or [H+]= 1 x 10](https://slidetodoc.com/presentation_image_h2/a5fc5e817f452cd4e6393103bb523ecf/image-18.jpg)
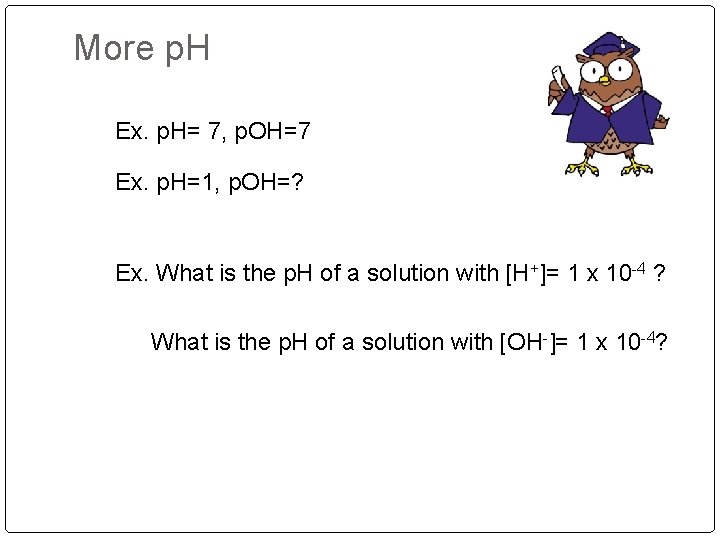
p. H and [H+] conversions Ex. p. H= 8 or [H+]= 1 x 10 -8 Ex. p. H = 10, [H+]= ? Ex. [H+]= 1 x 10 -8 , p. H? Ex. [H+]= 1 x 10 -4 [OH-]= 1 x 10 -10 Ex. [H+]= 1 x 10 -12 , [OH-]= ? p. H= -log 10 [H+] = 10 -p. H p. OH=-log 10 [OH-] p. H+ p. OH= 14 p. H+ p. OH= 1 x 10 -14

More p. H Ex. p. H= 7, p. OH=7 Ex. p. H=1, p. OH=? Ex. What is the p. H of a solution with [H+]= 1 x 10 -4 ? What is the p. H of a solution with [OH-]= 1 x 10 -4?

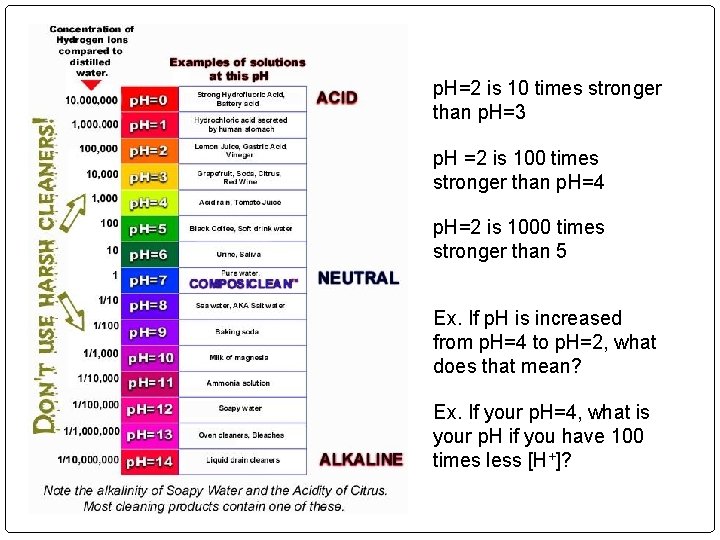
p. H=2 is 10 times stronger than p. H=3 p. H =2 is 100 times stronger than p. H=4 p. H=2 is 1000 times stronger than 5 Ex. If p. H is increased from p. H=4 to p. H=2, what does that mean? Ex. If your p. H=4, what is your p. H if you have 100 times less [H+]?
![Acids Strong versus Weak Acid donor of H increases H in soln Strong Acids- Strong versus Weak Acid- donor of [H+] , increases [H+] in sol’n ●Strong](https://slidetodoc.com/presentation_image_h2/a5fc5e817f452cd4e6393103bb523ecf/image-21.jpg)
Acids- Strong versus Weak Acid- donor of [H+] , increases [H+] in sol’n ●Strong Acids- completely dissociated in water ●Weak Acids- do not completely dissociate (Carbonic Acid) H 2 CO 3 HCO 3 - + H+ Carbonic Acid Bicarbonate Ion Hydrogen Ion ●
![Base Strong versus Weak Base acceptor of H dec H in soln Base- Strong versus Weak ● Base- acceptor of [H+] , dec. [H+] in sol’n](https://slidetodoc.com/presentation_image_h2/a5fc5e817f452cd4e6393103bb523ecf/image-22.jpg)
Base- Strong versus Weak ● Base- acceptor of [H+] , dec. [H+] in sol’n 1) Weak Base: reduces [H+] by accepting hydrogen ions NH 3 + H+ NH 4+ ● 2) Strong Base: reduces [H+] by dissociating to form Hydroxide ions [OH-] Na. OH Na+ + OH●

