Chemical Formulas and Equations NOTES MOLECULE A molecule


- Slides: 12

Chemical Formulas and Equations NOTES

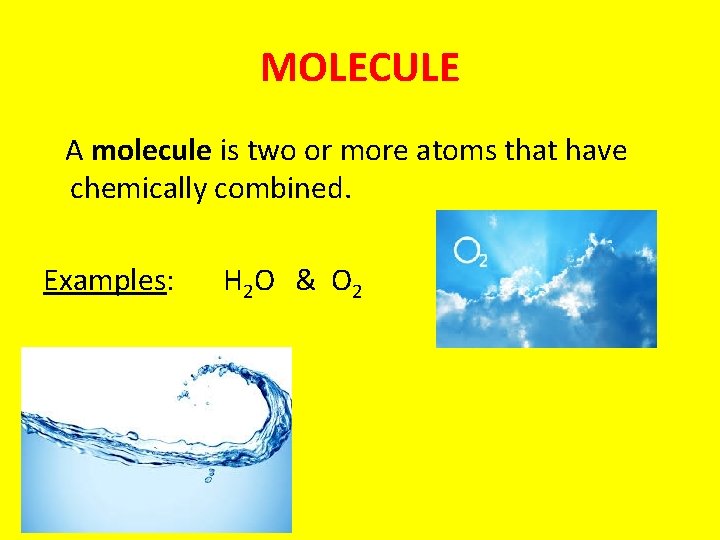
MOLECULE A molecule is two or more atoms that have chemically combined. Examples: H 2 O & O 2

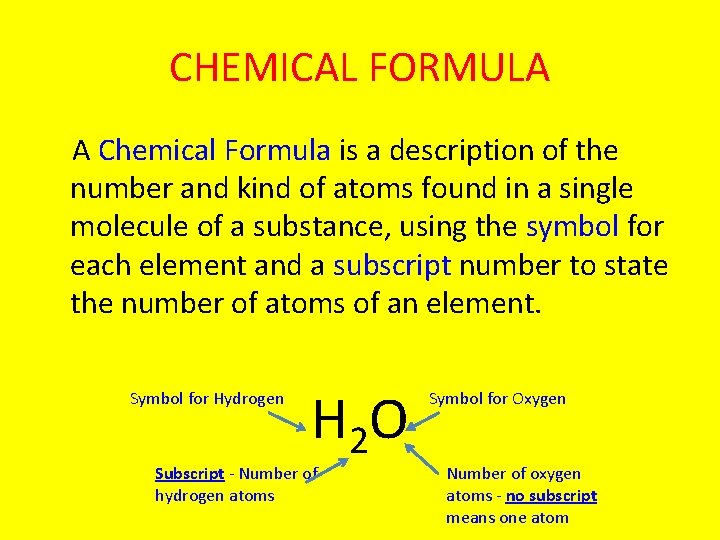
CHEMICAL FORMULA A Chemical Formula is a description of the number and kind of atoms found in a single molecule of a substance, using the symbol for each element and a subscript number to state the number of atoms of an element. Symbol for Hydrogen H 2 O Subscript - Number of hydrogen atoms Symbol for Oxygen Number of oxygen atoms - no subscript means one atom

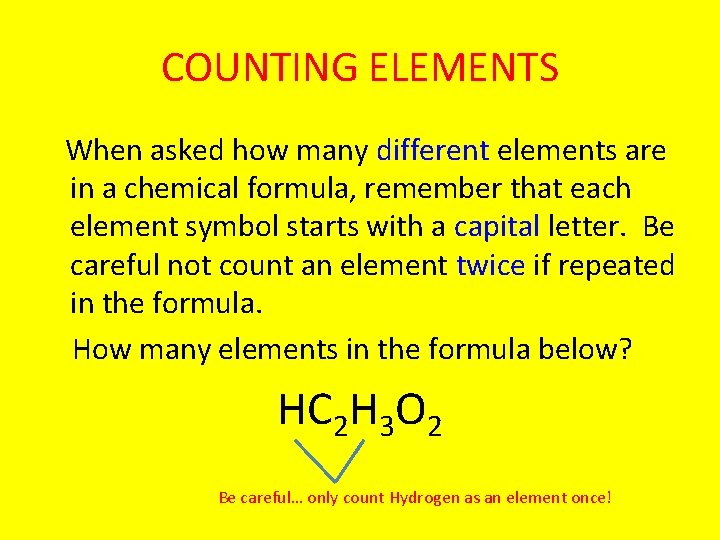
COUNTING ELEMENTS When asked how many different elements are in a chemical formula, remember that each element symbol starts with a capital letter. Be careful not count an element twice if repeated in the formula. How many elements in the formula below? HC 2 H 3 O 2 Be careful… only count Hydrogen as an element once!