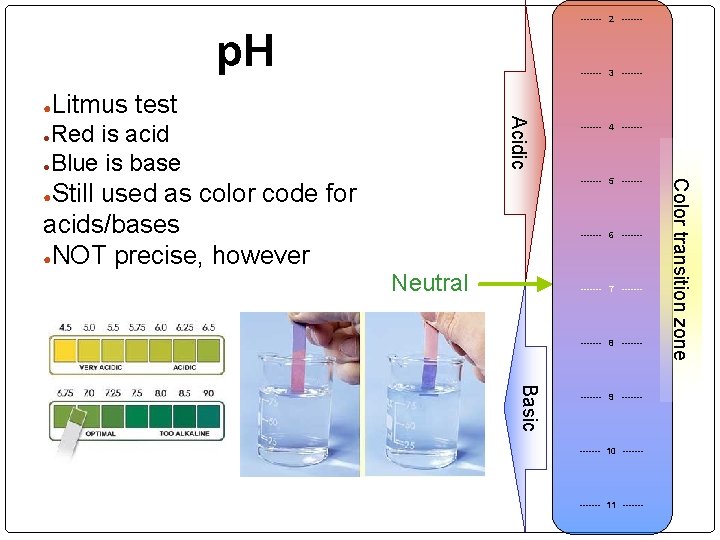
------- 2 ------- p. H ● ------- 3 ------- Acidic Litmus test Red is acid ●Blue is base ● ------- 5 ------- ● ------- 6 ------- Neutral ------- 7 ------- 8 ------- Basic ------- 9 ------- 10 ------- 11 ------- Color transition zone Still used as color code for acids/bases ●NOT precise, however ------- 4 -------

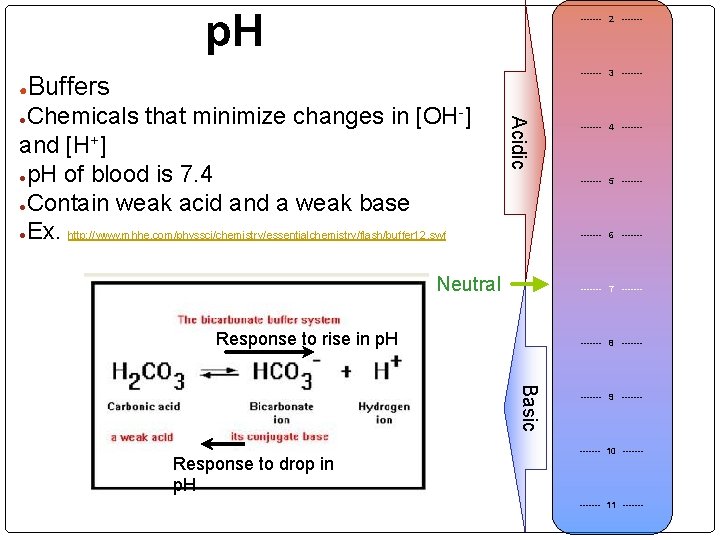
p. H ● ------- 2 ------- 3 ------- Buffers Acidic Chemicals that minimize changes in [OH-] and [H+] ●p. H of blood is 7. 4 ●Contain weak acid and a weak base ●Ex. http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/buffer 12. swf ● ------- 5 ------- 6 ------- Neutral ------- 7 ------- Response to rise in p. H ------- 8 ------- Basic Response to drop in p. H ------- 4 ------- 9 ------- 10 ------- 11 -------

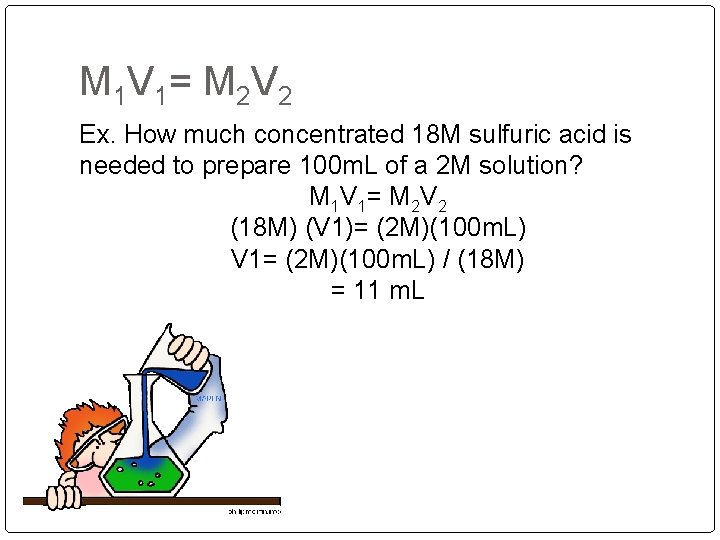
M 1 V 1 = M 2 V 2 Ex. How much concentrated 18 M sulfuric acid is needed to prepare 100 m. L of a 2 M solution? M 1 V 1 = M 2 V 2 (18 M) (V 1)= (2 M)(100 m. L) V 1= (2 M)(100 m. L) / (18 M) = 11 m. L

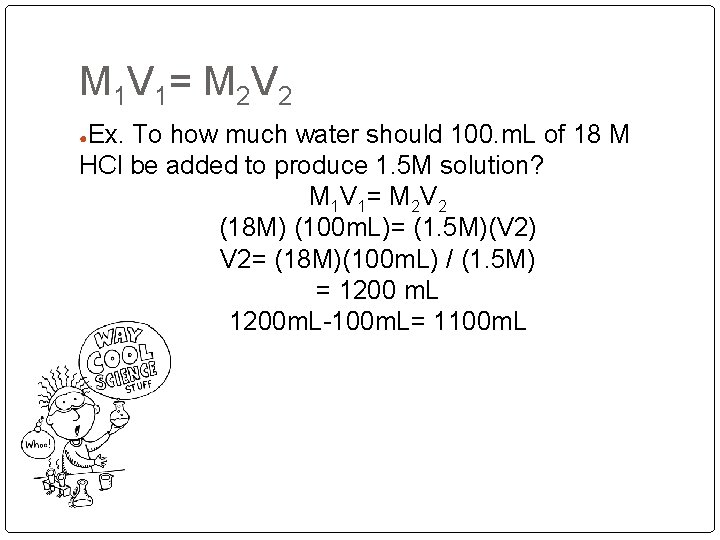
M 1 V 1 = M 2 V 2 Ex. To how much water should 100. m. L of 18 M HCl be added to produce 1. 5 M solution? M 1 V 1 = M 2 V 2 (18 M) (100 m. L)= (1. 5 M)(V 2) V 2= (18 M)(100 m. L) / (1. 5 M) = 1200 m. L 1200 m. L-100 m. L= 1100 m. L ●