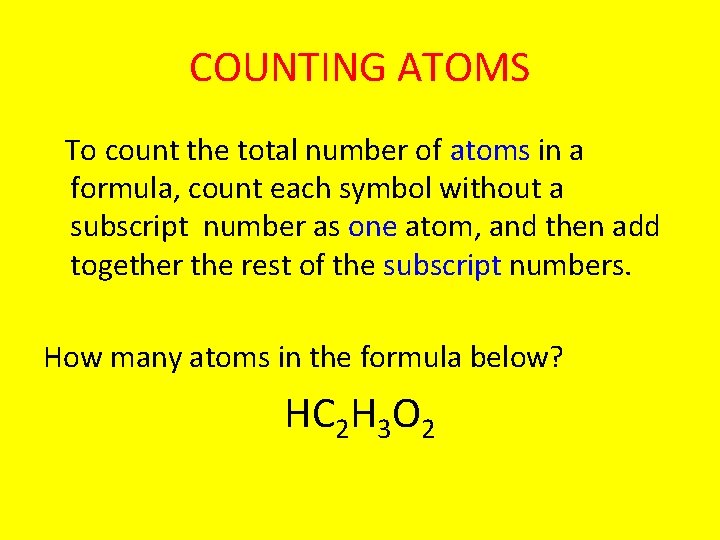
COUNTING ATOMS To count the total number of atoms in a formula, count each symbol without a subscript number as one atom, and then add together the rest of the subscript numbers. How many atoms in the formula below? HC 2 H 3 O 2

Law of Conservation of Matter In a chemical reaction, matter cannot be created or destroyed. The atoms that were present before the reaction took place simply rearranged themselves into new substances. In a baking soda/vinegar reaction the two ingredients Na. HCO 3 and HC 2 H 3 O 2, are rearranged into three different substances with the same number and kind of atoms: Na. C 2 H 3 O 2, H 2 O and CO 2

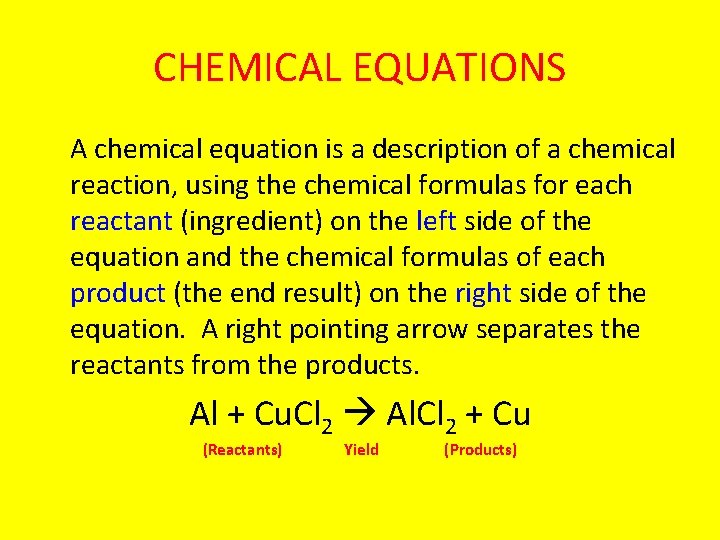
CHEMICAL EQUATIONS A chemical equation is a description of a chemical reaction, using the chemical formulas for each reactant (ingredient) on the left side of the equation and the chemical formulas of each product (the end result) on the right side of the equation. A right pointing arrow separates the reactants from the products. Al + Cu. Cl 2 Al. Cl 2 + Cu (Reactants) Yield (Products)

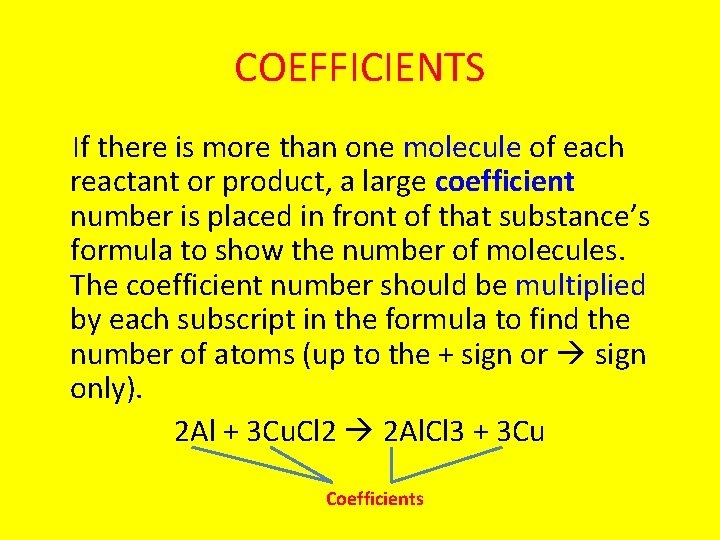
COEFFICIENTS If there is more than one molecule of each reactant or product, a large coefficient number is placed in front of that substance’s formula to show the number of molecules. The coefficient number should be multiplied by each subscript in the formula to find the number of atoms (up to the + sign or sign only). 2 Al + 3 Cu. Cl 2 2 Al. Cl 3 + 3 Cu Coefficients

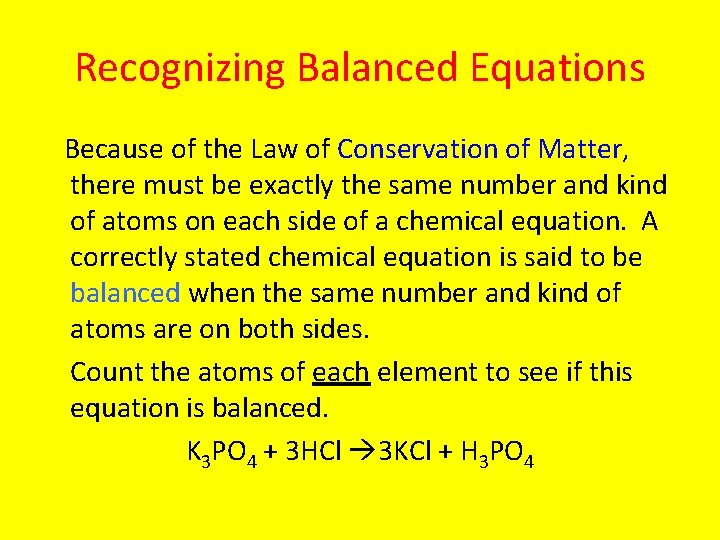
Recognizing Balanced Equations Because of the Law of Conservation of Matter, there must be exactly the same number and kind of atoms on each side of a chemical equation. A correctly stated chemical equation is said to be balanced when the same number and kind of atoms are on both sides. Count the atoms of each element to see if this equation is balanced. K 3 PO 4 + 3 HCl 3 KCl + H 3 PO 4

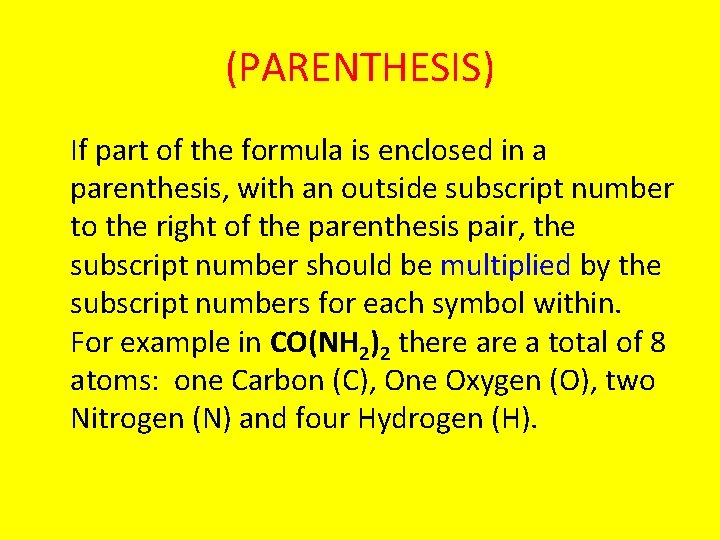
(PARENTHESIS) If part of the formula is enclosed in a parenthesis, with an outside subscript number to the right of the parenthesis pair, the subscript number should be multiplied by the subscript numbers for each symbol within. For example in CO(NH 2)2 there a total of 8 atoms: one Carbon (C), One Oxygen (O), two Nitrogen (N) and four Hydrogen (H).

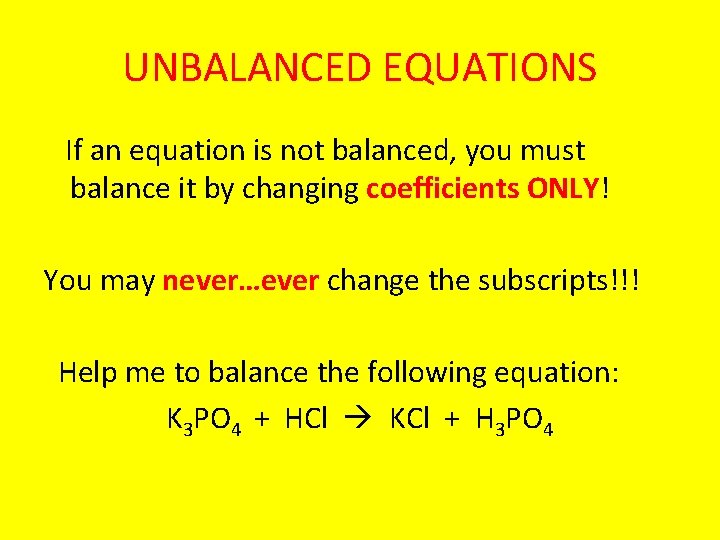
UNBALANCED EQUATIONS If an equation is not balanced, you must balance it by changing coefficients ONLY! You may never…ever change the subscripts!!! Help me to balance the following equation: K 3 PO 4 + HCl KCl + H 3 PO 4

Warm-up 11/9/15 What do Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium have in common? How are they different? What do you think more electrons might mean for reactivity? https: //www. youtube. com/watch? v= m 55 kgy. Ap. Yr. Y