0 A. The following are p. H values: cola-2; orange juice 3; beer-4; coffee-5; human blood-7. 4. Which of these liquids has the highest molar concentration of OH-? 1. 2. 3. 4. 5. cola orange juice beer coffee human blood

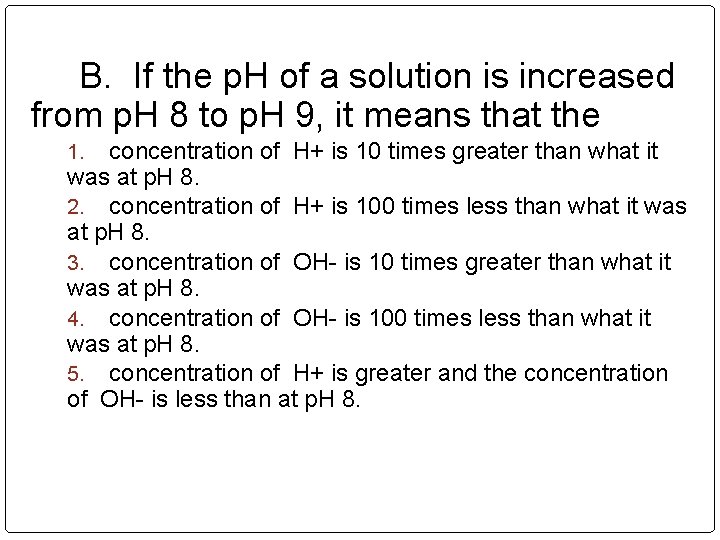
B. If the p. H of a solution is increased from p. H 8 to p. H 9, it means that the concentration of H+ is 10 times greater than what it was at p. H 8. 2. concentration of H+ is 100 times less than what it was at p. H 8. 3. concentration of OH- is 10 times greater than what it was at p. H 8. 4. concentration of OH- is 100 times less than what it was at p. H 8. 5. concentration of H+ is greater and the concentration of OH- is less than at p. H 8. 1.

C. Acid precipitation has lowered the p. H of a particular lake to 4. 0. What is the hydroxide ion concentration of the lake? 1. 2. 3. 4. 5. 10 -7 M 10 -4 M 10 -10 M 10 -14 M 10 M
 Coil visbreaking
Coil visbreaking Hot spot crm
Hot spot crm Geologia
Geologia Hot spot nabe
Hot spot nabe Incomplete casting defect
Incomplete casting defect New zealand biodiversity hotspot
New zealand biodiversity hotspot Earthquake jeopardy
Earthquake jeopardy In argon arc welding the electrode is made of
In argon arc welding the electrode is made of Transform boundary
Transform boundary White hot vs red hot temperature
White hot vs red hot temperature Advantages of cold working
Advantages of cold working Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Be either hot or cold
Be either hot or cold Dna the molecule of life
Dna the molecule of life Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Writ of certiorari ap gov example
Writ of certiorari ap gov example Nader amin-salehi
Nader amin-salehi Prisma diagram example
Prisma diagram example Narrative review vs systematic review
Narrative review vs systematic review Life review therapie
Life review therapie Life insurance review letter
Life insurance review letter Fitness for life chapter 3 review answers
Fitness for life chapter 3 review answers Chapter 2 the chemistry of life section 2-3 answer key
Chapter 2 the chemistry of life section 2-3 answer key Whats a thesis
Whats a thesis Iso weld symbol
Iso weld symbol Melt through weld definition
Melt through weld definition P n e u m a t i c r o a d t u b e s
P n e u m a t i c r o a d t u b e s Cbrn 1 report
Cbrn 1 report Eight points of a compass
Eight points of a compass Spot mr whoops' mistakes answers
Spot mr whoops' mistakes answers